Fig. 31.1
Seborrhoeic dermatitis affecting the scalp and face, especially the eyebrows and perinasal regions (photo courtesy of Prof. Ruiz-Maldonado, MD)
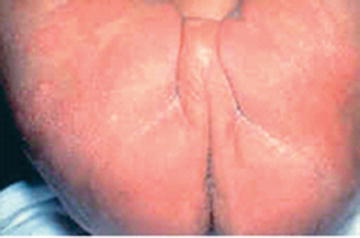
Fig. 31.2
Infantile seborrhoeic dermatitis affecting the diaper area
Some infants may only have scalp involvement which is also known as pityriasis capitis or “cradle cap” (Fig. 31.3). The scales can appear as white, off-white, yellow or brown, in darker children, and is usually asymptomatic in infancy, with no pruritus.
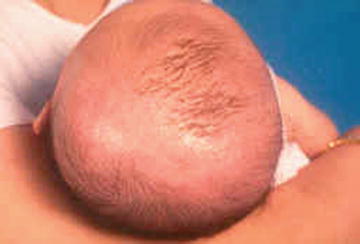

Fig. 31.3
Pityriasis capitis
Black and Hispanic children have an increased risk of tinea capitis. It may be confused with SD if the seborrhoea-like scalp scaling is mild with minimal pruritus and kerion is not present. With a gentle pull of the hairs, the whole hair shaft and hair bulb are obtained in SD in contrast to tinea capitis where the hair shaft breaks easily. Clinicians should examine for the presence of posterior lymphadenopathy, alopecia with broken hairs and black dots as it is associated with a positive predictive value of 97 % for tinea capitis [7]. In a study done on predominantly black and Hispanic children presenting with scalp hyperkeratosis only, the patients were seven times more likely to have tinea capitis compared to SD [8]. Isolation of Trichophyton tonsurans, Trichophyton verrucosum and Microsporum canis confirms the diagnosis of tinea capitis, although other dermatophyte species may also be responsible. The use of Wood’s light is useful as Microsporum ectothrix infections fluoresce green. Older children with scalp SD have loosely adherent, small white or grey flakes. In teenagers, SD may present dermatitis with erythema and white scaling. SD in Hispanic, Asian, and African adolescents may also present with hypopigmented scaly plaques on the eyebrow and perinasal regions [9].
Some infants who have SD in infancy develop atopic dermatitis (AD) and/or psoriasis on follow-up. The proportion of patients that develop AD varies greatly between studies, ranging from 4.5 to 37 % [6, 10–12]. It has been questioned whether SD is a variant of AD or two distinct clinical entities which may coexist. It may be clinically challenging to distinguish infantile AD from SD. However, the most obvious difference between the two conditions is the absence of itch in SD which is invariably present in AD patients [13]. Infantile AD may be distinguished from SD as it usually spares the diaper region which is moist, warm and occluded.
Some authors believe that infantile SD is a spectrum of clinical signs and symptoms which may be caused by infantile AD, infantile psoriasis, infantile candidiasis, Leiner’s disease, and Langerhans cell histiocytosis (LCH), the latter two being rare, but of significant concern given their severity [14].
Infantile SD can be indistinguishable from infantile psoriasis especially in the “inverse” pattern which occurs in the flexures as the usual dry white scales in psoriasis is not present. Instead, the red shiny glazed psoriatic plaques appear similar to SD.
The presence of satellite lesions, papules and pustules is useful in differentiating candidiasis from SD.
Patients with LCH have seborrhoeic dermatitis-like skin lesions which are resistant to treatment. Removing gently a seborrhoea-like scalp scale, a small drop of blood will be observed, which is never present in SD (Fig. 31.4). The presence of petechiae, purpuric papules or lymphadenopathy within the areas of dermatitis should suggest the possibility of LCH [15]. Furthermore, LCH does not clear with conservative therapy. In addition, patients may exhibit unifocal or multifocal bony lumps often on the skull and facial bones. Diabetes insipidus may also be a feature if there is pituitary involvement.
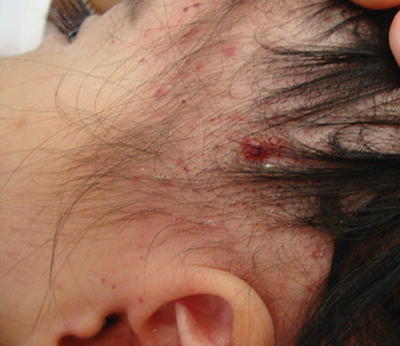

Fig. 31.4
Langerhans cell histiocytosis (photo courtesy of Dr. Carola Duran Mc. Kinster, MD)
Pathogenesis
The Malassezia sp. (previously known as Pityrosporum) is a lipophilic lipase-producing yeast [16]. The lipase splits triglycerides into fatty acids. The yeast organism ingests the saturated fatty acids, allowing the unsaturated fatty acids to penetrate the stratum corneum, causing inflammation [17]. A study of 31 Japanese adults showed that the population of genus Malassezia found was approximately three times more on SD skin than on non-lesional skin [18]. Studies have also shown good correlation between the Malassezia sp. count and severity of SD [19]. There is furthermore strong evidence of clinical response of SD to antifungal treatment in adult studies [20].
In a Chinese study carried out on adult patients with SD, M. globosa and M. restricta were found in 87.0 and 81.5 % of patients, respectively. 82.9 % of these patients also showed colonisation of two or more Malassezia sp. [21]. Similar results have been observed in infants with SD. A study done on Japanese infants aged 1 month showed that M. furfur and M. globosa were isolated from affected patients at significantly higher rates than from healthy infants [22].
Other studies have shown that M. furfur (previously known as P. ovale or P. orbiculae in the oval and round forms, respectively) has been isolated more frequently in infants with SD compared to controls [23]. Prolonged stay in the intensive care unit, parenteral nutrition, insertion of central lines in neonates are risk factors for colonisation of the infant skin with M. furfur [24, 25].
Infants with active SD have been found to have significant levels of a rare essential fatty acid 20:2w6. It is postulated that there is transient abnormal function of the enzyme delta-6-desaturase causing the elevated levels of 20:2w6. The normalisation of the high essential fatty acid levels correlated with a resolution of their SD [26].
Treatment
Most infants recover without treatment
Antifungal creams or shampoos with sometimes in combination with mild potency topical steroids are effective treatment for SD
Patients may require re-treatment as SD commonly recurs.
Most infants will not require any treatment due to the self-limiting nature of SD. Thick scales can be softened through the application of a sterile mineral oil to the scale which is massaged into the scalp for 5–10 min before washing the hair. In a cohort study on 34 children aged between 1 month and 10 years, 97 % of patients treated with bifonazole shampoo 1 % three times weekly for 4 weeks had good to complete resolution of their scalp SD [27]. There is a small study of 13 infants with scalp SD treated with ketoconazole shampoo for 4 weeks. During the course of the treatment, measured serum ketoconazole were undetectable and liver enzymes were not elevated, showing no evidence of significant systemic absorption of topical ketoconazole among infants [28]. Adult studies show that ketoconazole cream or shampoo for 3–4 weeks is efficacious and well tolerated in patients with SD [29, 30].
Alternatively, coal tar shampoo can be used to treat SD although coal tar use on the trunk may be limited by its odour and staining of skin and clothing. Selenium sulphide shampoo has been shown to be effective in treating SD in all regions in older children [31] but is generally not used for application to the face. Older children with thicker scalp scales may also benefit from salicylic acid 2 % cream for 2–3 h before shower. From the authors’ experience, the use of mild topical steroids such as hydrocortisone 1 % cream combined with topical antifungals (e.g. Daktacort®) can help to reduce the erythema associated with SD. Other options include application of desonide lotion in the morning and topical antifungals in the evenings.
For patients of Black or African American descent, it may be difficult to shampoo more frequently than once a week due to intrinsic dryness of the hair shaft. In these patients, usage of pomades with selenium sulphide and/or oil-based mid-potency topical corticosteroids (e.g. fluocinolone) may be less damaging to the hair shaft (Table 31.1).
Table 31.1
Management of seborrhoeic dermatitis
Site | Treatment |
|---|---|
Scalp | • For infants, spontaneous resolution occurs within first few months. If pityriasis capitis is not resolving, trial of bifonazole shampoo 1 % is safe and effective [27] • For older children, topical antifungals such as ketoconazole or coal tar shampoo are efficacious [29, 30] • If facial and scalp SD are present concurrently, the lather of the ketoconazole shampoo can be applied onto the facial SD for 10–15 min before washing off • The antifungal shampoo should be given daily for a few weeks until SD clears and then maintained two to three times a week in patients with recurrent SD • For thicker scalp scales, 2 % salicylic acid cream is useful to loosen the scales |
Face | • Ketoconazole cream combined with mild potency topical steroids can be given for facial SD in children [29, 32, 33] • Pimecrolimus cream for children above the age of 2 years who are refractory to antifungals or mild potency steroid creams [32–35] • A gentle skin cleanser is essential in inflamed facial SD |
Neck and flexures | • Ketoconazole cream or selenium sulphide suspension is effective in eradicating the Malassezia colonisation that causes SD • They may be combined with mild potency topical steroid creams |
Prognosis
The prognosis in infants is generally good as SD resolves on its own. For adolescents, a course of ketoconazole shampoo and cream for scalp and facial SD, respectively, often leads to excellent clinical improvement. Studies also show that the recurrence rate of SD in adults 3–4 months post-antifungal treatment is similar to those treated with topical steroids [29, 32, 33]. Although no formal studies have been done in adolescents, recurrence is not uncommon and patients will benefit from a repeat course of treatment.
In view of the diagnostic challenge in differentiating infantile SD from other chronic conditions such as AD and psoriasis, clinicians must always consider these possible differential diagnoses in patients who do not improve with standard treatment.
Ongoing Research
There has been recent research on the use of topical pimecrolimus and tacrolimus for facial and truncal SD. Topical calcineurin inhibitors were found to be as effective as topical steroids and antifungals in adults [32–35]. A small study also showed that the pimecrolimus group were associated with fewer and less severe relapses compared to topical steroids [35]. Patients on pimecrolimus tend to have more side effects than those treated with topical steroids. Mild transient burning sensation and skin irritation were reported in 10–45 % of patients on pimecrolimus and 0.1 % tacrolimus ointment which usually subsides within the first 72 h [36–40]. Due to the transient side effects, patients often do not discontinue treatment but they should be advised of these adverse effects especially as SD often affects the eyebrows and forehead. Rosaceiform dermatitis is also a rare side effect of pimecrolimus cream which has been reported in the literature [41]. Tacrolimus may cause acne formation if used on facial locations in acne prone pre-teens and teenagers. Furthermore, these agents bear a black box warning in the United States stating that they may be associated with cancer and should not be used in children under the age of 2 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


