Background
Intrauterine growth restriction is a condition in which the fetus has a birthweight and/or length <10th percentile for the gestational age. Intrauterine growth restriction can be associated with various causes, among which is low uteroplacental perfusion and chronic hypoxia during gestation. Often, intrauterine growth-restricted fetuses have increased oxidative stress; therefore, agents that decrease oxidative stress and increase utero, placental, and umbilical perfusion have been proposed as a beneficial therapeutic strategy. In this scenario, melatonin acts as an umbilical vasodilator and a potent antioxidant that has not been evaluated in pregnancies under chronic hypoxia that induce fetal growth restriction. However, this neurohormone has been proposed as a pharmacologic therapy for complicated pregnancies.
Objectives
The aim of this study was to determine the effects of prenatal administration of melatonin during the last trimester of pregnancy on the biometry of the growth-restricted lambs because of developmental hypoxia. Further, we aimed to determine melatonin and cortisol levels and oxidative stress markers in plasma of pregnant ewes during the treatment.
Study Design
High-altitude pregnant sheep received either vehicle (n = 5; 5 mL 1.4% ethanol) or melatonin (n = 7; 10 mg/kg –1 day –1 in 5 mL 1.4% ethanol) daily during the last one-third of gestation. Maternal plasma levels of melatonin, cortisol, antioxidant capacity, and oxidative stress were determined along treatment. At birth, neonates were examined, weighed, and measured (biparietal diameter, abdominal diameter, and crown-rump length).
Results
Antenatal treatment with melatonin markedly decreased neonatal biometry and weight at birth. Additionally, melatonin treatment increased the length of gestation by 7.5% and shifted the time of delivery. Furthermore, the prenatal treatment doubled plasma levels of melatonin and cortisol and significantly improved the antioxidant capacity of the pregnant ewes.
Conclusions
Our findings indicate that antenatal melatonin induces further intrauterine growth restriction but improves the maternal plasma antioxidant capacity. Additional studies should address the efficiency and safety of antenatal melatonin before clinical attempts on humans.
I ntrauterine growth restriction (IUGR) is defined as a condition in which the fetus has an estimated body weight and/or length at <10th percentile for the gestational age. This condition is associated markedly with increased perinatal morbidity and mortality rates. Therefore, IUGR needs careful prenatal and labor monitoring and appropriate neonatal assistance. Furthermore, IUGR programs several organs and systems through epigenetic mechanisms and triggers metabolic and cardiovascular diseases in neonates, juveniles, and adults. This is a worldwide relevant condition, because it is estimated that there are 150 million newborn infants per year and that 5-10% of them, depending on the area, will have low birthweight standardized for gestational age. Although many efforts have been made in the IUGR-related mechanisms and potential therapies, there is still no preventive or definitive curative treatment for these babies.
IUGR may be attributed to several causes that include oxygen and/or nutrition deprivation and exposure to toxins during gestation. The most common cause is blood flow restriction with low uteroplacental perfusion, which hampers oxygen and nutrient delivery to the fetus. Moreover, IUGR fetuses and their placentas have increased oxidative stress, which is postulated as an important mechanism involved in IUGR and cardiovascular dysfunction. Therefore, agents that increase umbilical and/or uteroplacental perfusion and decrease oxidative stress may be an advantageous therapeutic approach. In this context, melatonin is an effective umbilical vasodilator and a potent antioxidant in the perinatal period. These effects of melatonin that are supported by several studies on animal models have led to the proposal of pilot clinical trials to evaluate the effects of maternal melatonin administration on fetal oxidative stress and growth in human pregnancies that are affected by IUGR. However, the outcomes of studies of antenatal melatonin in humans have not been revealed yet.
Plasma melatonin levels are decreased in complicated pregnancies such as preeclampsia and IUGR. Moreover, IUGR and premature babies have a delayed development of melatonin rhythmicity. In addition, the maternal alteration of melatonin levels during the last one-third of pregnancy also affects the melatonin synthesis in the offspring. However, it is unknown, and still in debate, whether the decrease in melatonin is a cause or effect of the pathologic pregnancies.
Gestation under chronic hypobaric hypoxia induces a marked IUGR in human and animal models. We have developed a sheep model in which neonates experience the development of IUGR and impaired cardiovascular functions that are related to oxidative stress. Furthermore, a postnatal treatment with melatonin improved pulmonary and cerebral vascular functions that are associated with a significant fall in oxidative stress after 1 week of treatment. In the present study, we evaluated whether a treatment with antenatal melatonin during the last one-third of gestation under chronic hypobaric hypoxia would prevent neonatal IUGR in sheep.
Materials and Methods
All animal care, procedures and experimentation were approved by the Bioethics Committee of the Faculty of Medicine, University of Chile (CBA 0398), and were conducted in accordance with the ARRIVE guidelines and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Animals and sampling
Twelve time-mated pregnant sheep ( Ovis aries ) at high altitude (Putre Research Station, International Center for Andean Studies, University of Chile, 3600 m above sea level) were bred and maintained under standard housing conditions in the Research Station. Once verified that the fetal development was a single gestation, we randomly allocated melatonin-treated (MM; n = 7; 4 female and 3 male) and vehicle-treated (MC; n = 5; 3 female and 2 male) sheep. MM ewes received melatonin (10 mg.kg –1 day –1 in 5 mL 1.4% ethanol); MC ewes received 5 mL of 1.4% ethanol, orally at 18 hours in the last one-third of gestation (starting at 100 days; gestation length approximately 150 days). Melatonin administration was at dusk to preserve physiologic rhythmicity with nighttime increases. Maternal plasma samples were taken at 10 and 22 hours at 120, 130, and 140 days of gestation and kept with 0.005% butylated hydroxytoluene at –80°C until use. Pregnant ewes were left to deliver naturally, and no interventions were made during labor. Immediately after delivery, neonates were attended, weighed, and measured (biparietal diameter, abdominal diameter, and crown-rump length).
Plasma melatonin
Plasma levels of melatonin were obtained by radio immunoassay as previously described. Samples were collected under sterile conditions into chilled EDTA tubes (2 mL K+/EDTA; LIP, Ltd, Shipley, West Yorkshire, UK) and analyzed in duplicate at the same time. Plasma samples were extracted with diethyl ether before being assayed. The assay used melatonin antiserum (batch no. 704 8483; Guildhay Antisera Ltd, Guildford, Surrey, UK) and [O-methyl-3H]-labeled melatonin (85 Cimmol/L; Amersham Biosciences AB, Uppsala, Sweden) as a tracer.
Plasma cortisol
Blood samples (4 mL) were collected and placed in chilled polystyrene tubes that contained 200 μL of 0.5 mmol EDTA. The tubes were centrifuged for 20 minutes at 2800 g at 4°C. Plasma was separated and stored in aliquots at −80°C. Cortisol was measured by specific radioimmunoassay (Cortisol Coat-a-Count; Diagnostic Products Corp, Los Angeles, CA) as previously described.
Plasma 8-Isoprostanes
As a marker of lipid peroxidation, analysis of 8-isoprostanes (8-iso prostaglandin F2 alpha) was performed on plasma with the use of a commercial colorimetric kit (8-isoprostane EIA kit; Cayman Chemical Company, MI). The assay is based on competition between 8-isoprostane and 8-isoprostane acetylcholinesterase conjugate (8-isprostane tracer) with fixed attachment sites for specific serum antibody to 8-isoprostane. Because the concentration of the tracer is kept constant and the 8-isoprostane in the sample varies, the amount of tracer bound to serum antibody will be inversely proportional to the concentration of 8-isoprostane in the measuring plate. The product of the enzyme reaction was measured spectrophotometrically at 412 nm.
Reducing ability of plasma
Total antioxidant capacity of the plasma of mothers was assessed by the ferric reducing ability of plasma (FRAP). Briefly, the FRAP assay working reagent was prepared by mixing 300 mmol/L of acetate buffer (pH 3.6), 10 mmol/L of Tripyridil-s-triazine (TPTZ) solution, and 20 mmol/L of FeCl 3 × 6H 2 O in a 10:1:1 ratio and by subsequent heating of the resultant mixture to 37°C. The reaction mixture was composed of 750 μL FRAP solution, 75 μL H 2 O, and 25 μL samples and incubated in the dark at 25°C for 30 minutes; the absorbance was read at 593 nm. A standard curve that ranged from 50 μmol/L to 1.5 mmol/L of FeSO 4 was prepared for the quantitative determination of FeSO 4 as millimolar Fe 2+ and FeSO 4 equivalents that was produced in the samples.
Statistical analyses
All data were expressed as means ± SEM. Time of delivery was analyzed by a Watson-William test. Kolmogorov-Smirnov test was used to confirm normality of the data; comparisons were done by an unpaired t -test. Differences were accepted as significant at a probability value of ≤.05 (Prism 5.0; GraphPad Software Inc, La Jolla, CA).
Results
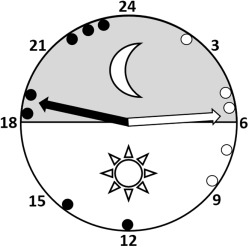
Melatonin antenatal administration induced changes in the normal development of pregnancy at chronic hypobaric hypoxia, increasing the length of pregnancy by 7.5% and shifting labor and delivery time from early morning deliveries to afternoon and evening events (MC, 5:38 ± 0:40 AM vs MM, 6:42 ± 1:06 PM; P < .05; Figure 1 ). This represents an average shifting time of approximately 12 hours. Additionally, melatonin caused a greater restriction of growth that manifested as a decreased birthweight, biparietal diameter, crown-rump length, and abdominal diameter ( Table ). However, calculation of the ratios between biparietal diameter/birthweight and between crown-rump length/birthweight, evidenced similarity of the IUGR pattern between groups ( Table ).
| Variable | Control group (n = 5) | Melatonin-treated group (n = 7) |
|---|---|---|
| Gestational length, d | 149 ± 1 | 155 ± 1 a |
| Birthweight, kg | 3.56 ± 0.16 | 2.88 ± 0.22 a |
| Biparietal diameter, mm | 64.98 ± 1.53 | 55.50 ± 2.06 a |
| Crown-rump length, cm | 47.98 ± 1.82 | 37.98 ± 1.40 a |
| Abdominal diameter, cm | 38.70 ± 1.53 | 32.57 ± 1.71 a |
| Biparietal diameter/birthweight, mm/kg | 18.73 ± 1.24 | 19.86 ± 1.68 |
| Crown-rump length/birthweight, cm/kg | 13.48 ± 1.17 | 13.60 ± 1.17 |
a Significant differences by unpaired t -test ( P < .05) vs the control group.
The maternal plasma samples that were taken at 10:00 AM and 10:00 PM to determine melatonin, cortisol, FRAP, and 8-isoprostanes showed similar values between 120, 130, and 140 days of gestation at these hours. Therefore, we decided to average the results as daytime and nighttime samples.
Daytime samples showed similar values of melatonin concentration between groups (MC, 138.7 ± 31.5 pg*mL –1 vs MM, 187.5 ± 31.5 pg*mL –1 ). In marked contrast, plasma melatonin raised 2-fold at nighttime in mothers who were treated with melatonin (MC, 654.2 ± 160.4 pg*mL –1 vs MM, 1481.0 ± 229.9 pg*mL –1 ; P < .05; Figure 2 A).
Maternal cortisol levels were similar between daytime and nighttime samples in control ewes. In contrast, melatonin induced increased cortisol levels in morning samples (MC, 1.422 ± 0.528 pg*mL –1 vs MM, 9.250 ± 1.366 pg*mL – 1; Figure 2 B).
In addition, melatonin treatment increased the maternal plasma antioxidant capacity during daytime (MC, 311.8 ± 23.7 μmol/L vs MM, 484.9 ± 45.3 μmol/L; P < .05) and nighttime (MC, 420.4 ± 66.7 μmol/L vs MM, 616.2 ± 44.9 μmol/L; P < .05; Figure 3 A). Moreover, melatonin administration clearly decreased plasma levels of 8-isoprostane at nighttime (MC, 16.12 ± 2.40 pg*mL –1 vs MM, 4.65 ± 0.40 pg*mL –1 ; P < .05); there was no difference in daytime samples between groups (MC, 13.18 ± 2.3 pg*mL –1 vs MM, 8.74 ± 0.8 pg*mL –1 ; Figure 3 B).
Results
Melatonin antenatal administration induced changes in the normal development of pregnancy at chronic hypobaric hypoxia, increasing the length of pregnancy by 7.5% and shifting labor and delivery time from early morning deliveries to afternoon and evening events (MC, 5:38 ± 0:40 AM vs MM, 6:42 ± 1:06 PM; P < .05; Figure 1 ). This represents an average shifting time of approximately 12 hours. Additionally, melatonin caused a greater restriction of growth that manifested as a decreased birthweight, biparietal diameter, crown-rump length, and abdominal diameter ( Table ). However, calculation of the ratios between biparietal diameter/birthweight and between crown-rump length/birthweight, evidenced similarity of the IUGR pattern between groups ( Table ).



