Reproduction and the Thyroid
 |
Thomas Wharton, in 1656, gave the thyroid gland its modern name (meaning oblong shield) because he believed the function of the thyroid was to fill vacant spaces and contribute to the shape and beauty of the neck, especially in women.1 For unknown reasons, thyroid disease is more common in women than in men. Because most thyroid disease is autoimmune in nature, an increased susceptibility to autoimmune diseases, perhaps secondary to the female endocrine environment, is a likely contributing factor.
The clinical objective is to detect and treat thyroid disease before the symptoms and signs are significant and intense. Subtle thyroid disease is easily diagnosed by the sensitive laboratory assessments now available. Therefore, the key to early diagnosis is to maintain a high index of suspicion and to readily screen for the presence of abnormal thyroid function. There is growing support to institute routine thyroid screening in two female populations: older women and pregnant women (preconceptual screening would be even better).
Normal Thyroid Physiology
Thyroid hormone synthesis depends in large part on an adequate supply of iodine in the diet. In the small intestine, iodine is absorbed as iodide that is then transported to the
thyroid gland. Plasma iodide enters the thyroid under the influence of thyroid-stimulating hormone (TSH), the anterior pituitary thyrotropin hormone. Within the thyroid gland, iodide is oxidized to elemental iodine, which is then bound to tyrosine. Monoiodotyrosine and diiodotyrosine combine to form thyroxine (T4) and triiodothyronine (T3). These iodinated compounds are part of the thyroglobulin molecule, the colloid that serves as a storage depot for thyroid hormone. TSH induces a proteolytic process that results in the release of iodothyronines into the bloodstream as thyroid hormone.
thyroid gland. Plasma iodide enters the thyroid under the influence of thyroid-stimulating hormone (TSH), the anterior pituitary thyrotropin hormone. Within the thyroid gland, iodide is oxidized to elemental iodine, which is then bound to tyrosine. Monoiodotyrosine and diiodotyrosine combine to form thyroxine (T4) and triiodothyronine (T3). These iodinated compounds are part of the thyroglobulin molecule, the colloid that serves as a storage depot for thyroid hormone. TSH induces a proteolytic process that results in the release of iodothyronines into the bloodstream as thyroid hormone.
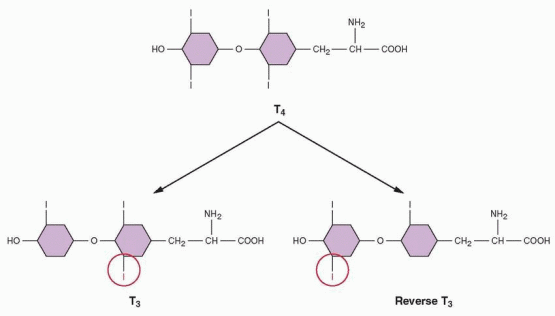 |
Removal of one iodine from the phenolic ring of T4 yields T3, whereas removal of an iodine from the nonphenolic ring yields reverse T3 (RT3), which is biologically inactive. In a normal adult, about one third of the T4 secreted each day is converted in peripheral tissues to T3, and about 40% is converted to the inactive, reverse T3. About 80% of the T3 generated is derived outside the thyroid gland, chiefly in the liver and kidney. T3 is 3-5 times more potent than T4, and virtually all the biologic activity of T4 can be attributed to the T3 generated from it. Although T4 is secreted at 20 times the rate of T3, it is T3 that is responsible for most if not all of the thyroid action in the body. T3 is more potent than T4 because the nuclear thyroid receptor has a 10-fold greater affinity for T3 than T4. Although T4 may have some intrinsic activity of its own, it serves mainly as a prohormone of T3. It is hard to think of a body process or function that does not require thyroid hormone for its normal operation, not only metabolism but also development, steroidogenesis, and most specific tissue activities.
Carbohydrate calories appear to be the primary determinant of T3 levels in adults. A reciprocal relationship exists between T3 and RT3. Low T3 and elevated RT3 are seen in a variety of illnesses such as febrile diseases, burn injuries, malnutrition, and anorexia nervosa. The metabolic rate is determined to a large degree by the relative production of T3 and RT3. During periods of stress, when a decrease in metabolic rate would conserve energy, the body produces more RT3 and less T3, and metabolism slows. On recovery, this process reverses, and metabolic rate increases.
Circulating thyroid hormones are present in the circulation mainly bound to proteins. Approximately 70% of thyroid hormones are bound to thyroxine-binding globulin (TBG), which, therefore, is the major determining factor in the total thyroid hormone concentration in the circulation. The remaining 30% is bound to thyroxine-binding prealbumin and albumin. The binding proteins have a greater affinity for T4 and, thus, allow T3 to have greater entry into cells. TBG is synthesized in the liver, and this synthesis is increased by estrogens. The passage of thyroid hormones into and out of cells is regulated by cell membrane thyroid hormone transporters; mutations in a key transporter are associated with elevated T3 levels and psychomotor retardation.2
The nuclear receptor for thyroid hormone is a member of the super family that includes the steroid hormone receptors (Chapter 2).3 The thyroid hormone receptor exists in several forms, the products of 2 genes located on different chromosomes. The a receptor gene is on chromosome 17, and the β receptor gene is on chromosome 3. The nuclear T3 receptor is truly ubiquitous, indicating the widespread actions of thyroid hormone throughout the body. Mutations in the gene for the thyroid receptor lead to the synthesis of a receptor that actually antagonizes normal receptors, a syndrome of thyroid resistance characterized by elevated thyroid hormone levels. TSH is elevated as well because of the impairment in thyroid hormone action.
The thyroid axis is stimulated by the hypothalamic factor, thyrotropin-releasing hormone (TRH) and inhibited by somatostatin and dopamine. Thyroid hormones regulate TSH by suppressing TRH secretion, but primarily by affecting the pituitary sensitivity to TRH (by reducing the number of TRH receptors). Pituitary secretion of TSH is very sensitive to changes in the circulating levels of thyroid hormone; a slight change in the circulating level of T4 will produce a many-fold greater response in TSH. TSH-secreting cells are regulated by T4, but only after the T4 is converted to T3 in the pituitary cells. Although modulation of thyroid hormone occurs at the pituitary level, this function is permitted by the hypothalamic releasing hormone, TRH. Although some tissues depend mainly on the blood T3 for their intracellular T3, the brain and the pituitary depend on their own intracellular conversion of T4. The measurement of T4 and TSH, therefore, provides the most accurate assessment of thyroid function.
The TSH response to TRH is influenced mainly by the thyroid hormone concentration in the circulation; however, lesser effects are associated with dopamine agonists (inhibition), glucocorticoids (inhibition), and dopamine antagonists (stimulation). Estrogen increases the TRH receptor content of the pituitary; hence, the TSH response to TRH is greater in women than in men, and greater in women taking estrogen-progestin contraceptives.
TRH also stimulates prolactin secretion by the pituitary. The smallest doses of TRH that are capable of producing an increase in TSH also increase prolactin levels, indicating a physiologic role for TRH in the control of prolactin secretion. However, except in hypothyroidism, normal physiologic changes as well as abnormal prolactin secretion can be understood in terms of dopaminergic inhibitory control, and TRH need not be considered.
Functional Changes with Aging
Thyroxine metabolism and clearance decrease in older people, and thyroxine secretion decreases in compensation to maintain normal serum thyroxine concentrations.4 With aging, conversion of T4 to T3 decreases, and TSH levels increase. The TSH response to TRH is normal in older women. TBG concentrations decrease slightly in postmenopausal women but not enough to alter measurements in serum.
Thyroid Function Tests
Free Thyroxine (FT4)
Assays that measure free T4 are usually displacement assays using an antibody to T4. The result is not affected by changes in TBG and binding. The free T4 level has a different range of normal values from laboratory to laboratory, but is usually 0.8-2.0 ng/dL.
Total Thyroxine (TT4)
The total thyroxine, both the bound portion to TBG and the free unbound portion, is measured by displacement assays, and, in the absence of hormone therapy or other illnesses, it estimates the thyroxine concentration in the blood. However, the free T4 is unaffected by factors that influence TBG and is preferred.
Free Thyroxine Index (FTI or T7)
The free thyroxine index is calculated from the TT4 and the T3 resin uptake measurements. This test has been replaced by the free T4 assay.
Total T3 and Reverse T3
Both of these thyronines can be measured by sensitive immunoassays. However, in most clinical circumstances they add little to what is learned by the free T4 and TSH measurements. The clinical situations in which measurement will be useful are discussed under the specific diseases and indicated on the algorithm.
Thyroid-Stimulating Hormone (TSH)
TSH (also called thyrotropin) is measured by highly sensitive assays using monoclonal antibodies, usually in a technique that uses two antibodies, one directed at the a subunit and one directed at the β subunit of TSH. The normal levels vary from laboratory to laboratory, but the sensitive TSH assay can detect concentrations as low as 0.01 μU/mL, and the normal range is usually 0.45-4.5 μU/mL. White people and the elderly normally have slightly higher TSH levels (individuals 80 years and older may have an upper limit as high as 7.5 μU/mL), making the upper limit for the normal range more difficult to interpret.5,6 TSH is a very sensitive indicator of thyroid hormone action at the tissue level because it is dependent on the pituitary exposure to T4. In the absence of hypothalamic or pituitary disease, the sensitive TSH assay will provide the best indication of excess or deficient thyroxine; slight changes in T4 are reflected in a many-fold greater response in TSH. Nearly all women with elevated TSH levels have hypothyroidism. Transient changes in TSH can be caused by systemic illnesses, major psychiatric disturbances, and pharmacologic treatment with glucocorticoid agents or dopamine.
TSH-Receptor Antibody
Antibodies that compete with TSH for its receptor are collectively known as TSH-receptor antibodies, known as TRAb. The assays measure the inhibition of TSH binding, but the percentage binding usually correlates with activation of the receptor by stimulating antibodies. Most patients with Graves’ disease will have detectable TRAb, TSH-receptor antibodies. This is an essential test in patients with hyperthyroidism to differentiate Graves’ disease from non-autoimmune hyperthyroidism, specifically toxic multinodular goiter, in order to select appropriate therapy. This test is not needed to monitor treatment.
Radioactive Iodine Uptake Scan
Because the thyroid gland is the only tissue that utilizes iodine, radioisotopes of iodine can be used as a measure of thyroid gland activity and to localize activity within the gland. The scan is obtained 24 h after the administration of I123 given orally.
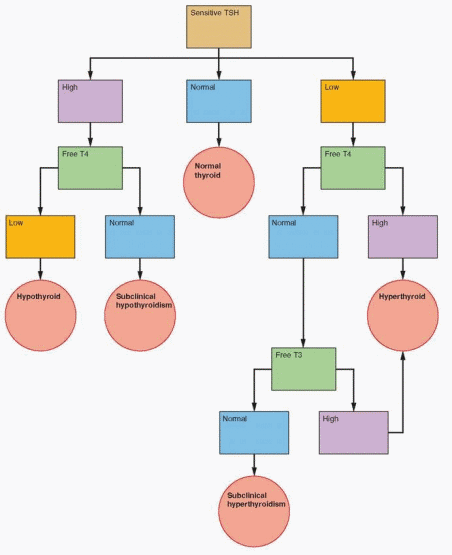
The Laboratory Evaluation
The algorithm represents a cost-effective and accurate clinical strategy. For screening purposes, or when there is a relatively low clinical suspicion of thyroid disease, the initial step is to measure TSH. A normal TSH essentially excludes hypothyroidism or hyperthyroidism. A high TSH requires the measurement of free T4 to confirm the diagnosis of hypothyroidism.
If the initial TSH is low, especially less than 0.08 μU/mL, then measurement of a high T4 will confirm the diagnosis of hyperthyroidism. If the T4 is normal, the T3 level is measured, because some patients with hyperthyroidism will have predominantly T3 toxicosis. If the T3 is normal, this compensated state is called subclinical hyperthyroidism. Some of these patients will eventually have increased T4 or T3 levels with true hyperthyroidism.
Hypothyroidism
In most cases of hypothyroidism, a specific cause is not apparent. It is believed that hypothyroidism is usually secondary to an autoimmune reaction, and, when goiter formation is present, it is called Hashimoto’s thyroiditis.7 Unless abnormal thyroid function can be documented by specific laboratory assessment, empiric treatment with thyroid hormone is not indicated, and it is especially worth emphasizing that thyroid hormone treatment does not help infertility in euthyroid women. Hypothyroidism can be a cause of recurrent miscarriages, and an assessment of thyroid function is worthwhile in these patients.
Hypothyroidism increases with aging and is more common in women.8 Up to 45% of thyroid glands from women older than age 60 show evidence of thyroiditis.9 In women admitted to geriatric wards, 2-4% have clinically apparent hypothyroidism. Therefore, hypothyroidism is frequent enough to warrant consideration in most older women, justifying screening even in asymptomatic older women. We recommend that older women be screened with the TSH assay every 5 years beginning at age 35, then every 2 years beginning at age 60, or with the appearance of any symptoms suggesting hypothyroidism.10
 |
Menstrual irregularities and bleeding problems are common in hypothyroid women. Amenorrhea can be a consequence of hypothyroidism, either with TRH-induced increases in prolactin or with normal prolactin levels. Other clinical manifestations of hypothyroidism include constipation, cold intolerance, psychomotor retardation, carpal tunnel syndrome, and decreased exercise tolerance. However, patients often are asymptomatic. Close evaluation can reveal mental slowness, decreased energy, fatigue, poor memory, somnolence, slow speech, a low-pitched voice, water retention, periorbital edema, delayed reflexes, or a low body temperature and
bradycardia. Hypothyroidism can cause hypertension, cognitive abnormalities, pericardial effusion, asymmetric septal myocardial hypertrophy, myopathy, neuropathy, ataxia, anemia, elevated cholesterol and LDL-cholesterol, or hyponatremia. Myxedematous infiltration can produce enlarged, cystic ovaries.11 The increase in cholesterol is due to impaired LDL-cholesterol clearance secondary to a decrease in cell membrane LDL receptors. The mechanism for this LDL effect is attributed to a thyroid response element in the LDL receptor gene.12
bradycardia. Hypothyroidism can cause hypertension, cognitive abnormalities, pericardial effusion, asymmetric septal myocardial hypertrophy, myopathy, neuropathy, ataxia, anemia, elevated cholesterol and LDL-cholesterol, or hyponatremia. Myxedematous infiltration can produce enlarged, cystic ovaries.11 The increase in cholesterol is due to impaired LDL-cholesterol clearance secondary to a decrease in cell membrane LDL receptors. The mechanism for this LDL effect is attributed to a thyroid response element in the LDL receptor gene.12
Serum enzymes may be elevated because of decreased clearance, including creatine phosphokinase (CPK), aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT), lactic dehydrogenase (LDH), and alkaline phosphatase, triggering a fruitless search for other organ disease. It is worth screening for hypothyroidism in any women with abnormal menses or with complaints of fatigue and depression. In addition, patients should be screened who have elevated levels of cholesterol and LDL-cholesterol.
Diagnosis of Hypothyroidism
With primary thyroid failure, the circulating thyroid hormone levels fall, stimulating the pituitary to increase TSH output. Elevated TSH and low T4 confirm the diagnosis. Hypothyroidism can occur due to pituitary failure in which case the TSH will be inappropriately low for the T4. The most common cause is autoimmune thyroid disease (elevated titers of antithyroid antibodies) in areas with normal iodine intake. However, making an etiologic diagnosis in women adds little to the clinical management.
Subclinical Hypothyroidism
In early hypothyroidism, with undetectable symptoms or signs, a compensated state can be detected by an elevated TSH (greater than the upper limit of the normal range of 0.45-4.5 μU/mL) and normal T4 (called subclinical hypothyroidism). The prevalence is greater in women. About 2 to 5% each year will eventually become clinically hypothyroid with low T4 concentrations.13,14 Subclinical hypothyroidism is present in 4 to 8.5% of U.S. adults, is less common in blacks, and increases with age, present in up to 20% of women over age 60.14
A good reason to treat subclinical hypothyroidism is to avoid the development of a goiter. Furthermore, some patients in retrospect (after treatment) recognize improved physical and mental well-being. Patients with subclinical hypothyroidism have alterations in energy metabolism in skeletal muscle.15 An improvement in impaired cognitive function and emotional behavior has been documented with thyroxine treatment of subclinical hypothyroidism.16 In those patients who are asymptomatic, it is worth measuring antithyroid antibodies. A positive test identifies those who are more likely to become clinically hypothyroid, at a rate of approximately 20% per year. With only very slight elevations of TSH (less than 10 μU/mL), it is reasonable not to treat asymptomatic patients, especially those over age 80, and to check thyroid function every 6 months to detect further deterioration; however symptomatic patients may benefit from treatment.13 Patients with an abnormal cholesterol-lipoprotein profile can show a rapid improvement with thyroxine treatment.17,18 and 19 Subclinical hypothyroidism is a strong risk factor for coronary heart disease.20 In addition, iron deficiency anemia is common in patients with subclinical hypothyroidism, and responds better when levothyroxine is added to iron treatment.21
The risk of pregnancy loss is increased for women with uncorrected overt or even subclinical hypothyroidism. The results of a study of pregnancy outcomes in women with hypothyroidism challenged the notion that subclinical hypothyroidism has no impact on pregnancy.22
The incidence of pregnancy loss was very low in treated hypothyroid women having normal thyroid indices, but markedly increased in women with elevated TSH levels, including both women with untreated subclinical disease and those with overt disease who received inadequate exogenous thyroid hormone replacement. These observations indicate that subclinical hypothyroidism is not entirely benign and further justify recommendations to include TSH screening in the evaluation of women with recurrent pregnancy loss.
The incidence of pregnancy loss was very low in treated hypothyroid women having normal thyroid indices, but markedly increased in women with elevated TSH levels, including both women with untreated subclinical disease and those with overt disease who received inadequate exogenous thyroid hormone replacement. These observations indicate that subclinical hypothyroidism is not entirely benign and further justify recommendations to include TSH screening in the evaluation of women with recurrent pregnancy loss.
Treatment of Hypothyroidism
Initial therapy is straightforward with synthetic thyroxine, T4, given daily. Mixtures of T4 and T3, such as desiccated thyroid, provide T3 in excess of normal thyroid secretion. It is better to provide T4 and allow the peripheral conversion process to provide the T3.23,24 “Natural” thyroid preparations are not better, and in fact are potentially detrimental. Patients taking biologic preparations should be switched to synthetic thyroxine. Studies have documented that adding T3 to T4 does not improve treatment outcomes.25,26 and 27 Because of a risk of coronary heart disease in older women, the initial dose should be 25-50 μg/day for 4 weeks, at which time the dose is increased by 25 μg daily every 4 weeks according to the clinical and biochemical assessment. Usually the dose required will be close to 1.5 μg/lb body weight, but it may be less in very old women.28 The average final dose required in the elderly is approximately 70% of that in younger patients. Patients who have been on thyroid hormone for a long time may have their medication discontinued. Recovery of the hypothalamic-pituitary axis usually requires 8 weeks at which time the TSH and free T4 levels can be measured.
Evaluation of Therapy
When the patient appears clinically euthyroid, evaluation of TSH levels will provide the most accurate assessment of the adequacy of thyroid hormone replacement. The goal is to maintain the TSH in the lower half of the normal range, between 0.45 and 2.0 μU/mL.7,29 Thyroid hormone requirements decrease with age. A patient being treated with thyroid hormone should be evaluated once every year with the TSH assay, and each patient should consistently remain on the same levothyroxine product. If the TSH level is low, then the free T4 should be measured to help adjust the thyroxine dose.30 The full response of TSH to changes in T4 is relatively slow; a minimum of 8 weeks is necessary between changes in dosage and assessment of TSH.
Hyperthyroidism
The two primary causes of hyperthyroidism are Graves’ disease (toxic diffuse goiter) and Plummer’s disease (toxic nodular goiter).31 Plummer’s disease is usually encountered in postmenopausal women who have had a long history of goiter. Twenty percent of hyperthyroid patients are older than 60, and 25% of older women with hyperthyroidism present with an apathetic or atypical syndrome.
Graves’ disease, about 5 to 10 times more common in women than men, is characterized by the triad of hyperthyroidism, ophthalmopathy, and pretibial myxedema and is caused by autoantibodies that have TSH properties and, therefore, bind to and activate the TSH receptor. The measurement of TSH-receptor antibodies is essential to distinguish Graves’ disease
from toxic goiter. Menstrual changes associated with hyperthyroidism are unpredictable, ranging from amenorrhea to oligomenorrhea to normal cycles (hence, the amenorrhea in a thyrotoxic woman can be due to pregnancy).
from toxic goiter. Menstrual changes associated with hyperthyroidism are unpredictable, ranging from amenorrhea to oligomenorrhea to normal cycles (hence, the amenorrhea in a thyrotoxic woman can be due to pregnancy).
The classic symptoms of thyrotoxicosis are nervousness, disturbed sleep, heat intolerance, weight loss, sweating, palpitations, and diarrhea. These symptoms are associated with typical findings on physical examination: proptosis, lid lag, tachycardia, tremor, warm and moist skin, and goiter. Women in the reproductive years usually present with the classic picture. In postmenopausal women, symptoms are often concentrated in a single organ system, especially the cardiovascular or central nervous system. Goiter is absent in 40%. Sinus tachycardia occurs in less than half, but atrial fibrillation occurs in 40% and is resistant to cardioversion or spontaneous reversion to sinus rhythm. In old women, there is often a coexistent disease, such as an infection or coronary heart disease that dominates the clinical picture.
Hyperthyroidism in older women is sometimes described as “apathetic hyperthyroidism” because the clinical manifestations are different. The triad of weight loss, constipation, and loss of appetite, suggesting gastrointestinal malignancy, occurs in about 15% of older patients with hyperthyroidism. Ophthalmopathy is rare in older patients. The clinician should consider the diagnosis in older patients with “failure to thrive”; in patients who are progressively deteriorating for unexplained reasons; and in patients with heart disease, unexplained weight loss, and mental or psychological changes.
Psychological changes are not unusual in hyperthyroid women. Women who complain of emotional lability and nervousness should be screened for hyperthyroidism.
Diagnosis of Hyperthyroidism
The diagnosis of hyperthyroidism requires laboratory testing. A suppressed TSH (below 0.4 μU/mL) with a high T4 or a high T3 confirms the diagnosis. About 2% of U.S. adults will have subclinical hyperthyroidism, more common in women and blacks.14 Progression to overt hyperthyroidism is essentially limited to patients with a TSH level lower than 0.1 μU/mL. Graves’ disease is associated with the presence of TSH receptor autoantibodies, TRAb. The measurement of TRAb in all patients with hyperthyroidism is important in order to confirm a diagnosis of Graves’ disease.32 Most patients should have a radioactive iodine thyroid uptake and scan after laboratory confirmation of the diagnosis. If the uptake is suppressed then drug therapy is indicated. The scan will indicate whether the patient has a diffuse toxic goiter, a solitary hot nodule, or a hot nodule in a multinodular gland. Toxic multinodular goiters occur more frequently in the elderly. TSH hypersecretion as a cause of hyperthyroidism is extremely rare; the combination of a normal or elevated TSH and elevated thyroid hormone will be the clue to this possibility.
Subclinical Hyperthyroidism
By definition, patients with subclinical hyperthyroidism have normal T4 and T3 levels, but subnormal concentrations of TSH. TSH levels can be suppressed to 0.1-0.5 μU/mL by general illnesses and drugs such as glucocorticoids, dopamine, and anticonvulsants; however, this suppression does not extend below 0.1 μU/mL. Values below 0.1 μU/mL are regarded as nondetectable, and patients with overt hyperthyroidism usually have undetectable TSH. Subclinical hyperthyroidism is half as common in older people as subclinical hypothyroidism (excluding the most common cause, treatment with excessive doses of thyroxine). Keep in mind that the dose of thyroxine required to treat hypothyroidism declines
with age (because of the decrease in metabolic clearance with age); all patients being treated with thyroid hormone should have their TSH levels assessed every year. Atrial fibrillation is a common cardiovascular problem associated with subclinical hyperthyroidism, especially in older women when the TSH is less than 0.1 μU/mL.14,33 Progression to overt hyperthyroidism is uncommon. Therefore, TSH levels less than 0.1 μU/mL should be treated to avoid bone loss and atrial fibrillation in older women, or in those at risk for osteoporosis and heart disease. With TSH levels of 0.1-0.4 μU/mL, treatment is indicated only in older patients but TSH follow-up in younger women is warranted every 6 months.14,34,35
with age (because of the decrease in metabolic clearance with age); all patients being treated with thyroid hormone should have their TSH levels assessed every year. Atrial fibrillation is a common cardiovascular problem associated with subclinical hyperthyroidism, especially in older women when the TSH is less than 0.1 μU/mL.14,33 Progression to overt hyperthyroidism is uncommon. Therefore, TSH levels less than 0.1 μU/mL should be treated to avoid bone loss and atrial fibrillation in older women, or in those at risk for osteoporosis and heart disease. With TSH levels of 0.1-0.4 μU/mL, treatment is indicated only in older patients but TSH follow-up in younger women is warranted every 6 months.14,34,35
Treatment of Hyperthyroidism
There are multiple objectives of therapy: control of thyroid hormone effects on peripheral tissues by pharmacologic blockade of beta-adrenergic receptors, inhibition of thyroid gland secretion and release of thyroid hormone, and specific treatment of non-thyroidal systemic illnesses that can exacerbate hyperthyroidism or be adversely affected by hyperthyroidism.36 Antithyroid drugs are administered first to achieve euthyroidism before definitive therapy is accomplished by radioactive iodine treatment only when symptoms are severe or when radioactive iodine therapy must be delayed. Of course, it is important to ensure that a woman is not pregnant before treatment with radioactive iodine, and pregnancy should be postponed for several months after treatment. Monitoring treatment response requires a full 8-week interval for stabilization of the hypothalamic-pituitary-thyroid system.
Antithyroid Drugs
The drug of choice in most circumstances (except in pregnant women, as discussed later) is methimazole because it has fewer adverse effects. The drug inhibits organification of iodide and decreases production of T4 and T3. The oral dose is 10-20 mg daily. The onset of effect takes 2-4 weeks. Remember that the half-life of thyroxine is about 1 week, and the gland usually has large stores of T4. Maximal effect occurs at 4-8 weeks. The dose can be titrated down once the disease is controlled to a maintenance dose of 5-10 mg daily. The major side effects are rash, gastrointestinal symptoms, and agranulocytosis (an idiosyncratic reaction). Propranolol and other beta-blockers are effective in rapidly controlling the effects of thyroid hormone on peripheral tissues. The dose is usually 20-40 mg, every 12 h orally, and the dose is titrated to maintain a heart rate of about 100 beats/ min. The drug may cause bronchospasm, worsening congestive heart failure, fatigue, and depression. Rarely inorganic iodine is needed to block release of hormone from the gland. Lugol’s solution, 2 drops in water daily, is sufficient. The onset of effect is 1-2 days, with maximal effect in 3-7 days. There may be an escape from protection in 2-6 weeks, and the drug can cause rash, fever, and parotitis. Iodine precludes radioiodine administration for several months.
Most patients to be treated with radioactive iodine are not pretreated with antithyroid drugs. Some patients with hot nodules in multinodular glands require surgery because of the size of the gland and because the hyperthyroidism tends to recur in new nodules after the ablation of the original hot nodule. This can result in repetitive treatments with substantial doses of radioactive iodine, and surgery may be preferable. All patients definitively treated for hyperthyroidism must be monitored for the onset of hypothyroidism.
Osteoporosis and Excessive Thyroxine
Because postmenopausal women are at increased risk for osteoporosis and frequently develop hyperthyroidism or receive levothyroxine treatment for hypothyroidism, it is important to understand how thyroid hormone affects bones. Thyroid hormone excess alters bone integrity via direct effects on bone and gut absorption of calcium and indirectly through the effects of vitamin D, calcitonin, and parathyroid hormone.37
Thyroid hormone increases bone mineral resorption. In addition, total and ionized calcium increase in hyperthyroid women, leading to increases in serum phosphorus; alkaline phosphatase; and bone Gla protein (osteocalcin), a marker of bone turnover. Parathyroid hormone decreases in response to the increased serum calcium, and this results in decreased hydroxylation of vitamin D. Intestinal calcium and phosphate absorption decrease, while urinary hydroxyproline and calcium excretion increase. The net effect of excessive thyroid hormone is increased bone resorption and a subsequent decrease in bone density—osteoporosis.38
These effects become more clinically important in prolonged exposure to excessive thyroid hormone.39 Women who have had hyperthyroidism are at greater risk for fractures and experience postmenopausal fractures earlier than usual.40,41 Postmenopausal women with subclinical hyperthyroidism have lower bone density measurements and experience a higher rate of fractures.42,43
A major concern is that mild chronic excess thyroid hormone replacement, especially in postmenopausal women, might increase the risk of osteoporosis, and indeed this has been documented.44 Bone density has been found to be reduced (9%) in premenopausal women receiving enough thyroxine to suppress TSH for 10 years or more.45 A meta-analysis of the literature on this subject concluded that premenopausal women treated for long durations did not suffer a clinically significant loss of bone (probably because of the protective presence of estrogen); nevertheless, postmenopausal women lose an excess of bone if thyroid treatment results in TSH levels below the normal range.46 Case-control and cohort studies, however, have been unable to detect an increase in fractures associated with thyroid administration.47,48 and 49
Because this is a problem easily avoided, we believe exposure to excessive thyroxine must be added to the risk factors for osteoporosis. It makes sense to monitor patients (both premenopausal women and especially postmenopausal women) receiving thyroxine with TSH levels to ensure that levothyroxine doses are “physiologic.” Some patients who require TSH suppressive doses of thyroxine, such as patients with cancer, must be considered at increased risk of osteoporosis. It would be wise to assess bone density in women on long-term thyroid treatment and in women receiving high-dose thyroxine suppression of TSH. The use of hormone therapy, exercise programs, and possibly bisphosphonate treatment must be seriously considered for these patients. In a cross-sectional study of elderly women, the bone loss associated with long-term thyroid treatment was avoided in those women also taking estrogen.50
Thyroid Nodules
The major concern with thyroid nodules is the potential for thyroid cancer.51 Single nodules are 4 times more common in women, and carcinoma of the thyroid is nearly 3 times more
common in women than in men. The incidence rises steadily after the age of 55. Mortality from thyroid cancer occurs predominantly in the middle-aged and the elderly. There are 4 major types of primary thyroid carcinoma: papillary, follicular, anaplastic, and medullary. In solitary nodules that are “cold” (those that do not take up radioactive iodine or pertechnetate on thyroid scan), 12% prove to be malignant. This also means that the majority are
benign. Surgical excision of nodules can result in vocal cord paralysis, hypoparathyroidism, and other complications. Therefore, the goal is to select patients for curative surgery who have the greatest likelihood of having cancer in the nodule.
common in women than in men. The incidence rises steadily after the age of 55. Mortality from thyroid cancer occurs predominantly in the middle-aged and the elderly. There are 4 major types of primary thyroid carcinoma: papillary, follicular, anaplastic, and medullary. In solitary nodules that are “cold” (those that do not take up radioactive iodine or pertechnetate on thyroid scan), 12% prove to be malignant. This also means that the majority are
benign. Surgical excision of nodules can result in vocal cord paralysis, hypoparathyroidism, and other complications. Therefore, the goal is to select patients for curative surgery who have the greatest likelihood of having cancer in the nodule.
 |
Epidemiologic and Clinical Data
The major risk factors for thyroid cancer are family history of this disease or a history of irradiation to head or neck. In those who have received thyroid irradiation, about one-third will have thyroid abnormalities, and about one-third of those with abnormalities will have thyroid cancer (about 10% overall). The carcinogenic risk has been estimated to be 1% per 100 rads in 20 years. A rapidly growing nodule, a hard nodule, the presence of palpable regional lymph nodes, or vocal cord paralysis greatly increase the probability of thyroid cancer.
Thyroid nodules in multinodular thyroid glands, not previously exposed to thyroid irradiation, have no greater risk of thyroid carcinoma than normal glands. Therefore, predominant thyroid nodules in multinodular glands should be followed, and, if a nodule grows, then biopsy or surgery should be considered.
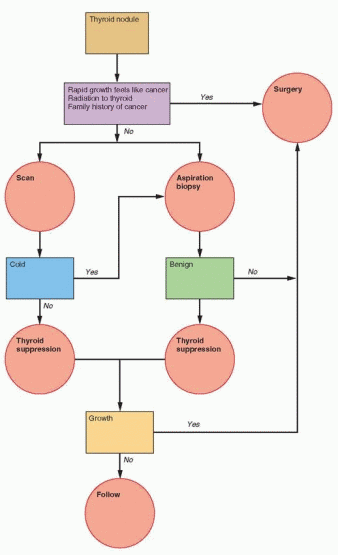
Diagnostic Strategy
In patients with a thyroid nodule, laboratory assessment of thyroid function is essential. When abnormal thyroid function is present, the nodule is almost always benign. Ultrasonography of the thyroid is necessary to identify the presence of nonpalpable nodules and to guide fine-needle biopsy. Detection of a thyroid nodule is followed by clinical characterization of the nodule; examination of the lymph nodes; and inquiry regarding rapid growth, family history, and history of thyroid irradiation. In the presence of any positive findings, surgery is recommended for excision of the nodule. If none of these is present, proceed directly to fine-needle aspiration biopsy. Suppression with levothyroxine treatment is no longer recommended for two reasons: poor efficacy and an inability to differentiate benign lesions from thyroid cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


