Background
Cytomegalovirus infection is the most common perinatal viral infection that can lead to severe long-term medical conditions. Antenatal identification of maternal cytomegalovirus infections with proven fetal transmission and potential postnatal clinical sequelae remains a major challenge in perinatology. There is a need to improve the prenatal counseling offered to patients and guide future clinical management decisions in cases of proven primary cytomegalovirus infection.
Objective
We sought to evaluate the accuracy of fetal ultrasound for predicting sequelae in fetuses infected with congenital cytomegalovirus after maternal primary infection.
Study Design
We conducted a prospective observational study from 1996 through 2012 in pregnant women with serological evidence of primary cytomegalovirus infection and proven vertical transmission to the fetus, based on viral load in the amniotic fluid. Fetal ultrasound was performed in all patients. Pregnancy termination was presented as an option for infected fetuses. Hearing and neurological clinical assessments were performed for all neonates with cytomegalovirus-positive urine samples.
Results
A total of 67 patients (69 fetuses) with proven vertical transmission were included in this study, including 64 singleton and 3 twin pregnancies. Eight fetuses were lost to follow-up. Of the remaining 61 fetuses, termination of the pregnancy was performed for 26, including 11 with fetal ultrasound anomalies. Autopsy provided histological evidence of fetal cytomegalovirus infection in all cases. In the 15 terminated fetuses without ultrasound anomalies, histological evidence of damage caused by fetal infection was detected in 13 cases. Among the 35 live-born infants, 12 had fetal ultrasound anomalies suggestive of congenital infection. Of these 12 infants, 6 had normal clinical evaluations, whereas 6 presented with either hearing and/or neurological anomalies, classified as severe in 4 cases. Among the 23 live-born infants with normal prenatal ultrasound, 5 developed hearing impairments and 1 showed mild neurological developmental delay.
Conclusion
Fetal ultrasound anomalies were detected in 37.7% of pregnant women with primary cytomegalovirus infection acquired in early pregnancy and proven fetal infection, and were confirmed by autopsy or postnatal clinical evaluation in 73.9%. Autopsy or postnatal clinical evaluation also detected cytomegalovirus-related anomalies in 55% of infants with normal fetal ultrasound evaluations.
Introduction
Human cytomegalovirus (CMV) is a beta-herpes virus that can cause congenital infections, with an estimated overall birth prevalence of 0.64%. Fetal transmission can occur after both primary and nonprimary maternal infections, with the incidence of congenital CMV (cCMV) infection at birth increasing to 30% when maternal primary CMV infection occurs during the first trimester. CMV infection is the most common fetal viral infection, with a broad range of postnatal outcomes from completely normal infants to perinatal losses. However, children born with cCMV infection are at increased risk of developing clinical sequelae such as sensorineural hearing loss (SNHL) and neurodevelopmental disabilities.
We initiated an experimental CMV screening program in 1996 to detect congenitally infected neonates, and demonstrated that serological screening was a reliable method for detecting antenatal CMV infections. This serological screening allows a distinction to be drawn between primary and nonprimary infections. Detection of CMV DNA in the amniotic fluid by amniocentesis is the gold standard for confirming fetal infection. Detection of CMV DNA by polymerase chain reaction (PCR) is associated with high positive predictive values (PPV), but false-negative results are possible if amniocentesis is performed before excretion of CMV into the amniotic fluid. The risk of false-negative results is reduced if amniocentesis is performed >20 weeks, and if a period of 6 weeks between maternal primary CMV infection and amniotic-fluid sampling is observed. However, detection of CMV viral load in the amniotic fluid or CMV strain polymorphisms are not accurate enough to identify fetuses that will be symptomatic at birth, and systematic ultrasound (US) examinations of the fetus and placenta, including biometrics and the detection of anomalies characteristic of cCMV infection, are therefore recommended. US may detect CMV-related anomalies in the central nervous system (CNS), as well in other organs. The presence of ventriculomegaly, periventricular hyperechogenicity with or without cysts, brain calcifications, intraventricular adhesions, abnormal cortical development, callosal or cerebellar insults, brain atrophy, and hemorrhage are the most characteristic cerebral features ( Figure 1 ). Affected fetuses can also present with intrauterine growth restriction, echogenic bowels and kidneys, ascites, hepatosplenomegaly, hepatic calcifications, pericardial effusion, and cardiomegaly, with placentomegaly, placental calcifications, and polyhydramnios as possible additional findings. However, the main US findings mentioned above are not specific to fetal CMV infection, and fetal US studies from prenatally diagnosed infants are scant and contradictory. Some studies found a correlation between the presence of abnormal brain findings on US examination and poor neurodevelopmental outcome, while other studies were unable to identify fetuses at risk of severe neurodevelopmental sequelae based on prenatal US findings.
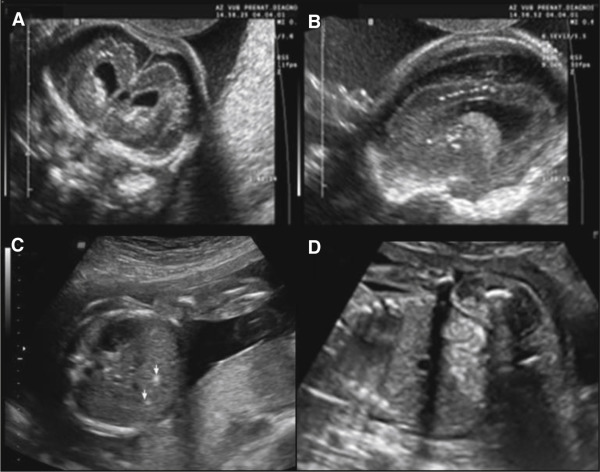
The aim of this study was to determine the outcome of pregnancies with documented fetal CMV infection according to the presence or absence of abnormal US findings. The results of this study may thus improve the prenatal counseling offered to patients, and guide future clinical management decisions in cases of proven primary CMV infection.
Materials and Methods
Our institution has been conducting an ongoing prospective observational study of cCMV infection since 1996. Patients followed up from 1996 through 2012 with CMV infection occurring <20th week of pregnancy and associated with proven fetal transmission were selected from a cohort of women with primary CMV infection. All pregnant women followed up in our center underwent an experimental serological screening program for CMV at the first prenatal visit to detect antenatal CMV infections. When pregnant women showed clinical symptoms suggestive for CMV infection or reported a high-risk contact with an infected child an additional blood screening was performed. Maternal CMV infection is defined as primary when maternal seroconversion of IgG antibodies occurs during pregnancy. Initial CMV serology is highly suggestive for primary CMV infection if IgM antibodies are present with no or very-low-titer IgG antibodies, followed by a subsequent rise in IgG and decrease in IgM. For patients testing positive for both IgM and IgG, a serum IgG avidity test was performed. Serum IgG avidity index can be determined to help distinguish between a recent or past infection. CMV IgM detection is a sensitive marker for primary CMV infection. Nevertheless, the specificity of CMV IgM production remains poor because these antibodies are also produced during viral reactivations or recurrent infections. Moreover, persisting CMV IgM are not uncommon even a long time after a primary infection. Therefore, serum CMV IgG avidity index has been developed to help distinguish between a recent or past infection. IgG avidity is defined as the strength with which IgG antibody binds to the antigen with gradual maturation that takes place in the months following a primary infection. During the first few months following a primary infection, freshly produced IgG antibodies are characterized by a low avidity, whereas older antibodies produced by 6 months after an infection exhibit a high avidity. Low CMV IgG avidity is an accurate indicator for primary infection that occurred within the preceding 3 months. Contrariwise, high CMV IgG avidity is an accurate marker to exclude a primary infection within the preceding 3 months. The avidity index cut-off point used in our institution for defining low avidity is typically <40%, whereas the avidity index cut-off point for defining high avidity is typically >65%. Bodeus et al showed that the mean avidity index for a primary infection was significantly lower than the mean avidity index for a recurrent infection or a reactivation. All our patients were selected out of cohort women with primary infection during pregnancy. For patients testing positive for both IgM and IgG, a serum IgG avidity test was performed. Only patients with low IgG avidity index were included. No patients were tested for CMV using PCR technique. The gestational age (GA) at which primary maternal infection occurred was estimated from serological results. Diagnosis of fetal transmission was based on the detection of CMV DNA by PCR and culture of amniotic fluid samples obtained by amniocentesis. Amniotic fluid sampling was performed 6-8 weeks after the estimated maternal infection and not before week 20 of gestation. Patients with a positive diagnosis were counseled regarding fetal and perinatal risks by a fetal-medicine specialist. Termination of pregnancy (TOP) was offered in patients with proven fetal CMV infection. Patients who opted for TOP were scheduled for extensive counseling, including psychological support, and signed informed consent for autopsy. Patients who decided to continue the pregnancy underwent targeted US examination, and were offered clinical follow up for the offspring. All neonates born symptomatic at birth (petechiae, jaundice, microcephaly, hepatosplenomegaly) were treated with antiviral therapy (intravenous ganciclovir and/or valganciclovir by mouth). There are insufficient evidence-based results to motivate prenatal therapies as prevention or treatment of a cCMV fetal infection. Therefore, none of the patients received antiviral therapy or hyperimmune anti-CMV globulins during their pregnancy.
Prenatal US examination
Targeted US examinations of the fetus and placenta were performed every 4 weeks until delivery. Transabdominal and transvaginal examinations were carried out using high-frequency US equipment (Voluson 730 or E8; GE Medical Systems, Zipf, Austria). The following findings were considered suggestive of cCMV infection and were recorded: intrauterine growth restriction (defined as birthweight <10th percentile for GA), hydrops or ascites, echogenic bowel, pleural or pericardial effusion, hepatosplenomegaly, intrahepatic calcifications, CNS abnormalities such as ventriculomegaly of ≥10 mm at the level of the atrial diameter, periventricular hyperechogenicity with or without cysts, brain calcifications, and intraventricular adhesions. Hepatosplenomegaly was defined as a subjective enlargement of the liver and spleen and registered when the 2 organs occupied most of the abdomen and when the liver deformed the anterior abdominal wall causing a dip at the level of thoracoabdominal junction. We also used nomograms to detect growth disorders of the fetal liver and spleen by US measurements.
Amniotic-fluid volume and placental appearance (volume and echogenicity) were also recorded. The findings were categorized into 4 groups: normal; isolated extracerebral anomalies (including 1 of the following: oligohydramnios, intrahepatic calcification, echogenic bowel, hepatosplenomegaly); combination of different extracerebral anomalies; and CNS abnormalities (including ≥1 of the following: ventriculomegaly, microcephaly, echogenic foci, septa, cysts or hyperechogenic periventricular halo, irregular lining of the ventricles).
Confirmation of cCMV infection
Urine or saliva samples were collected from newborns within 7 days after birth and cCMV was confirmed by culture methods.
Evaluation of adverse sequelae in live-born infants
Live-born, congenitally infected infants underwent physical examination to detect symptoms of CMV infection. These infants were also included in a follow-up program involving audiologic and neurological assessments.
Audiologic assessment
Infants with cCMV infection underwent hearing evaluations within 1 month after birth, at 5 months, at 1 year, and yearly thereafter, with additional hearing tests performed if necessary. Hearing tests involved auditory brainstem responses, distortion product otoacoustic emissions, and behavioral pure tone audiometry, as described previously. Infants were categorized into 3 groups: normal hearing, unilateral SNHL, and bilateral SNHL (hearing threshold in the best ear of ≥40 decibel hearing level).
Neurological assessment
Neurological evaluation based on a standard series of measurements was performed to assess cognitive, motor, language, and emotional-behavioral development in infants and toddlers. The Bayley Scales of Infant Development II and Wechsler Intelligence Scale for Children III were mainly used. The results of neurodevelopmental tests were categorized as follows: normal (scores within normal limits); mild neurological impairment (abnormal neurological findings slightly affecting normal function); and severe neurodevelopmental disability (major psychomotor impairment, intellectual disability [IQ <70], or cerebral palsy). If no follow-up was performed at the hospital, the results of audiologic and neurologic assessments were obtained from hospital charts and by telephone interviews with the parents.
Evaluation of fetal anomalies in terminated pregnancies
Fetuses and placentas from terminated pregnancies underwent histopathological examination. Histological diagnosis of fetal CMV infection was based on the presence of intranuclear amphophilic inclusion bodies surrounded by a clear halo, or the presence of basophilic granular cytoplasmic inclusion bodies, both defined as cytomegalic inclusion bodies. The presumptive diagnoses were: no sequelae (normal histopathological findings); mild sequelae (CMV inclusions present in several organ systems with no signs of neuropathological abnormalities); and severe sequelae (CMV inclusions present in several organ systems with neuropathological findings).
The study protocol was approved by the Committee of Medical Ethics of the Universitair Ziekenhuis Brussel.
Results
Among a cohort of 355 pregnant women with primary CMV infection, 67 (64 singleton pregnancies and 3 bichorial-biamniotic twin pregnancies) with CMV infections that occurred <20 weeks of gestation and proven fetal transmission were included for analysis. Diagnosis of fetal transmission was based on the detection of CMV DNA by PCR and culture of amniotic fluid samples. There were no discrepancies found between PCR and CMV culture in the results after amniocentesis. One twin pregnancy was associated with discordant PCR results after amniotic-fluid sampling (one fetus was infected and the other was healthy).
Patients were grouped according to their decision to undergo TOP or to continue the pregnancy ( Figure 2 ). After multidisciplinary counseling, 31 patients (32 congenitally infected fetuses) decided to terminate the pregnancy, and TOP was performed between 21-27 weeks of gestation. Four patients were excluded from the analysis because TOP was performed in another hospital, and a further 2 were excluded because they declined fetal autopsy, leaving 26 fetuses available for histological analysis. In Belgium, patients <12 weeks of GA are legally allowed to ask for a TOP without any medical reason. After 12 weeks of GA, indications of TOP for medical maternal or fetal reasons are submitted to a multidisciplinary perinatal staff council. Postnatal prognosis is discussed until consensual approval is reached. For complex cases without consensus, the ethical committee of the hospital is asked to give its opinion whether or not the long-term medical conditions of the fetus or the maternal condition are eligible for a TOP indication.
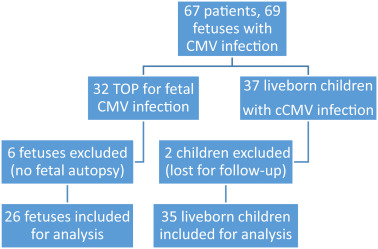
In all, 36 patients (37 fetuses) decided to continue the pregnancy. Two infants were lost to follow-up and their outcomes were excluded from the analysis. Follow-up information for 35 live-born infants was therefore retrieved and included in this report.
A total of 61 congenitally infected fetuses were included in the final analysis.
Prenatal US findings and correlation with clinical outcome among patients who continued their pregnancy (n = 35)
Detailed US findings for the live-born children and their clinical outcomes are described in Table 1 . None of the fetuses had US findings suggestive of CMV infection before serum test or amniocentesis. Targeted serial prenatal US revealed abnormal findings in 12 fetuses (34.3%), including 6 with major fetal CNS anomalies with or without abnormal extracerebral US images (50%). Of these, 2 children (cases 1 and 2) developed major neurological sequelae, 3 had severe audiological deficits (cases 3 and 4 with bilateral SNHL, case 5 with unilateral SNHL), and 1 (case 6) had a strictly normal clinical follow-up, despite apparently abnormal prenatal US results. Six other children showed isolated extracerebral anomalies during the prenatal US assessment (cases 7-12), 1 of whom had severe, late-onset unilateral SNHL with mild psychomotor retardation. The other 23 infants showed no abnormalities during their prenatal US assessments. Five of them presented with audiological sequelae (21.7%), including 1 (case 13) with bilateral SNHL associated with mild developmental delay, and 4 (cases 14-17) with unilateral SNHL. One of these 23 cases (case 18) was diagnosed with mild neurological sequelae.



