40 Polycystic Ovary Syndrome, Hirsutism, and Virilization Robert L. Barbieri Polycystic ovary syndrome (PCOS) is a common gynecologic endocrine disorder that is characterized by both reproductive abnormalities, including excess ovarian secretion of androgens and oligo-ovulation, and metabolic abnormalities such as insulin resistance, the metabolic syndrome, and an increased risk for diabetes mellitus. The key to understanding PCOS is to recognize the dual defects in reproductive and metabolic function associated with the syndrome. Two major approaches to the definition of PCOS are in clinical use (Table 40.1). According to the National Institutes of Health (NIH) definition, hyperandrogenism plus oligo-ovulation must both be present to diagnose the condition. Hyperandrogenism can be diagnosed by clinical examination, based on the presence of significant hirsutism, or by laboratory testing with the demonstration of elevated levels of serum testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS). Oligo-ovulation can usually be diagnosed by history based on the report of menstrual cycles greater than 35 days. Most women with PCOS report fewer than six menses per year. In addition, the NIH definition suggests that other diseases which cause hyperandrogenism should be excluded, such as nonclassical adrenal hyperplasia due to 21-hydroxylase deficiency. Exclusion of the latter disease is best accomplished by demonstrating that the serum 17-hydroxyprogesterone level is less than 2 ng/mL in the early morning during the follicular phase of the menstrual cycle. In 2003, the European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) jointly proposed a new approach to the definition of PCOS. In this new approach, two of the following three criteria must be present to diagnose PCOS: 1) hyperandrogenism, 2) oligo-ovulation or anovulation, 3) transvaginal ultrasound demonstration of the presence of more than 12 small follicles in each ovary (Fig. 40.1).
Definition and Diagnosis
| NIH criteria | ESHRE/ASRM criteria ** |
| 1.Hyperandrogenism as evidenced by clinical findings, such as hirsutism, and/ or laboratory findings such as elevated serum testosterone, androstenedione, or DHEAS plus | 1.Hyperandrogenism |
| 2.Oligomenorrhea or amenorrhea and | 2.Oligomenorrhea or amenorrhea |
| 3.Rule out other causes of hyperandrogenism such as nonclassical adrenal hyperplasia due to 21-hydroxylase deficiency by demonstrating a normal 17-hydroxyprogesterone level (<2 ng/mL) | 3. Multifollicluar ovary demonstrated by highresolution transvaginal ultrasonography More than 12 small follicles 2–9 mm in diameter in each ovary An alternative ultrasonographic criterion is an ovarian volume >10 mL |
DHEAS, dihydroepiandrosterone sulfate
* The author prefers the NIH criteria for diagnosing polycystic ovary syndrome
** The diagnosis is made when two of the three listed conditions are present
The strength of the NIH definition is that diagnosis can largely be made using clinical history (oligomenorrhea) and physical examination (hirsutism). The ESHRE/ ASRM definition requires transvaginal ultrasound examination of the ovary, a resource-intensive test. The ESHRE/ ARMS definition also permits the diagnosis of PCOS if no hyperandrogenism is present, but the patient has both oligo-ovulation and a multifollicluar ovary on ultrasound examination. Many authorities believe that hyperandrogenism is the key feature of PCOS and the diagnosis should not be made unless hyperandrogenism is present. TheE-SHRE/ASRM definition would increase the prevalence of PCOS by about 50%, with many women being diagnosed with very mild forms of the syndrome.
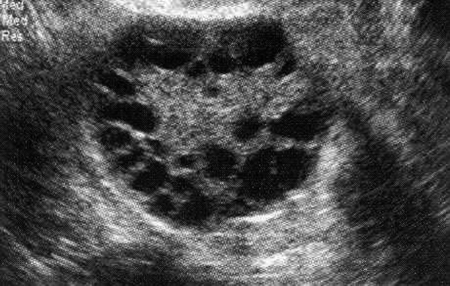
Fig. 40.1 A commonly used definition of a “multi-follicular” ovary is the presence of 12 or more small follicles with diameters of 2–9 mm in each ovary Image obtained by transvaginal ultrasound scan of one ovary: more than 20 small follicles are demonstrated, clearly indicating that this is a multifollicular ovary
Prevalence and Epidemiology
PCOS is a common gynecologic endocrine problem. Approximately 6–8% of women of reproductive age (15–45 years) have PCOS as defined by the NIH criteria.
Etiology and Pathophysiology
The etiology of PCOS remains a mystery. PCOS is characterized by defects in both the reproductive and metabolic systems. Most women with PCOS have abnormally increased gonadotropin-releasing hormone (GnRH) pulse frequency, suggesting that a hypothalamic neuroendocrine abnormality is a key cause of the syndrome. The cause of the increased GnRH pulse frequency is not known. Increased GnRH pulse frequency results in increased pituitary secretion of luteinizing hormone (LH) which, in turn, stimulates the ovarian theca and stroma to over-secrete androstene-dione and testosterone. Increased LH secretion results in increased ovarian androgen production which, in turn, causes hirsutism by stimulating the growth of the pilose-baceous unit in androgen dependent areas such as the face. Increased LH and ovarian androgen secretion causes stunted growth of ovarian follicles, resulting in the accumulation of small follicles, 2–9 mm in diameter. In the absence of the growth of a large follicle (20–25 mm in diameter), the LH surge does not occur and ovulation is not triggered. In the absence of ovulation, the menstrual pattern is oligomenorrhea or amenorrhea. The stunted growth of the ovarian follicles results in the accumulation of many small follicles 2–9 mm in diameter that can be detected by transvaginal ultrasonography. The ESRHE/ASRM definition of PCOS requires that more than 12 small follicles, 2–9 mm in diameter, be detected in each ovary by sonography.
Many women with PCOS also have excessive adrenal secretion of the androgen precursors, androstenedione, and DHEAS. These androgen precursors can be converted to the potent androgens, testosterone (Fig. 40.2) and dihydrotestosterone.
The metabolic abnormalities of PCOS include:
- insulin resistance and a compensatory hyper-insulinemia
- the metabolic syndrome (Table 40.2) characterized by centripetal obesity, increased visceral fat, dyslipidemia, and mild hypertension
- increased risk for developing diabetes mellitus
- dyslipidemia
- elevated serum concentrations of markers of endothelial inflammation, such as C-reactive protein, interleukin-6, interleukin-18, and endothelin-1
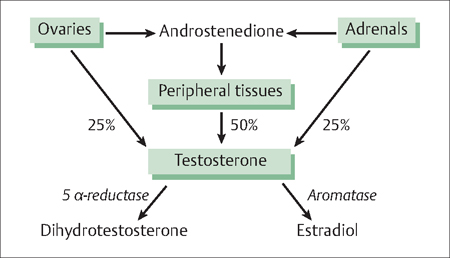
Fig. 40.2 Both the ovary and adrenal glands secrete testosterone and androstenedione In turn, androstenedione can be converted to testosterone in peripheral tissues In the pilosebaceous unit, testosterone is converted to the potent androgen, dihydrotestosterone
| Findings from physical examination |
| 1 Abdominal obesity Waist circumference greater than 88 cm (35 in) in women and 102 cm (40 in) in men |
| 2 Elevated blood pressure Blood pressure greater than 130/85 mmHg or drug treatment for elevated blood pressure |
| Findings from laboratory testing |
| 3 Elevated blood glucose Fasting plasma glucose higher than 100 mg/dL or drug treatment for elevated blood glucose |
| 4 Elevated triglycerides Fasting serum triglycerides 150 mg/ dl or higher or drug treatment for elevated triglycerides |
| 5 Low level of high-density lipoprotein–cholesterol (HDL-C) Fasting serum HDL-C =50 mg/dL for women and =40 mg/ dL for men, or drug treatment for low HDL-C |
Source: Grundy SM, Cleeman JL, Daniels SR, et al Diagnosis and management of the metabolic syndrome: an American Heart Association / National Heart Lung and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752
Recently, women with PCOS have also been documented to be at increased risk for sleep apnea and nonalcoholic steatohepatitis.
For women with insulin resistance and adequate pancreatic beta-cell function, a glucose load (or other mixed meal) results in a marked increase in insulin secretion and circulating insulin in an attempt to overcome the peripheral resistance to insulin action. Both lean (Fig. 40.3) and obese women with PCOS often have insulin resistance. In general, obese women with PCOS have more severe insulin resistance than obese normally-ovulating women or lean women with PCOS.
History
Menstrual Dysfunction
Women with PCOS typically have fewer than six menses per year. In most women with PCOS, the menstrual dysfunction begins during the teen years. The differential diagnosis of oligomenorrhea is extensive. Other causes of oligomenorrhea include pregnancy, stress or exercise-induced oligomenorrhea (hypothalamic amenorrhea), eating disorders including anorexia or bulimia, abnormally reduced body fat with a normal body mass index (BMI), premature ovarian failure, hyperprolactinemia, and non-classical adrenal hyperplasia.
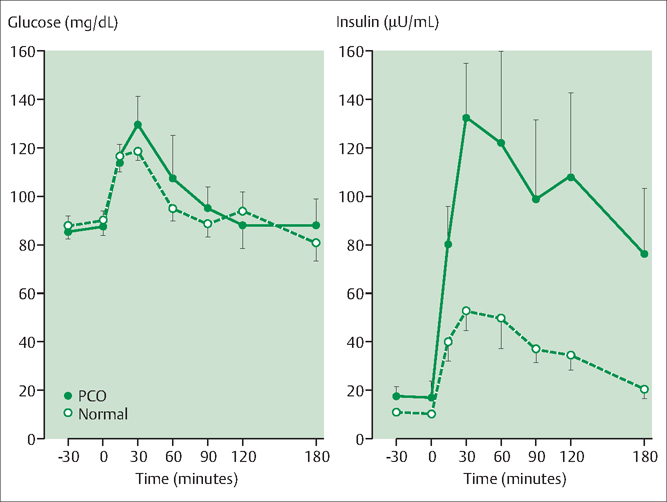
Fig. 40.3 Many women with poly-cystic ovary syndrome (PCOS) are insulin resistant In an insulin-resistant woman the ingestion of glucose results in the excess secretion of insulin in an attempt to overcome the peripheral insulin resistance In this study normally cycling women (open circles) and lean women with PCOS (closed circles) were given an oral glucose load Glucose and insulin were frequently sampled for 2 hours The lean PCOS women had markedly increased serum insulin levels compared with the normally cycling women Given the normal levels of glucose in both groups, this indicates peripheral insulin resistance in the lean women with PCOS Source: Chang RJ, Nakamura RM, Judd HL, Kaplan SA Insulin resistance in nonobese patients with polycystic ovary syndrome J Clin Endocrinol Metab. 1983;57:356–9
Oligo-ovulation
Moliminal symptoms such as dysmenorrhea, breast tenderness, and abdominal bloating typically occur in association with ovulatory cycles. Due to anovulation, women with PCOS may report fewer moliminal symptoms with their menses.
Physical Examination
Hyperandrogenism
Women with PCOS typically have signs of hyperandrogenism, the most frequent of which are hirsutism and acne. Hirsutism is often quantitatively assessed using the Ferriman—Gallwey scoring system. Nine different body areas are graded 0 (no hirsutism) to 4 (marked hirsutism), and the individual score for each body part is summed for a total score (Fig. 40.4). Approximately 5% of the female population has a total score greater than or equal to 8. Severe hyperandrogenism can lead to signs of virilization including male pattern hair loss, severe hirsutism, increased upper body muscle mass, deepening of the voice, and clitoromegaly.
Obesity and Insulin Resistance
Approximately 50% of women with PCOS have a BMI greater than 30 kg/m2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


