Pulmonary Disorders in Pregnancy
Brian A. Mason
Karen Dorman
Pregnancy causes profound changes in the physiology of respiration such that the physiologic adaptations of pregnancy may cause respiratory sequelae in any pregnant woman. Beyond the normal respiratory changes, there are certain pathologic pulmonary complications which are unique to pregnancy and are explored at length in this chapter. In addition to the pulmonary conditions unique to pregnancy, pregnant women may be subject to the entire host of respiratory complications which occur in the non-pregnant population as well. The pathophysiology and treatment of these conditions can be altered by pregnancy, and a number of these conditions and their treatments are outlined in this chapter.
Maternal Cardiopulmonary Changes in Pregnancy
It is impossible to speak of respiratory changes in pregnancy in isolation from the cardiovascular changes. The maternal cardiovascular system undergoes changes to allow it to support the increased oxygen and nutrient delivery demands of the growing fetus. During an average singleton pregnancy, maternal blood volume increases by at least 40 percent.1,2 While red cell mass rises as well, it increases less than 30 percent above baseline, which causes a 10 percent apparent drop in hematocrit values. This is sometimes referred to as a physiologic anemia of pregnancy.2,3 As a result, the oxygen carrying capacity of an individual milliliter of maternal blood is slightly less than that of her non-pregnant counterpart. Furthermore, as intravascular volume expands, plasma protein density decreases, which leads to a decrease in plasma colloid osmotic pressure (COP).
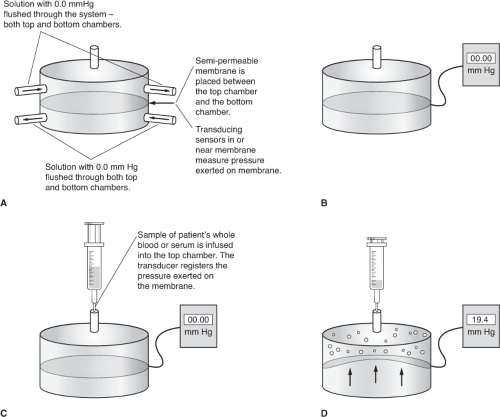
With the advent of membrane transducer systems developed in the 1960s, measurement of COP became a reality. A number of commercial devices are available for the determination of plasma COP. A schematic of such a device is represented in Figure 9-1. A semipermeable membrane, usually selectively permeable to proteins with 30,000 particle weight, separates two chambers. A sensitive pressure transducer which incorporates a Wheatstone bridge is located in the membrane. The chamber below the membrane is the reference chamber, and that above the membrane is the sample chamber. First, both chambers are filled with isotonic saline and the device is zeroed. In addition, daily (or more frequent) calibration of various COP values is performed through the use of a water manometer connected to the sample chamber or through the use of a standard solution with a known COP value. The sample to be processed is placed in the upper chamber and the solutions equilibrated for a short time. From the reference chamber, saline is drawn across the semipermeable membrane and into the sample chamber. This movement is a result of the presence of the colloid protein molecules. The net movement of fluid results in a negative pressure in the reference chamber. After amplification of the electronic signal, a value for the oncotic pressure of the sample is displayed. Several determinations are then made and the results averaged. A comparison of COP values in non-pregnant and pregnant women is presented in Table 9-1. In addition to measurement via a commercial device, COP may be estimated based on a calculation from an equation. Equations for this calculation are presented in Box 9-1.
Interest in the clinical application of COP resulted in multiple studies which have demonstrated the role of COP in the development of pulmonary edema. The alterations in COP associated with pregnancy greatly increase the patient’s risk for pulmonary edema, even at lower intravascular hydrostatic pressures. There is an additional fall in COP in the first 24 hours after delivery, which is likely due to blood loss and mobilization of extravascular fluid. This makes the first 24 hours after parturition potentially hazardous for a woman predisposed to pulmonary edema.
Table 9.1 Colloid Osmotic Pressure Values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Box 9-1. Formulas for Calculating Colloid Oncotic Pressure
These formulae can be used to calculate colloid osmotic pressure (COP) within ± 10%. The top formula has a 75% correlation, and the bottom formula has an 80% correlation/
The hemodynamic alterations that occur during pregnancy, specifically the increased intravascular volume, are largely accommodated by an increase in vascular capacitance. This increased capacitance, coupled with an increase in left ventricular compliance, allows the central venous pressure (CVP) and pulmonary capillary wedge pressure (PCWP) to remain essentially unchanged in pregnancy. This leads to an increased left ventricular end-diastolic volume and an increase in stroke volume. The increase in vascular capacitance causes systemic vascular resistance (SVR) to decrease. Despite the fact that the cardiac ejection fraction is essentially unchanged in pregnancy, the increased stroke volume and decreased SVR, in addition to the physiologic increase in heart rate, lead to a significant increase in cardiac output. Cardiac output at rest increases by at least 45 percent by 32 weeks of gestational age.
Oxygen delivery to tissues is determined by cardiac output and arterial oxygen content. In pregnancy, despite a slight decrease in oxygen-carrying capacity related to hemodilution, arterial oxygen content actually increases. The net effect of these normal physiologic changes is a significant increase in cardiac output with a consequent increase in oxygen delivery. This is largely accomplished through changes in cardiorespiratory physiology during pregnancy.3,4 Concepts related to hemodynamic and oxygen transport physiology are described in depth in Chapter 4 of this text.
There are a number of mechanical as well as physiologic alterations in the respiratory system during pregnancy. Although the diaphragm becomes elevated by at least 5 centimeters near term, its excursion does not decrease. Further, there is a 50 percent increase in the average costal angle resulting in an increase in the circumference of the lower chest wall.5 The net effect of these changes is a slight decrease in total lung capacity. However, vital capacity does not change secondary to intrapulmonary dilation, possibly related to the influence of progesterone and its resultant slight decrease in airway resistance. The intrinsic increase in pulmonic volume leads to a 35 percent increase in tidal volume, which in turn leads to an increase in minute ventilation, despite a minimal increase in respiratory rate (less than 10 percent) late in pregnancy. This large increase in tidal volume, in the face of a relatively fixed or slightly decreased total lung volume, causes the gravida to utilize respiratory reserves. This results in a significant decrease in functional residual capacity (FRC). During normal pregnancy, the 30 percent increase in oxygen consumption related to maternal and fetal metabolic requirements is well balanced with the increase in minute ventilation, cardiac output, and oxygen delivery. However, should oxygen demand increase or respiratory function be compromised, there is extremely limited respiratory reserve in the pregnant woman. There is an even greater loss of FRC near term when the patient is recumbent. The reduction in FRC results in a loss of the “oxygen reservoir” function of end-expiratory lung volume and causes a much more rapid hemoglobin desaturation during periods of respiratory compromise. This makes maintaining oxygenation during endotracheal intubation considerably more difficult in the pregnant woman, especially in the supine position and at advanced fetal gestational age.
There are dramatic changes in minute ventilation and respiratory drive in pregnancy. Central respiratory drive is increased as early as 13 weeks gestation and continues to increase until approximately 37 weeks gestation. It does not fully return to normal until approximately 4 months postpartum. These changes in respiratory drive are believed to be related to increased sensitivity of the respiratory center to the partial pressure of carbon dioxide (CO2) in the blood or because of the direct respiratory stimulation effect of progesterone.6,7 Minute ventilation increases so dramatically that a respiratory alkalemia develops, despite the increased CO2 production in pregnancy. The respiratory alkalemia is partially compensated by renal bicarbonate wasting; therefore, a normal arterial blood gas in a pregnant woman reflects a partially compensated respiratory alkalemia. A comparison of normal arterial blood gases during pregnancy and in non-pregnant women is presented in Table 9-2.
As a result of these cardiorespiratory changes, the pregnant woman has very limited cardiopulmonary reserve. It is therefore relatively easy for her to demonstrate pulmonary decompensation in the presence of any respiratory compromise.
Although the etiologies of various pulmonary disorders differ, the clinical signs and symptoms of pulmonary compromise are similar. Thus, it is extremely important for the care provider to understand potential etiologies and predisposing factors of these complications in order to facilitate correct diagnosis and treatment modalities. Early detection and resolution of the disease process is important for optimizing maternal and fetal outcomes.
Table 9.2 Arterial Blood Gas Values During Pregnancy | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Physiologic Pulmonary Complications
Dyspnea of Pregnancy
Dyspnea, synonymous with shortness of breath or air hunger, is a common feature of normal pregnancy affecting up to 70 percent of gravidas. Patients may experience dyspnea as early as the first trimester, although symptoms typically peak between 28 and 32 weeks of gestation. This occurs independent of exertion and may occur spontaneously at rest. As common as this finding is, its etiology is not fully understood. It does not correlate well to increasing abdominal girth, as has been previously suggested. However, some studies have demonstrated that women who have a higher baseline pre-pregnancy arterial partial pressure of carbon dioxide (PaCO2) have a higher tendency toward dyspnea. In addition, there appears to be a correlation between falling PaCO2 in pregnancy and symptoms of shortness of breath or air hunger. This is likely a manifestation of rising levels of progesterone on oxygen chemoreceptors in the carotid body which increase the physiologic set point of oxygen tension.
In a pregnant woman who presents with a chief complaint of shortness of breath or air hunger, absent any identified history or evidence of a concomitant cardiac or respiratory complication, and in the presence of an arterial oxygen saturation (SaO2) of 95 percent or greater via pulse oximetry on room air or a normal arterial PaO2 for pregnancy, it is reasonable to reassure her that this may be a normal effect of pregnancy. It represents a normal physiologic adaptation to increased partial oxygen tension, thus increasing the O2 diffusion gradient. This facilitates oxygenation of the developing fetus. If no adverse change in maternal or fetal status is evident, no additional evaluation or treatment is necessary.8,9 It may be difficult, however, to differentiate benign dyspnea of pregnancy from other pathologic causes of shortness of breath or air hunger. Pulmonary edema, pulmonary embolism, cardiomyopathy, and even asthma may present similarly. As always, proper evaluation begins with a meticulous history and physical examination. The inherent need for effective collaboration between health care providers cannot be over-emphasized.
Special attention should be paid to ascertaining if there is a history of asthma or other chronic airway disease as well as preexisting cardiac disease. These chronic conditions may be exacerbated by the increased cardiorespiratory work of pregnancy. A sudden onset of shortness of breath or air hunger with or without chest pain might suggest the possibility of pulmonary embolism as a cause for the adverse symptoms. Wheezing and coughing, especially with a history suggestive of a restrictive airways disease such as asthma, would make this a likely cause. Physical findings such as an abnormal chest X-ray, adventitious breath sounds such as respiratory crackles on auscultation, the presence of a cardiac murmur, tachycardia, elevated jugular venous distention (JVD) with tachypnea, suggest a possible cardiac etiology.
Pulmonary function testing may assist in the confirmation of a diagnosis of asthma because an obstructive pattern will most likely be evident. Echocardiography should be performed if findings from the history and physical examination suggest a potential cardiac etiology. Other adjuncts to facilitate diagnosis include an evaluation of hemoglobin to rule out the presence of anemia. The assessment of SaO2 with a pulse oximeter at rest and with moderate exertion is an easy non-invasive method to differentiate benign dyspnea from more worrisome pathophysiology. If the patient is able to maintain a SaO2 of 95 percent or greater with moderate exertion, the probability of major cardiorespiratory compromise is greatly reduced. If the patient is unable to meet this standard or if results are equivocal, the measurement of arterial blood gas values may be helpful but is rarely essential in the initial evaluation.
Radiographic studies such as chest X-ray, spiral computed tomography (CT), ventilation/perfusion (V/Q) scanning, or angiography should not be withheld simply because the patient is pregnant. The relative risk of ionizing radiation is minimal, compared to the potentially life-threatening conditions which these techniques may reveal.10
Pathologic Pulmonary Complications
Pulmonary Edema
Etiology
Pulmonary edema results when the normal homeostasis of fluid between the pulmonary capillaries and alveoli is disrupted. The net effect is an increase in the amount of fluid in the alveoli. A distinction is often made between hydrostatic (cardiogenic) and osmotic (non-cardiogenic) causes of pulmonary edema. The two primary mechanisms for development of pulmonary edema are increased hydrostatic pressure, and increased capillary permeability caused by endothelial damage or lowered COP. A comparison of these two mechanisms is presented in Table 9-3. Hydrostatic pulmonary edema results when there is a net increase in hydrostatic pressure within the capillary. This is reflected by a high pulmonary capillary wedge pressure (PCWP). Fluid is forced across the semipermeable membrane of the capillary secondary to increased intracapillary hydrostatic
pressure. This phenomenon is sometimes referred to as congestive heart failure. Osmotic pulmonary edema, also referred to as noncardiogenic or nonhydrostatic pulmonary edema, results when factors other than elevated intravascular pressure are responsible for the net flux of protein and fluid across the capillary wall into the alveolus. In order to make the diagnosis of osmotic pulmonary edema, hydrostatic contribution must be ruled out. This means there should be no evidence of a cardiac causative factor and that the PCWP should be normal or low for pregnancy.11 Reasonable efforts should be made to distinguish between these two types of pulmonary edema because treatment may be significantly different, depending on the underlying mechanism. Unfortunately, it is often difficult to distinguish between the two types based strictly on chest X-ray or the presence of clinical signs and symptoms. Compounding this dilemma is the fact that the two may exist concomitantly.
pressure. This phenomenon is sometimes referred to as congestive heart failure. Osmotic pulmonary edema, also referred to as noncardiogenic or nonhydrostatic pulmonary edema, results when factors other than elevated intravascular pressure are responsible for the net flux of protein and fluid across the capillary wall into the alveolus. In order to make the diagnosis of osmotic pulmonary edema, hydrostatic contribution must be ruled out. This means there should be no evidence of a cardiac causative factor and that the PCWP should be normal or low for pregnancy.11 Reasonable efforts should be made to distinguish between these two types of pulmonary edema because treatment may be significantly different, depending on the underlying mechanism. Unfortunately, it is often difficult to distinguish between the two types based strictly on chest X-ray or the presence of clinical signs and symptoms. Compounding this dilemma is the fact that the two may exist concomitantly.
Table 9.3 Comparison of Hydrostatic and Nonhydrostatic Pulmonary Edema | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
There are a number of physiologic changes in pregnancy that predispose the gravida to the development of pulmonary edema. The progressive increase in blood volume and cardiac output, in combination with other factors such as pain, multiple gestations, etc., may significantly increase cardiac work and lead to a rise in pulmonary arterial pressure (PAP), even though absolute values may remain within the normal limits for pregnancy. The subsequent increase in hydrostatic pressure within the vessel, in the setting of other pregnancy-associated changes, may predispose the pregnant woman to pulmonary edema. As previously described, there is a significant drop in plasma COP in pregnancy secondary to the increase in plasma free water, which decreases the concentration of plasma proteins. This can be seen when serum albumin levels are measured. A progressive decrease is also evident with advancing gestation. Without the pull of plasma COP, there tends to be a net filtration of fluid out of the vessel across the capillary membrane. Although the drop in plasma COP is progressive throughout pregnancy, there is an additional, significant decrease immediately after delivery. This is believed to be attributed in part to normal blood loss that occurs with delivery. This drop in COP may be as much as 20 percent below baseline values. Predisposition alone does not cause pulmonary edema. There are a number of inciting events that may lead to osmotic pulmonary edema in pregnancy. Some of these are naturally occurring and others are iatrogenic.
Up to 3 percent of patients with preeclampsia will develop pulmonary edema at some point during their
illness, with roughly 70 percent occurring within the first 72 hours following delivery. This may lead to a maternal mortality rate as high as 10 percent. There are several mechanisms at play in pulmonary edema caused by preeclampsia. The endothelial damage characteristic of severe preeclampsia disrupts the normal endothelial barrier and may lead to a leaky capillary syndrome. The intense vasospasm caused by preeclampsia leads to increased afterload or SVR. Although rare, this in turn may lead to significant diastolic dysfunction with a decrease in myocardial contractility.12,13,14,15,16,17 This may add a significant hydrostatic component to pulmonary edema that develops in women with preeclampsia. Furthermore, there is an additional decrease in plasma COP in preeclampsia greater than that in normal pregnancy. This combination of factors can easily increase the risk that the woman with preeclampsia may develop fulminant pulmonary edema. A detailed presentation of hypertensive disorders and associated secondary complications during pregnancy is presented in Chapter 7 of this text.
illness, with roughly 70 percent occurring within the first 72 hours following delivery. This may lead to a maternal mortality rate as high as 10 percent. There are several mechanisms at play in pulmonary edema caused by preeclampsia. The endothelial damage characteristic of severe preeclampsia disrupts the normal endothelial barrier and may lead to a leaky capillary syndrome. The intense vasospasm caused by preeclampsia leads to increased afterload or SVR. Although rare, this in turn may lead to significant diastolic dysfunction with a decrease in myocardial contractility.12,13,14,15,16,17 This may add a significant hydrostatic component to pulmonary edema that develops in women with preeclampsia. Furthermore, there is an additional decrease in plasma COP in preeclampsia greater than that in normal pregnancy. This combination of factors can easily increase the risk that the woman with preeclampsia may develop fulminant pulmonary edema. A detailed presentation of hypertensive disorders and associated secondary complications during pregnancy is presented in Chapter 7 of this text.
Tocolysis has also been cited as a possible cause of pulmonary edema. The practice of volume loading patients as the first-line treatment of preterm uterine contractions is of dubious benefit, yet is widely practiced. In addition, tocolytic agents such as magnesium sulfate and beta-andrenergic agents such as terbutaline are also associated with an increased risk of pulmonary edema. The major side effects of the beta-sympathomimetic tocolytic agents fall into two broad categories:
metabolic—including glucose, sodium, potassium, and water homeostasis
cardiopulmonary—including cardiac arrhythmias, pulmonary edema, and myocardial ischemia.
Increased myocardial work related to the beta-agonist–induced increase in cardiac output may also lead to myocardial depression and a resultant hydrostatic component to pulmonary edema. These effects are generally not seen when beta-agonists are used in non-pregnant individuals.18,19,20,21,22,23,24,25 A detailed presentation of pharmacologic agents used in the care of pregnant women with complications or critical illness is found in Chapter 6 of this text.
Aspiration syndrome and aspiration pneumonia are both of great concern in pregnancy and may cause pulmonary edema. There is significantly decreased lower esophageal sphincter pressure in pregnant women as well as delayed gastric emptying. Chemical pneumonitis and bacterial pneumonias may ensue and result in significant endothelial damage and disruption of normal lung water homeostasis resulting in pulmonary edema. Maternal hemorrhage, especially when associated with a need for massive red blood cell transfusion, may lead to pulmonary edema through a variety of mechanisms. Colloid osmotic pressure is decreased by replacement of whole blood loss with packed red blood cells and crystalloids, which lack the osmotically active proteins present in normal serum. There is also a tendency to exceed actual volume deficit with resultant volume overload and increased hydrostatic pressure. In addition, there is a systemic inflammatory response to massive hemorrhage which may be associated with endothelial damage, making the lung more permeable to the influx of fluid from the capillary. This may lead to an osmotic pulmonary edema. Transfusion-related acute lung injury (TRALI), thought to be caused by a reaction to leukocyte antibodies present in the plasma of blood products, causes fluid to rapidly leak into the lungs, producing acute pulmonary edema. It has become the number one cause of transfusion reaction mortality, and is further discussed in Chapter 15. Rare but potentially catastrophic causes of pulmonary edema include amniotic fluid embolism, also referred to as anaphylactoid syndrome of pregnancy, as well as sepsis. Detailed discussions of these complications are found in Chapters 19 and 18, respectively. Acute lung injury or pneumonia may progress to acute respiratory distress syndrome. In addition, bacterial infections outside the lung which are systemic may lead to pulmonary edema. There is a strong increased tendency for development of sepsis-related pulmonary edema in pregnant women, which is most likely related to an increased sensitivity of pregnant women to endotoxins as well as altered capillary permeability and the natural decrease in plasma COP. This is often seen in cases of pyelonephritis in which up to 10 percent of pregnant women subsequently develop pulmonary edema.26,27,28,29,30
Clinical Signs and Symptoms
The initial signs and symptoms of pulmonary edema may be very subtle. Frequently, the diagnosis of pulmonary edema is delayed because of the tendency of pregnant women to maintain cardiorespiratory stability better than the average non-pregnant patient with pulmonary edema. This is secondary to their relative youth and overall good health. This often will cause the health care provider to attribute clinical signs and symptoms of pulmonary edema to less significant causes such as dyspnea of pregnancy, asthma, or lower respiratory tract infections. An important presenting symptom is dyspnea, especially if it is associated with a degree of anxiety or tachypnea. As the alveoli progressively fill with fluid, the patient will display the classic signs of a cough, wheeze, or diffuse crackles upon auscultation of the lungs. However, early in the course of the process, chest auscultation may reveal clear breath sounds. Although pregnant women have an elevated resting heart rate, a pulse greater than 100 beats per minute (tachycardia) is a common symptom associated with pulmonary edema.
Non-invasive assessment of oxygen saturation via pulse oximetry is increasingly available in obstetric units. This is an extremely useful adjunct to diagnosis and treatment, but acquired data should be interpreted with caution. In normal pregnancy, SaO2 ranges from 98 to 100 percent saturation. A SaO2 of 90 percent reflects a PaO2 of 60 mmHg. A PaO2 of 70 mmHg is consistent with early ventilatory failure in a pregnant woman. Should it be necessary to obtain arterial blood gases in a patient with pulmonary edema, these will generally reflect a decreased PaO2 as well as PaCO2 secondary to initial hyperventilation. However, as the pulmonary edema progresses, PaCO2 increases as the patient is no longer able to maintain adequate minute ventilation. PaO2 will also continue to decrease. A PaCO2 greater than 40 mmHg in a pregnant woman is strongly suggestive of ventilatory insufficiency and indicative of impending respiratory failure.
The chest X-ray is another important adjunct to the diagnosis of pulmonary edema in pregnant women and should not be withheld because of concerns of radiation exposure to the mother or fetus. The amount of radiation used in a standard posterior to anterior projection chest X-ray is well below levels considered safe in pregnancy. Unfortunately, chest X-ray findings in pulmonary edema often lag behind the onset of clinical symptoms. It may take hours from the onset of symptoms before pulmonary infiltrates, pleural effusions, and other typical findings are visualized on a radiograph.
In all suspected cases of pulmonary edema, a complete blood count with differential and platelets should be obtained, in order to rule out anemia or systemic infection as a possible underlying cause. In addition, if preeclampsia is suspected, laboratory studies to rule out HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) and distinguish the hypertension of preeclampsia from chronic hypertension should be conducted. This includes analysis of uric acid, urinalysis for protein, aspartate aminotransferase analysis, and complete blood count.
Clinical Management
Treatment for pulmonary edema in pregnancy should be initiated promptly, even if the underlying etiology has not yet been determined. Certain principles apply in treating this condition in pregnancy regardless of the underlying cause. The first goal is to maintain adequate maternal oxygenation with a PaO2 of 70 mmHg or greater. This may be reasonably approximated by keeping the maternal SaO2 above 95 percent. This is initially accomplished by maternal positioning in an upright posture with lateral uterine displacement as well as the addition of supplemental oxygen as needed. Next, provided there is no contraindication, intravenous morphine sulfate is generally safe and effective in improving patient comfort, decreasing maternal anxiety, and decreasing pulmonary congestion by relaxing pulmonary vasculature. Fluid volume should be carefully monitored and recorded using intake and output balance sheets. Most pregnant women with pulmonary edema are not intravascularly volume overloaded; however, administration of low-dose diuretics (e.g.,10 mg of intravenous furosemide) should lower the PCWP and decrease the hydrostatic component of pulmonary edema. Furosemide may be safely administered, provided a normal blood pressure is maintained, in most patients with pulmonary edema regardless of the underlying etiology. Caution should be employed if cardiac lesions such as mitral stenosis, aortic stenosis, and/or idiopathic hypertrophic subaortic stenosis (IHSS) exist with the pulmonary edema. Judicious use of diuretics in pregnant women is highly advisable, since aggressive diuresis of these patients, in the absence of invasive hemodynamic confirmatory data, can lead to significant intravascular hypovolemia, impaired placental perfusion, and fetal compromise. This is especially true of the patient with preeclampsia who, in spite of having extensive peripheral edema and an elevated total body water, may be intravascularly contracted. Pregnant women without underlying renal disease and who have not been exposed to loop diuretics may be extremely sensitive to these agents and may have a brisk response to even small amounts of furosemide.31,32,33
Once these initial measures have been implemented, a review of the differential diagnosis of pulmonary edema and the search for its underlying cause should be undertaken to further direct therapy. In addition, other causes of respiratory failure should be ruled out. For example, unknown valvular heart disease, peripartum cardiomyopathy, or in rare instances ischemic heart disease may all present in a similar fashion to osmotic pulmonary edema. In addition to history and physical examination, including auscultation of heart sounds, an electrocardiogram should be performed. As many as half of all pregnant women with pulmonary edema have an unsuspected cardiac abnormality, and, for this reason, echocardiography may be routinely performed in any pregnant woman presenting with pulmonary edema. If there is any question as to the underlying cause of pulmonary edema in the gravida, such readily available and non-invasive testing should be performed.
Acute cocaine intoxication can lead to cocaine-associated pulmonary edema and should be suspected in a patient presenting with pulmonary edema and severe hypertension. Likewise, heroin intoxication may also lead to pulmonary edema. Both these etiologies can be ruled out through evaluation of a drug screen. Even prescription medications such as nitrofurantoin may lead to an acute pulmonary reaction and respiratory failure. This underscores the need for a careful
drug history in any patient with pulmonary edema. History and physical examination should help to differentiate a possible acute pneumonia from pulmonary edema. Patients who present with a history of chills and rigors or who are found to have fever and purulent sputum in addition to pulmonary edema likely suffer from infectious pneumonia. In these patients, in addition to the initial therapies for pulmonary edema, empiric antibiotic therapy should be initiated.
drug history in any patient with pulmonary edema. History and physical examination should help to differentiate a possible acute pneumonia from pulmonary edema. Patients who present with a history of chills and rigors or who are found to have fever and purulent sputum in addition to pulmonary edema likely suffer from infectious pneumonia. In these patients, in addition to the initial therapies for pulmonary edema, empiric antibiotic therapy should be initiated.
Once the underlying etiology of pulmonary edema is identified, further therapy should be initiated. For example, if preeclampsia is present, delivery of the fetus may be considered. Afterload should be reduced to optimize cardiac output with blood pressure maintained at or below 160/100 mmHg with a vasodilating antihypertensive agent (e.g., hydralazine). Unfortu-nately, seizure prophylaxis with magnesium sulfate (MgSO4) may exacerbate pulmonary edema secondary to the tendency of MgSO4 to lower COP and because of the high volume of fluid often administered with the medication. For this reason MgSO4 should be concentrated as much as possible with careful attention to limiting extraneous intravenous fluid administration. As with all cases of pulmonary edema, close attention to fluid balance should be observed, particularly at the time of labor and delivery and in the immediate postpartum period.
If pulmonary edema is believed to be related to tocolytic agents, then these should be discontinued even if the patient is at an unfavorably early gestation and is having preterm contractions. Tocolytics are of limited proven efficacy for long-term prolongation of pregnancy and should not be continued when there is a clear contraindication to them on the basis of a potentially dangerous side effect. Additionally, hypoxemia can severely exacerbate uterine contractility, and proper oxygenation is essential to uterine quiescence. Finally, fetal status is dependent upon maternal status; therefore, every effort should be made to appropriately treat the mother.
If the pulmonary edema appears to be related to an infectious cause, then the site of infection should be discerned. Abdominal pain and tenderness may suggest intra-abdominal infection such as appendicitis. Uterine tenderness and contractions may indicate chorioamnionitis or endometritis. Pyelonephritis is particularly important to rule out and should be evaluated by assessing for flank tenderness with percussion and performing a urinalysis and urine culture. If pneumonia is suspected, initial empiric treatment with broad-spectrum antibiotics is advisable. Antibiotic regimens may be adjusted and tailored to individual organisms identified. If a source is not immediately found and sepsis is suspected, empiric treatment with broad-spectrum antibiotics should be initiated.
If pulmonary embolism is suspected, anticoagulation should be begun as soon as possible. The basic treatment for pulmonary edema should also be implemented as the patient is anticoagulated and the diagnosis of embolism should be confirmed with ventilation perfusion studies or CT studies.
Pleural Effusion
Pleural effusion may complicate 45 to 65 percent of normal pregnancies.34




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree