Source
Location
Population
No. of patients
Incidence
Male:female ratio
Age range
Peak age
Seasonal variation
Jacyk [6]
Nigeria
All
138
2.42/100 Dermatologic patients
1.0:1.12
18 months to 55 years
15–24 years
No variation
Messenger et al. [15]
England
All
126
Not reported
1.0:1.8
Not reported
Not reported
Higher incidence in winter months
Chuang et al. [7]
Rochester, MN
All
939
172.2/100,000 Person-years
1.0:1.76
10 months to 78 years
10–35 years
Significantly higher in colder months
Ahmed [16]
Sudan
All
81
1.09/100 Dermatologic patients
1.0:1.53
Not reported
6–30 years
Peaked in cold and dry season
Olumide [17]
Lagos
All
152
4.80/100 Dermatologic patients
1.0:1.20
91 % between 5 and 35 years
10–14 years
Peaked during early part of rainy season (March to July)
Cheong and Wong [8]
Singapore
All
214
Not reported
1.85:1.0
1–61 years
20–24 years
Higher incidence in March, April, and November
Harman et al. [4]
Eastern Anatolia
All
399
0.75/100 Dermatologic patients
1.0:1.21
87 % between 10 and 39 years
20–29 years
Peaked during spring, autumn, and winter
Nanda et al. [18]
Kuwait
Children aged 12 and below
117
1.17/100 Aged 12 and below
1.0:1.38
Not reported
Not reported
Not reported
Tay and Goh [5]
Singapore
All
368
0.65/100 Dermatologic patients
1.19:1
9 months to 82 years
20–29 years
No variation
Chuh et al. [9]
Hong Kong
All
41
Not reported
1.0:1.05
5–54 years
Mean age 25.9 years
Slightly higher incidence in colder months and months with less rainfall
But noted to be insignificant
Kyriakis et al. [10]
Athens
All
479
0.95/100 Dermatologic patients
1.0:1.4
35 days to 96 years
First peak male 6–10 years
Second peak male 36–40 years
First peak female 21–25 years
Second peak female 31–35 years
Not reported
Sharma and Srivastava [11]
India
All
200
0.25/100 Dermatologic patients
2.0:1.0
1.5–65 years
13–36 years
Higher incidence from September to December
Gunduz et al. [19]
Turkey
Pediatric
51
Not documented
1.1:1.0
Not reported
6–11 years
Mean age 8 years
Peaked in winter
Ayanlowo et al. [12]
Lagos
All
427
3.7/100 Dermatologic patients
1.0:1.55
Not reported
10–29 years
Peaked in rainy season
Pathophysiology
As the condition is typically a self-limiting disorder, with seasonal clustering, predominance during cold or winter months [7, 9, 11, 15–17, 19], and few recurrences, it is thought to be due to an infection, usually precipitated by an upper respiratory tract infection, with long-lasting immunity [4, 8, 15]. Many infective agents have been implicated, e.g., Influenza A, H1N1 [20, 21], cytomegalovirus [22], Epstein–Barr virus [23], parvovirus B19 [24] and Mycoplasma pneumoniae [25]. However, prospective studies have failed to show a significant change in serological titers in acute and convalescent stages for mycoplasma, cytomegalovirus, Epstein–Barr virus and parvovirus B19 [26, 27]. There are reports of the association of human herpesvirus-7 (HHV-7) with polymerase chain reaction (PCR) analysis and less so human herpesvirus-6 (HHV-6) with pityriasis rosea [28–31]. But they remain controversial [32–34].
Clinical Presentation
A herald patch may be present
Distribution of rash is typically over Langer’s cleavage lines over the trunk
Atypical presentations over the scalp, face, limbs, palms, and soles can be found
A herald patch, which is a single isolated lesion, can be found classically on the trunk but can also occur on the upper arm, neck, or thighs. The herald patch is seen in 17–94 % of cases [5, 26]. This oval patch varying from about 1 to 10 cm with collarette scaling can be mistaken for tinea corporis or eczema. Approximately 5 % of patients may feel a mild prodrome of headache, fever, malaise, or arthralgias [35]. Within a few days after the herald patch, there is a subsequent secondary eruption over the trunk and proximal extremities of lesions in a T-shirt and shorts distribution with peripheral collarette scaling (Figs. 33.1 and 33.2) which has been reported to be enhanced by epiluminescence dermatoscopy [36].
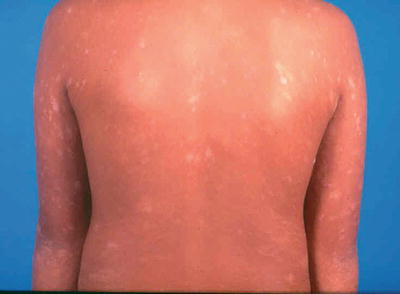
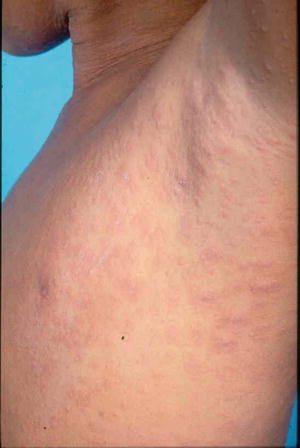

Fig. 33.1
Papules and plaques over the trunk with peripheral collerette scaling in a ‘fir tree’ pattern

Fig. 33.2
Papules and plaques over the trunk with peripheral collerette scaling
The orientation of these lesions on the posterior trunk is referred to as a “fir tree” or “Christmas tree” pattern [37]. “Langer’s cleavage lines” is now proposed to be the most appropriate term with the V-shaped pattern on the upper chest and back, circumferential pattern around the shoulders and hips, and transverse pattern on the lower anterior trunk and lower back (Fig. 33.3) [38]. Atypical forms of pityriasis rosea include an inverse pattern where the face and extremities are affected more than the trunk [39]. African patients have been reported to have a more extensive rash with more frequent involvement of the face and scalp [6]. It has been reported to present in the scalp in children [40] and when presenting on the soles can mimic secondary syphilis [41]. Other atypical forms of presentation include a vesicular eruption [42], erythema multiforme-like rash [43], urticarial [44], and purpuric variants [44, 45].
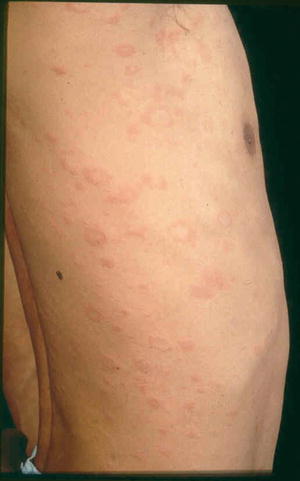

Fig. 33.3
Papules and plaques in a circumferential pattern along Langer’s cleavage lines
Treatment
Symptomatic treatment includes antihistamines and topical corticosteroids
Phototherapy or natural sunlight exposure may help ease pruritus and hasten resolution of rash in some patients, but there is an increased risk of post-inflammatory hyperpigmentation especially in darker skin types
Erythromycin or acyclovir can be tried to hasten resolution of the rash and decrease pruritus
As the condition is self-limiting in about 6–8 weeks, treatment may not be necessary (Table 33.2). However, pruritus can be bothersome and oral antihistamines and topical corticosteroids may be helpful. These provide symptomatic relief but do not help in the clearance of the rash [46]. Systemic steroids have not been shown to be effective [46, 47]. Exposure to UV-B therapy starting at 80 % of the minimum erythrogenic dose may relieve pruritus in as little as 24 h but may increase the incidence of post-inflammatory hyperpigmentation [48–50]. Alternatively, low-dose (10–30 J/cm2) UV-A1 phototherapy given 2–3 times a week until resolution may be tried [51]. Early administration of erythromycin 1 g taken orally in four divided doses for adults or 25–40 mg/kg divided four times daily in children for 2 weeks in a double-blind placebo controlled trial led to early resolution of symptoms [52]. However, subsequent studies did not find erythromycin or azithromycin useful [53, 54]. There is some evidence that oral acyclovir 1 g five times a day for 7 days at the onset in adults has been shown to shorten the duration of the disease [55, 56]. Subsequently, lower dosages of acyclovir 400 mg five times a day for 1 week was shown to be equally effective [57]. Recent studies have shown that despite its effectiveness in herpes simplex virus infection, acyclovir was not as effective as ganciclovir and forcarnet against HHV-6 and 7 [58]. This is because acyclovir is thymidine kinase-dependent whereas HHV-7 lacks the thymidine kinase gene [59]. There are yet to be published data on the effectiveness of ganciclovir and forscarnet in pityriasis rosea.
Table 33.2




Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


