Introduction
The rate of breastfeeding worldwide has continued to increase since the early 1970s. Now approximately 77% of American women opt to breastfeed their infants, at least initially. In many other countries, breastfeeding rates approach 95%. It has simply become clear to mothers around the globe that breastfeeding provides the best source of nutrition for their infant. Key benefits to the infant include perfect nutrition, enhanced neurocognitive development, stronger immune function, and significant reductions in infectious disease such as upper respiratory infections, otitis media, sudden infant death syndrome, and necrotizing enterocolitis [1–8].
Breastmilk is packed with large quantities of secretory IgA, interferons, antimicrobial proteins such as lactoferrin, lysozyme, and lipopolysaccharides. The presence of numerous growth factors (IgF-1), cytokines and gastric hormones (gastrin, motilin) enhances development of protective barriers in the gastrointestinal (GI) tract and stimulates elimination of meconium present during gestation.
While recent studies have suggested that the number of women who choose to breastfeed their infant is rising, the number of women who discontinue breastfeeding in order to take a medication is simply too high. Surveys of Western countries indicate that 90–99% of women who breastfeed will receive a medication during the first week post partum [9]. Another study of Scandinavian women suggests that in mothers who discontinue breastfeeding, the use of medications is a major reason and that 17–25% of breastfeeding women in this study had taken a medication during the prior 2 weeks [10]. The most frequently used drugs included analgesics, hypnotics, and methylergometrine [11].
Because so many women ingest medications during the early neonatal period, is it not surprising that one of the most common questions encountered by pediatricians and obstetricians is concerning the use of specific drugs during lactation. While in the past two decades we have developed extensive literature on the transfer of drugs into human milk, little of this information seems to have transferred to the practicing clinician. Too often, clinicians simply read the package insert, which always suggests discontinuing breastfeeding. Discontinuing breastfeeding is often the wrong decision and most mothers could easily continue to breastfeed and take the medication without risk to the infant.
In the past 20 years, we have developed a proficient understanding of the kinetics of drug entry into human milk. Most of the properties of drugs that facilitate transfer (molecular weight, pKa, lipophilicity) are known, but ultimately the degree of transfer of the medication must be determined in humans. Rodent studies have proven virtually worthless in comparison to human studies.
The following review covers a basic model for understanding how drugs enter milk, and numerous recommendations for drugs of choice for breastfeeding mothers.
The parenchyma of the breast consists of approximately 10–15 ductal regions which ultimately drain toward the nipple. The alveolar unit (Figure 31.1) is lined with a specialized epithelial cell, formerly called the alveolar epithelial cell but now called the lactocyte. The entire alveolar unit is thoroughly perfused with capillaries and lymphatics and is innervated with small nerves. Closely juxtaposed to the basal membrane of the alveolus are numerous capillaries that serve as the primary source of nutrients, fats, and many other components (including drugs) needed for the production of human milk. Surrounding the alveolus like a basket is a specialized smooth muscle cell called the myoepithelial cell. This contains receptor sites for oxytocin. Upon the release of oxytocin from the pituitary, the let-down process occurs and forces milk down the ductal system toward the nipple.
Figure 31.1 Typical mammary alveolus with secretory alveolar epithelium lining the lumen. Contractile myoepithelium cells are arrayed on the surface along with vascular supply. The alveolus empties into the mammary ductal system.
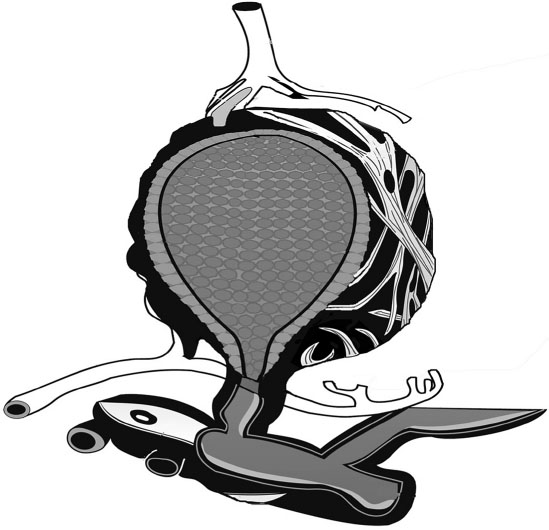
Plasma cells from the lymphatics surround the entire alveolar unit and provide most of the immunoglobins present in milk. Each day, an infant receives 800–1200 mg of secretory IgA and smaller amounts of IgM and IgG. Millions of living cells also enter milk, including lymphocytes, T-cells, and macrophages, all of which function to prepare the infant gut for exposure to microbes.
The ability of a medication to transfer into the milk compartment is largely determined by a few physicochemical properties, such as molecular weight, lipophilicity, plasma protein binding, and pKa. Maternal factors include the concentration of medication in the plasma compartment, with higher transfer at higher plasma levels (particularly Cmax–the peak plasma concentration of a drug).
The transfer of drugs into human milk is usually facilitated by passive diffusion down a concentration gradient. In the breast tissue, only a few transport systems (for drugs) are known to exist. Thus, most drugs normally transfer from areas of high concentration (plasma) to areas of low concentration (milk) by passive diffusion between compartments. During the first few days post partum, drugs may transfer into milk at slightly higher concentrations due to the lack of a tight junctional system. The retrograde diffusion of drugs from the milk back into the plasma is well documented and is probably controlled by the same kinetic factors as entry (size, pKa, lipophilicity). As the maternal plasma level of medication increases, so does the transfer into milk. As the maternal plasma level of medication drops, most drugs diffuse out of the milk compartment and back into the maternal plasma for elimination. Few drugs are actually trapped in milk but those that are include iodine, cimetidine and nitrofurantoin, among others.
The physicochemical factors most important include the following.
- Molecular weight: the lower the molecular weight, the more the drug enters the milk compartment. Drugs >800 daltons are virtually excluded from milk.
- pKa: drugs with a basic pKa may become highly ionized at the pH of milk (7.2) and thus become trapped in the milk compartment (e.g. barbiturates).
- Protein binding

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


