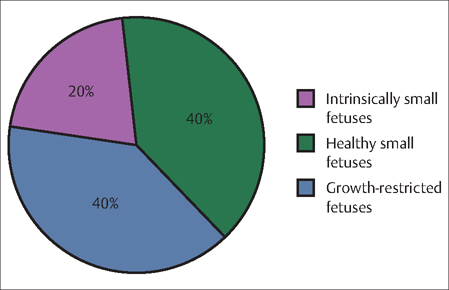
19 Preterm Birth and Fetal Growth Restrictions E. Albert Reece During pregnancy, a mother and her fetus act in harmony. The mother provides for the needs of the developing fetus, while at the same time the fetus lends support to the mother as she experiences a myriad of physiologic changes. The growth of a normal fetus is, thus, controlled by a delicate balance of genetic, placental, and maternal factors. The prevailing view in embryology is that a fetus has an inherent growth potential. Under normal circumstances, it will develop into a healthy, appropriately sized newborn. However, if there is an imbalance in one or more of these critical growth and development factors, the baby may fail to reach its appropriate size and weight. Inhibition of the fetus’ growth potential, particularly its growth in weight, is analogous to “failure to thrive” in the infant. However, low birth weight is not necessarily associated with long-term adverse consequences for the infant (Fig. 19.1). In the absence of obvious risk factors, a small but otherwise normally developed fetus should not be considered pathologic, and every effort should be made to distinguish that from a truly abnormal condition. Approximately 40% of growth-restricted fetuses develop serious complications (Fig. 19.1). Infants who are born weighing less than 2500 g (5 lb, 8 oz) at term have a perinatal mortality rate that is 5 to 30 times greater than that of infants whose birth weights are at the 50th percentile. The mortality rate is 70 to 100 times higher in infants who weigh less than 1500 g (3 lb, 5 oz) compared to normal-weight babies. Two major pathological conditions associated with poor fetal development are preterm birth and fetal growth restriction, which are the focus of the rest of this chapter. Fig. 19.1 Proportion of low birth weight babies with normal (purple and green) and abnormal (blue) medical profiles. Most pregnancies last around 40 weeks, and babies born between 37 and 42 completed weeks of pregnancy are considered full term. Babies born before completion of 37 weeks of pregnancy are called premature, or preterm. Most preterm babies (71.2%) are born between 34 and 36 weeks of gestation. These are known as “late” preterm births. Almost 13% of preterm babies are born between 32 and 33 weeks of gestation, about 10% between 28 and 31 weeks, and about 6% at less than 28 weeks. A number of biochemical markers of preterm labor and delivery have been studied and most are associated with overall poor predictive values (Table 19.1), except the level of fetal fibronectin (fFN). In symptomatic women, a positive fFN test (defined as >50 ng/mL) in cervicovaginal secretions after 20 weeks of gestation indicates potential decidual disruption. It does not guarantee, however, that a woman is about to give birth or that she will deliver early. The fFN test has an even higher negative predictive value (as high as 99.7% or a one in 333 chance of delivery within 1 week of a negative test result). A negative result on the fFN test means it is highly unlikely that the woman will give birth in the next week or two (See Evidence Box 19.1).
Preterm Birth
Definition
Diagnosis
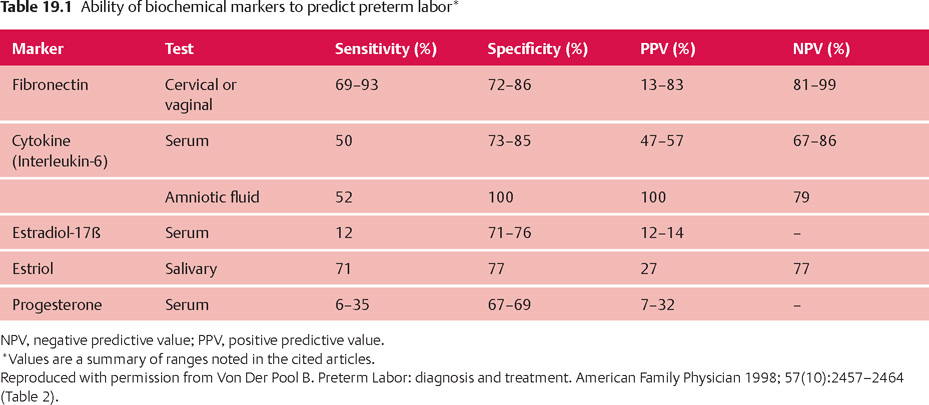
| Maternal factors | Lifestyle factors | Medical factors |
| Previous preterm birth | Smoking | Late or no prenatal care |
| Pregnant with twins, triplets or more | Drinking alcohol | Infections (including urinary tract, vaginal, sexually transmitted, and possibly other infections) |
| Having certain uterine or cervical abnormalities | Taking illicit drugs | High blood pressure |
| Short periods between pregnancies (<6–9 months between birth and beginning of next pregnancy) | Domestic violence (including physical, sexual, or emotional abuse) | Diabetes |
| Pregnant with a single fetus after in vitro fertilization | Exposure to the drug DES | Clotting disorders (thrombophilia) |
| Being underweight before becoming pregnant | Lack of social support | Certain birth defects in the baby |
| Being overweight or obese | Extremely high levels of stress | Bleeding from the vagina Standing for long periods |
This negative predictive value is extremely useful, because in the early stages of preterm labor, it is very difficult to tell if a woman really is in labor based on her symptoms and a pelvic exam (see History Taking section below). The test’s greatest value in the future may be to identify symptomatic women who may be able to continue their pregnancies without drug intervention.
Fetal Growth Restriction
Definition
The most widely used definition of fetal growth restriction (FGR) is a fetus whose estimated weight is below the 10th percentile for its gestational age, and whose abdominal circumference is below the 2.5th percentile. The birth-weight cutof for being classifed as fetal-growth restricted is 2500 g (5 lb, 8 oz) at term.
FGR is usually the result of placental insufficiency and is associated with a high rate of perinatal mortality. In the United States, for example, it is linked to a 6–10-fold increased rate of perinatal mortality. Often referred to in the scientific literature as intrauterine growth restriction, FGR is classifed typically as either symmetric or asymmetric. Symmetric growth restriction refers to a fetus with a small, but proportionally developed, body. Asymmetric growth restriction refers to an undernourished fetus that is forced to use up all of its energy to just maintain the growth of its vital organs, such as the brain and heart, at the expense of its liver, muscle, and fat.
The greatest risk of adverse perinatal outcomes with FGR occurs among growth restricted fetuses/infants with weights below the third percentile for gestational age. In addition, FGR appears to be a precursor of some cases of hypertension, hyperlipidemia, coronary heart disease, and diabetes mellitus when the child reaches adulthood.
Diagnosis
Diagnosing FGR is heavily dependent on accurate dating in early pregnancy. It is based on a certain date for the last menstrual period in a woman with regular cycles or ultrasound assessment of gestational age performed no later than the 20th week, when the margin of error is only 7–10 days. The most accurate method for estimating gestational age is to perform a sonographic evaluation at 8–13 weeks of gestation. Although ultrasonography performed later in the pregnancy is valuable for estimating fetal weight, it is only accurate in predicting gestational age within about 3 weeks when performed at term.
A common—and potentially dangerous—medical error is changing a patient’s due date based on a third-trimester ultrasound assessment. Doing so can, and often does, result in failure to recognize FGR.
Prenatal screening for FGR in general obstetrical populations also involves identifying risk factors for impaired fetal growth (Table 19.2) and physically assessing fetal size. Once FGR is suspected, based upon risk factors or physical examination, it should be followed by a detailed sonographic assessment of the fetus, placenta, and amniotic fluid.
Fortunately, advances in obstetrics and neonatology (the branch of pediatrics that deals with newborns) have improved the chances of survival for even the smallest babies. Late preterm babies generally have few or mild problems. Babies born before about 32–34 weeks’ gestation, however, may have a number of complications, ranging from mild to severe. These babies usually are very small, and their organs are less developed than those of babies born later. Their symptoms may include:
- respiratory distress syndrome
- apnea
- intraventricular hemorrhage
- patent ductus arteriosis
- necrotizing enterocolitis
- retinopathy
- jaundice
- anemia
- chronic lung disease (also called bronchopulmonary dysplasia)
- infections
Although a fetus with asymmetric FGR has normal head dimensions, its abdomen will be proportionally small due to decreased liver size. It also will have spindly limbs due to decreased muscle mass, and thin skin due to decreased fat.
If growth-restricting conditions are sustained long enough, or are severe enough, the fetus may lose the ability to compensate and will become symmetrically growth-restricted. Of greatest concern to the developmental potential of the fetus is arrested head growth, which offers a poor prognosis for mental development.
Prevalence and Epidemiology
Preterm labor and FGR are the two leading causes of perinatal morbidity and mortality. In the United States, pre-term birth afects 8–10% of births each year—totalling more than half a million babies, and causes at least 75% of neonatal deaths, excluding those related to congenital malformations.
Despite four decades of research, the rate of premature births has not changed, and some data indicate that the rate may be worsening. Because of technological advances in perinatal and neonatal medicine, survival rates have increased and morbidity has decreased. On the other hand, costs have soared. The health care expenditures for management of preterm labor and preterm birth account for more than $3 billion per year.
FGR is the second leading cause of perinatal morbidity and mortality, with an estimated incidence of approximately 5% of births. However, the incidence varies, depending on the population under examination (including its geographic location) and the standard growth curves used as reference.
Etiology and Pathophysiology
Preterm Birth
Preterm labor is characterized by cervical effacement and/or dilatation, and increased uterine irritability that occurs before 37 weeks of gestation. In most cases, the precise causes of preterm labor are not known. The strength of the association of each identified risk factor has been shown to vary, which has evoked a significant amount of debate in the literature. Women with a history of previous preterm delivery have the highest recurrence risk, estimated to be between 17% and 37%.
Most preterm births result from spontaneous preterm labor, either by itself or following spontaneous premature rupture of the membranes (PROM), when the amniotic sac inside the uterus that holds the baby breaks prematurely. The causes of preterm labor and PROM are not fully understood. The latest research suggests that many cases are triggered by the body’s natural response to certain infections, including infections involving the amniotic fluid and fetal membranes (see Chapter 20). However, in about 40% of all cases of preterm birth, the cause cannot be determined.
About 25% of preterm births result from early induction of labor or cesarean delivery due to pregnancy complications or health problems in the mother or the fetus. In most of these cases, early delivery is probably the safest approach for mother and baby.
Nevertheless, preterm birth is a serious health problem for the baby. In the short term, many preterm babies are at increased risk for common newborn health complications. They often must be cared for in a neonatal intensive care unit, which has specialized medical staff and equipment that can deal with the multiple problems they face. In the longer term, preterm babies may face lasting disabilities, such as mental impairment, cerebral palsy, lung and gastrointestinal problems, vision and hearing loss, and even premature death.
Any woman can deliver a preterm baby, but some women are at greater risk than others. Certain groups of women, certain life style factors, or certain medical conditions may put a woman at greater risk for preterm labor (Table 19.2).
Fetal Growth Restriction
It is important to identify the etiology of FGR whenever possible because such information additionally benefits the patient. For instance, knowing that there may be a chromosomal or genetic etiology may enable the patient to prepare better for her delivery, as well as plan for future pregnancies. This is also true when fetal anomalies are present.
FGR may be caused by fetal, placental, or maternal factors. There is significant overlap among these entities. Fetuses with trisomies, particularly for chromosomes 13 and 18, often have FGR. Triploidy also is a risk factor for FGR (Fig. 19.2). Others, including sex chromosome abnormalities such as 45 XO fetuses, and those with mosaic cell lines are often seen with growth restriction. Overall, chromosomal and genetic causes of FGR account for 5–15% of growth restricted fetuses and are more often seen with symmetric rather than asymmetric FGR.
Congenital abnormalities with or without chromosomal abnormalities are also seen with FGR. Most common are cardiac malformations such as tetralogy of Fallot and transposition of the great vessels. Other abnormalities, including gastroschisis and omphalocele, are also seen with altered fetal growth.
Among the agents that cause FGR, some of the strongest associations are infections caused by rubella, cytomegalovirus, and toxoplasmosis (see also Chapter 23). These infections involve multiple organs of the fetus that lead to growth restriction. Infections account for 5–10% of all FGR cases.
In multiple gestation pregnancies, FGR results from abnormal placental development and possibly abnormal cord insertions. In addition, nutritional factors and uteroplacental blood flow variations contribute to the growth restriction in such pregnancies (see Chapter 18).
Nutritional deficiencies, particularly protein intake, are among the most significant maternal factors associated with FGR, as are maternal medical pthologies, including cardiovascular, endocrine (e.g., diabetes, thyroid disorders), autoimmune, and renal diseases.
Environmental agents can pose significant risks to fetal growth as well, and include adverse effects from cigarette smoking, alcohol, and cocaine abuse. Women exposed to organic solvents and other toxins in the workplace have similar, elevated risks for FGR. High altitude, another risk factor for FGR, leads to an increase in the fetal hematocrit and results in fetal hypoxia. Table 19.3 lists some of the most common causes of FGR.
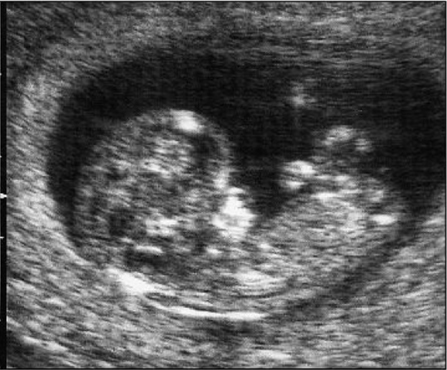
Fig. 19.2 Utrasonogram of severe asymmetrical growth restriction in a 13-week-old fetus with triploidy, showing normal head but small, underdeveloped torso and limbs
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


