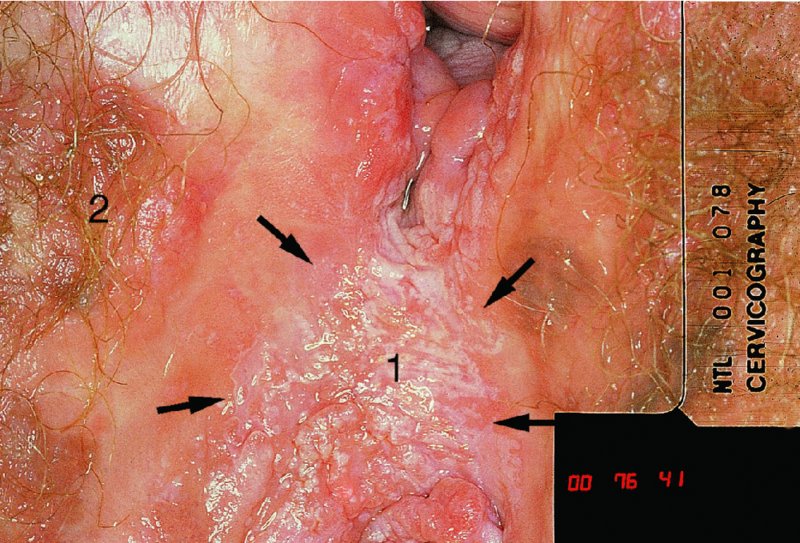
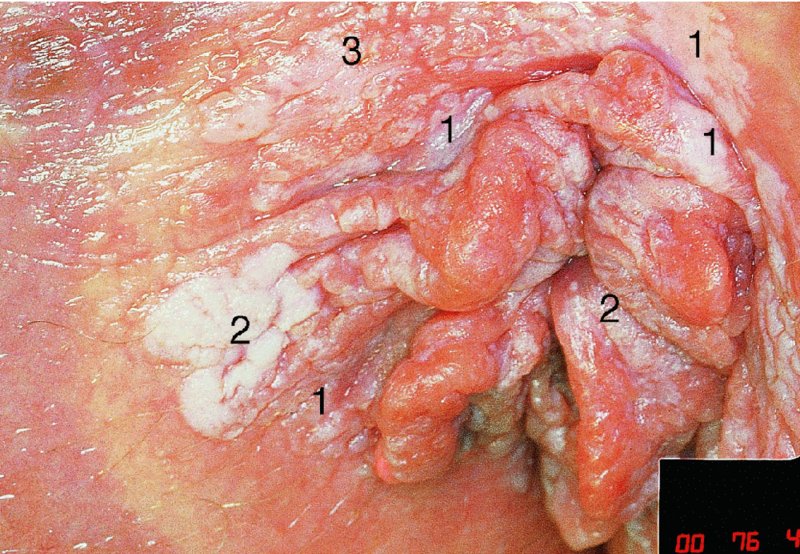
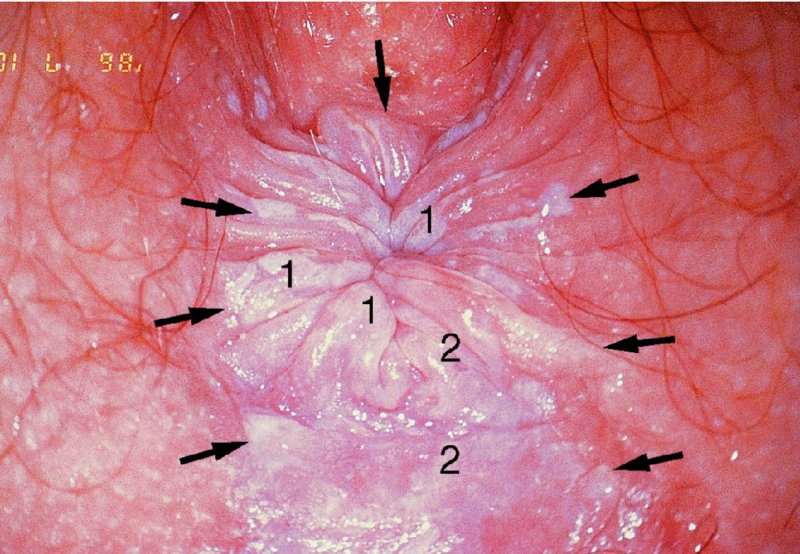
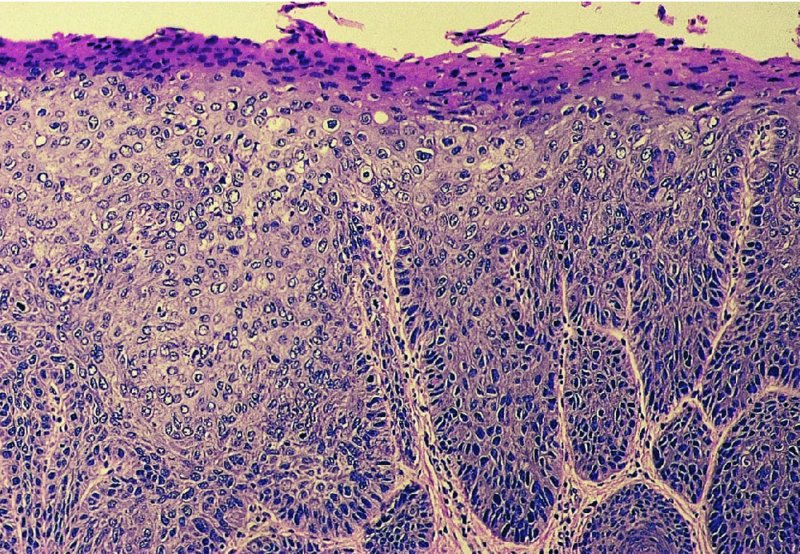
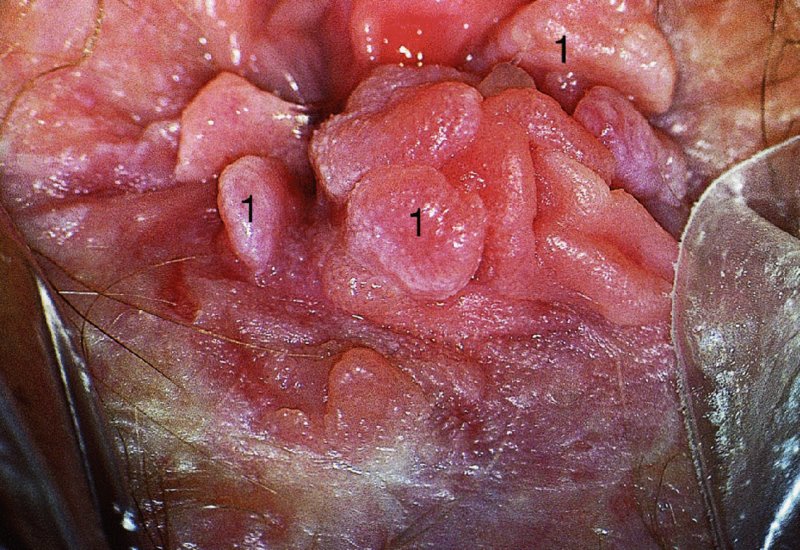
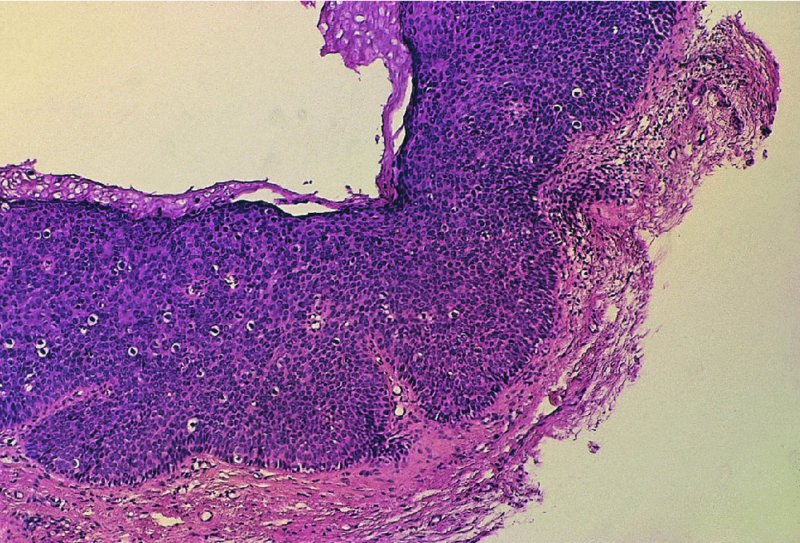
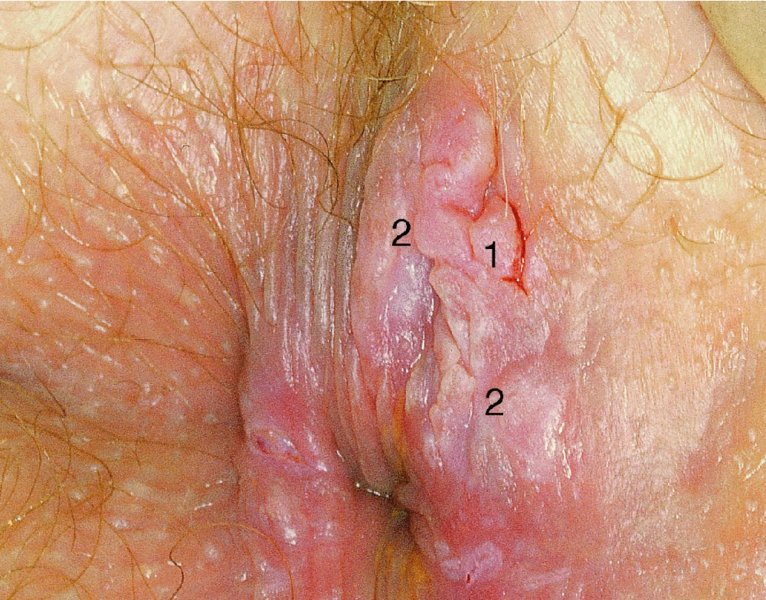
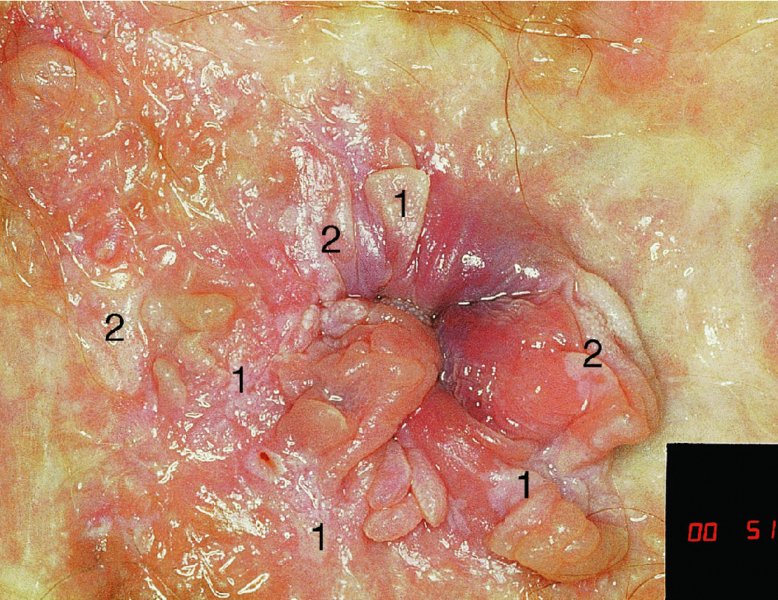
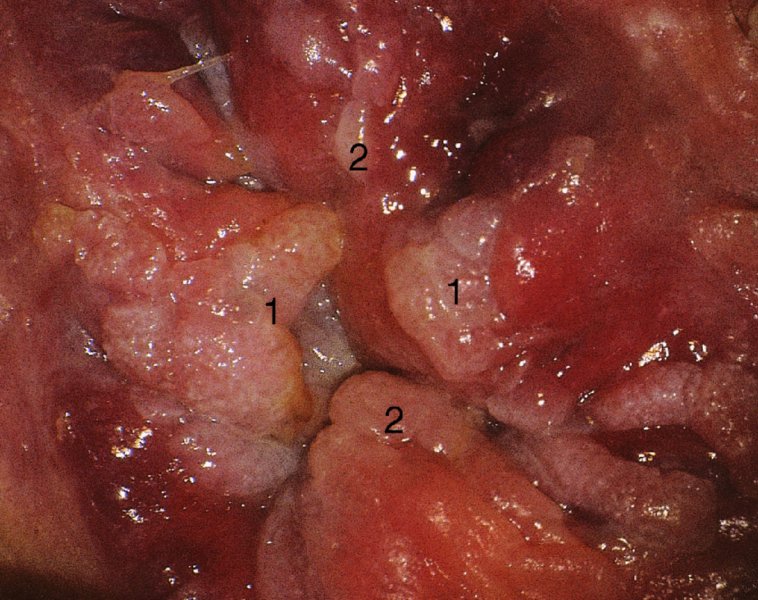
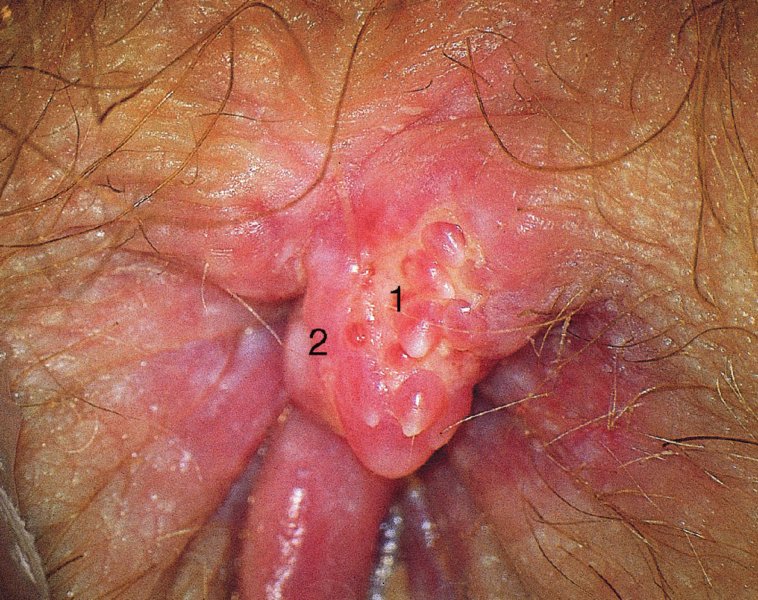
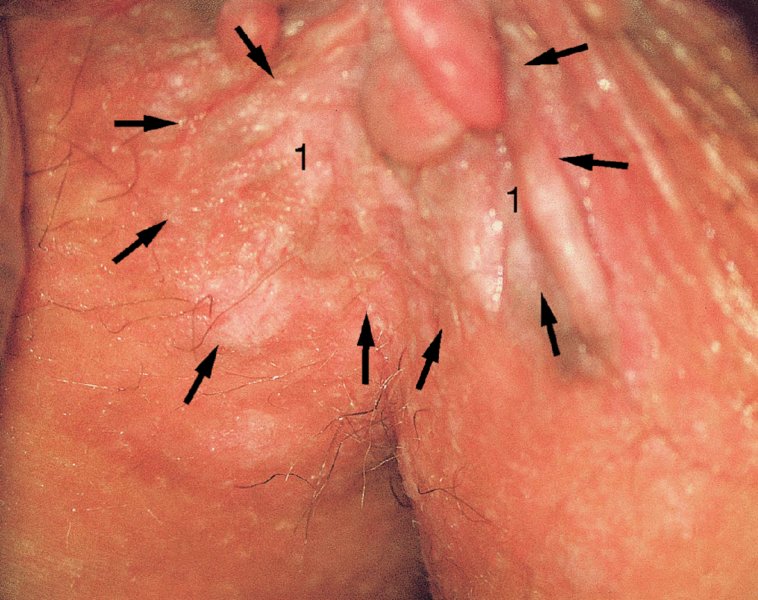
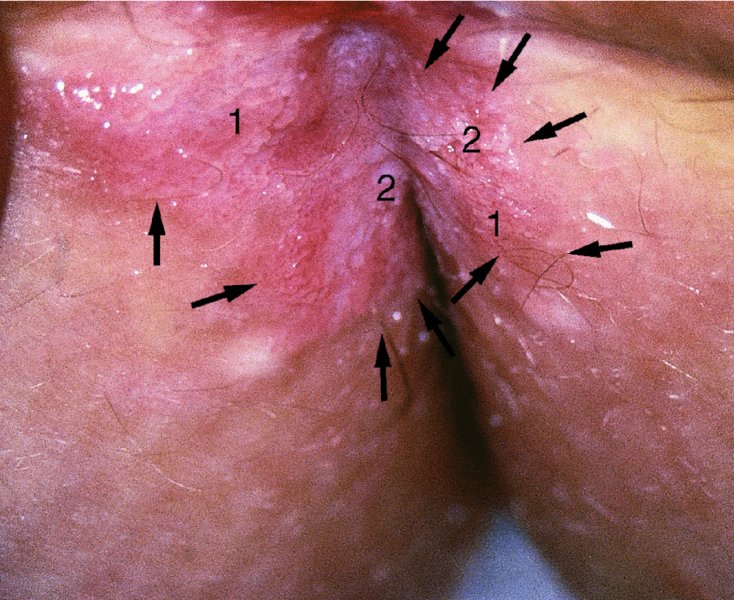
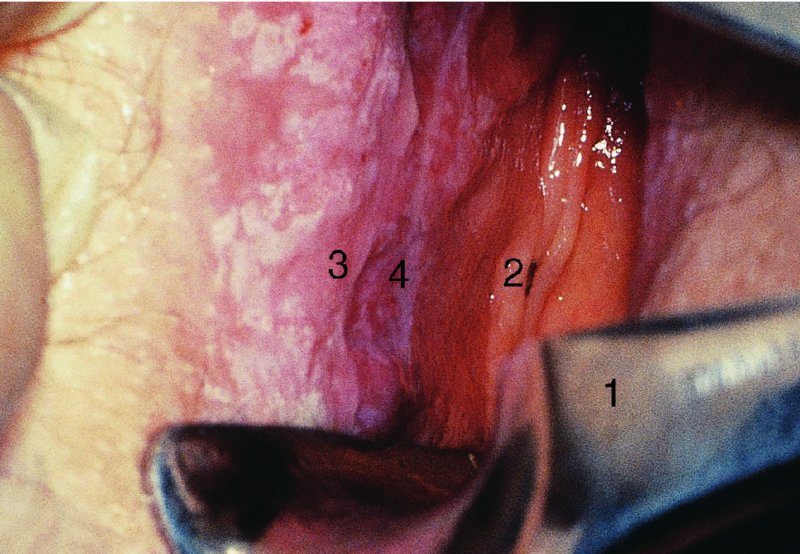
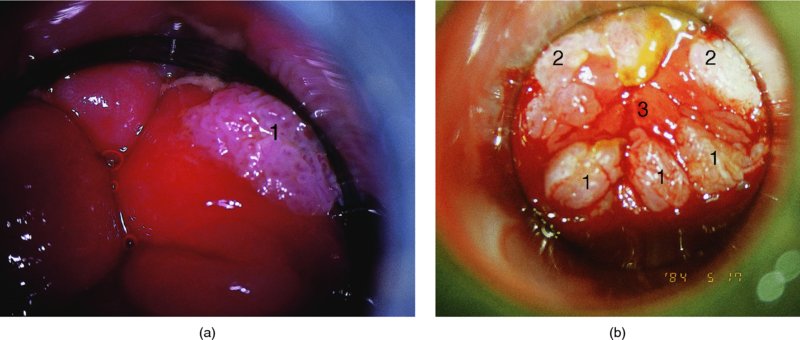
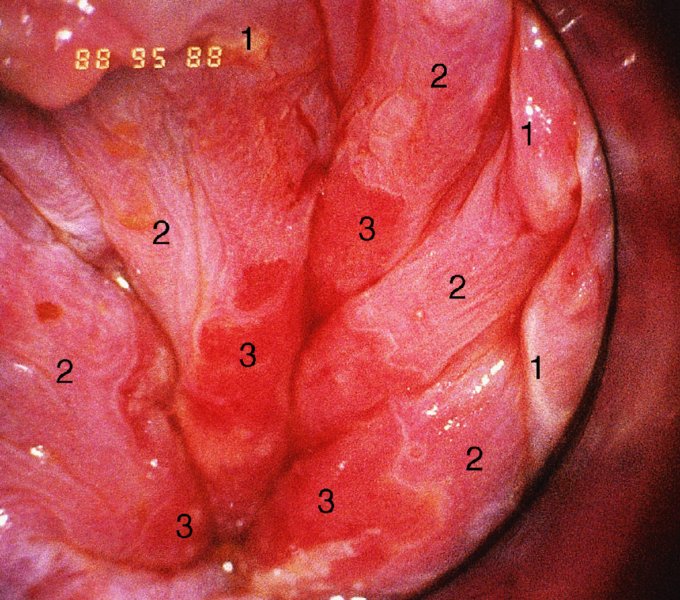
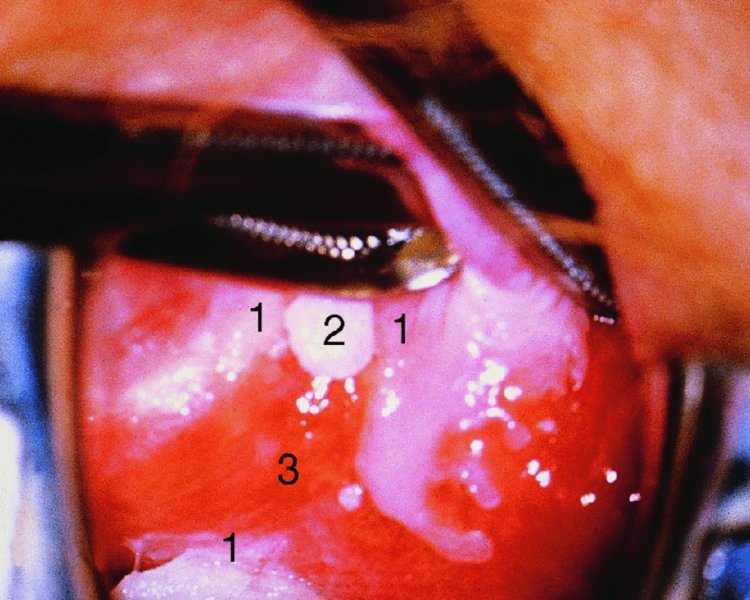
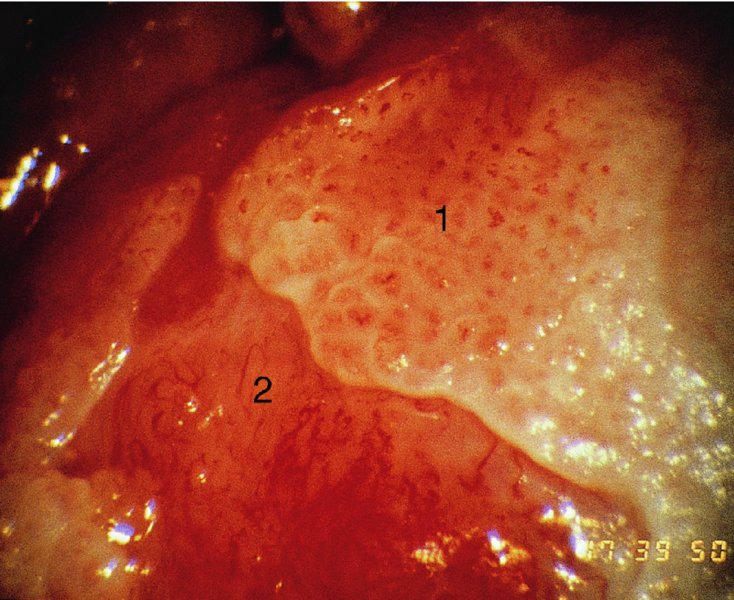
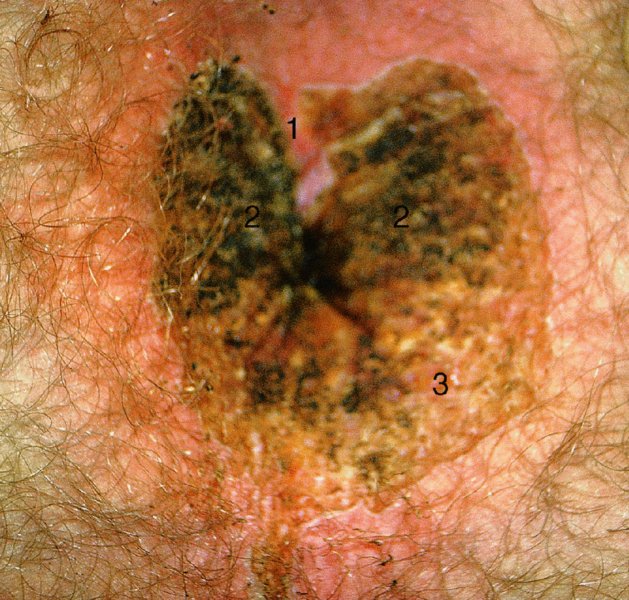
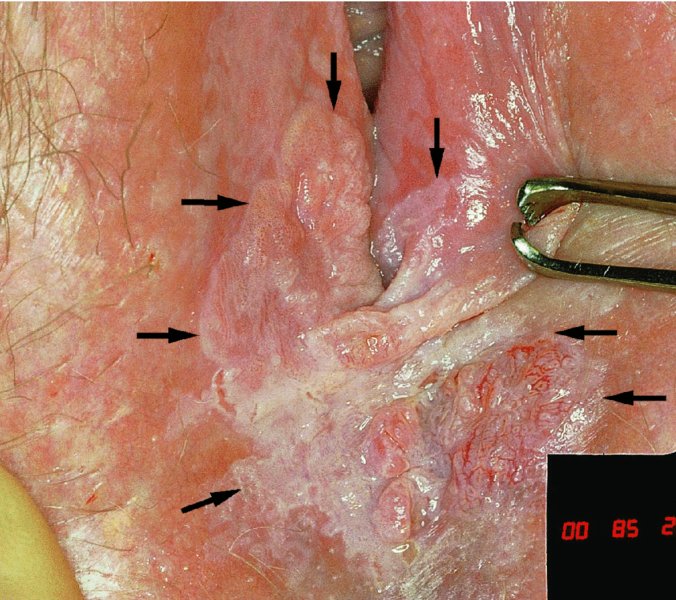
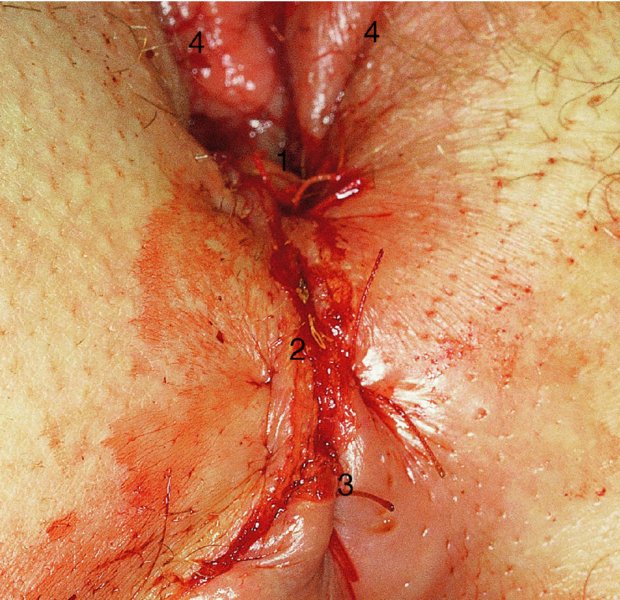
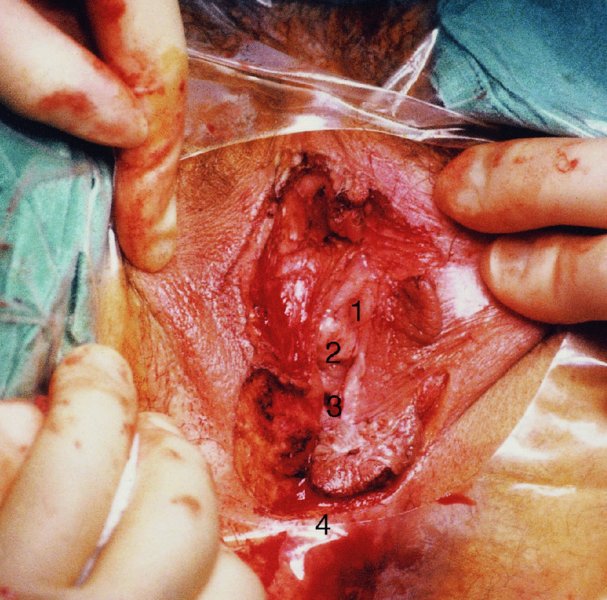
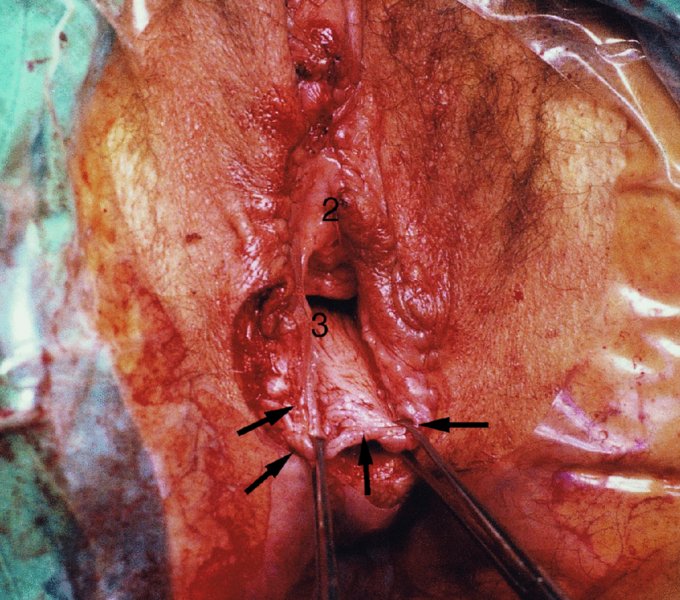
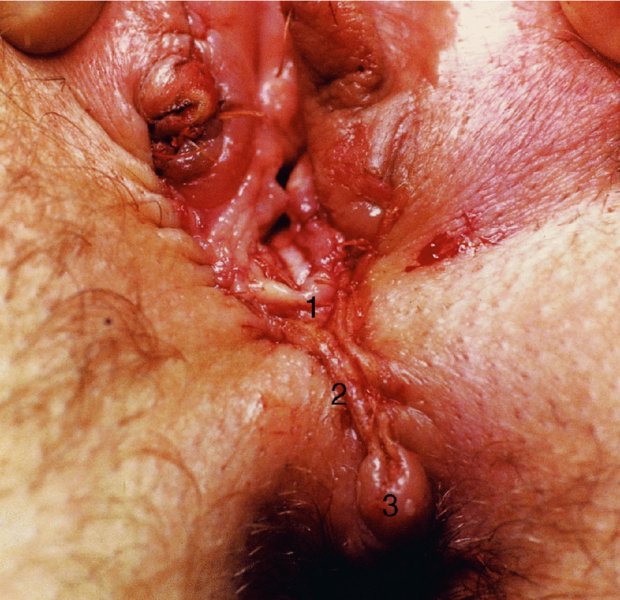
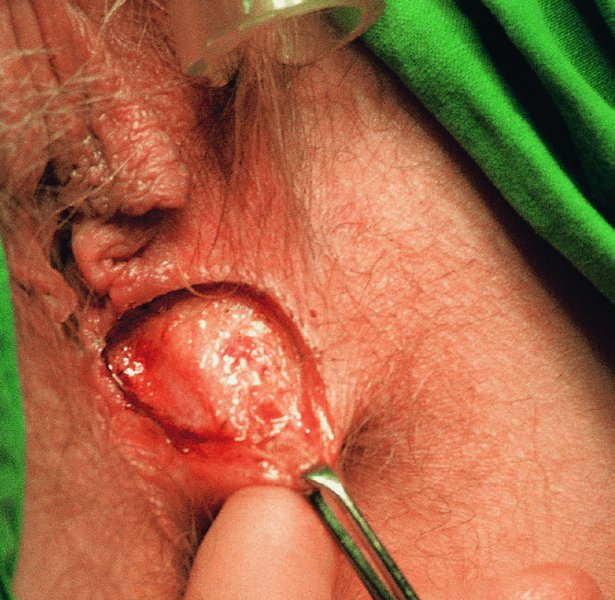
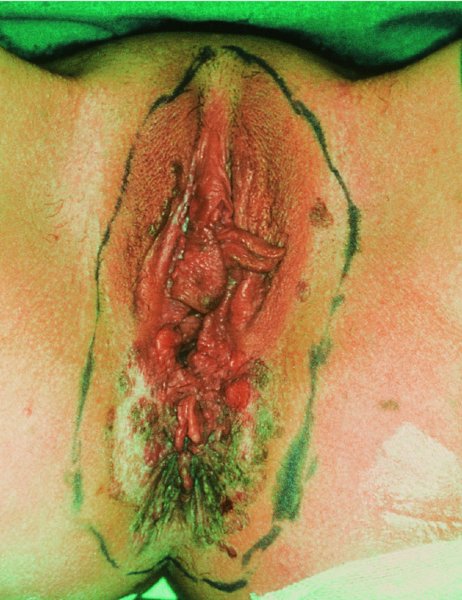
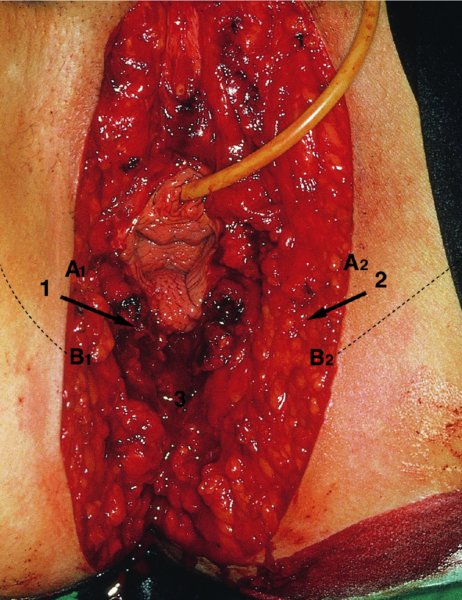
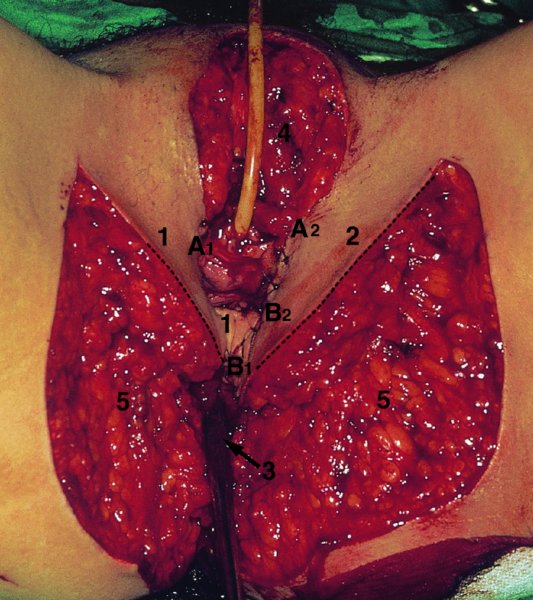
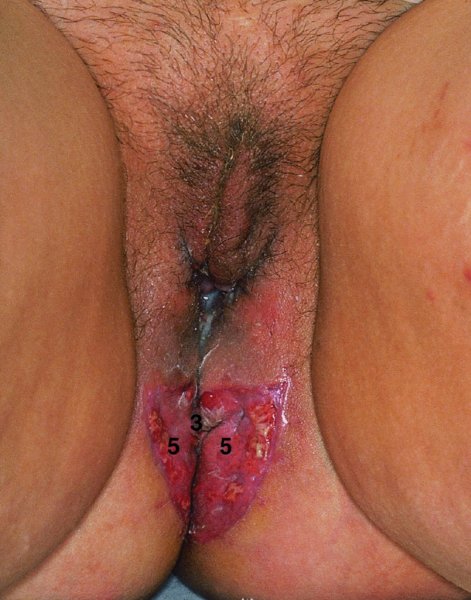
CHAPTER 10 The natural history of perianal and anal in situ intraepithelial neoplasia is uncertain in comparison with cervical intraepithelial neoplasia (CIN), in which the progression to invasive cancer occurs in about 30–40% of cases over a 20 year period. A well-defined premalignant stage of anal cancer is recognized in the form of an intraepithelial neoplastic lesion of the anus. It is referred to as anal intraepithelial neoplasia (AIN); it is also called a squamous intraepithelial lesion (SIL) in the USA. Histologically the lesion is characterized by nuclear and cellular abnormalities within the epithelium bounded by the basement membrane. AIN3 is a full-thickness epithelial change and is recognized as representing high-grade dysplasia or carcinoma in situ, being analogous to its cervical counterpart. AIN1 and 2 show these nuclear and cellular abnormalities confined, respectively, to the lower one-third and two-thirds, respectively. When the anal SIL terminology is used, the term LSIL represents a low-grade lesion, namely AIN1, and HSIL refers to a high-grade set of lesions, namely AIN2 and 3. A new classification for anogenital squamous lesions has recently been proposed. The prevalence of AIN is estimated to be less than 1% in the general population but evidence indicates a rising incidence in line with changing lifestyle and behavioral changes such as smoking, a high rate of human papillomavirus (HPV) infections and anal intercourse. There are a number of groups at high risk for the development of AIN. High on the list are those with immunocompromising conditions, the most well known of which is human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), transplant recipients, long-term steroid users, and those with other genital intraepithelial lesions. Anal carcinoma is much less common than its cervical counterpart, accounting for some 4% of all large bowel malignancies. However, there would seem to be evidence that the rate of anal carcinoma is indeed increasing, especially in the USA, where the incidence increased from 0.8 to 1.7 per 100 000 during the period 1975–2002. The only way of possibly reducing this potential risk with its corresponding morbidity and mortality would be to introduce a program of diagnosis and eradication of the intraepithelial disease. There is now ample evidence to indicate that the AINs (as a precancerous change) and anal carcinoma are part of one spectrum. This chapter will be concerned with presenting the clinical features and characteristics of the premalignant lesions and recommending appropriate management. There has been evidence for a number of years that HPV types 16 and 18 have an important role in the etiology of most lower genital tract precancers and cancers, including those related to the perianal and anal areas. It is suggested that the transmission of the HPV infection throughout the genital tract is by direct spread from adjacent areas and in this situation it would be from the vulva to the perianal and anal areas. Anal intercourse is likewise an obvious common method of transmission. The presence of HPV16 and 18 DNA in perianal and anal preneoplastic lesions was suggested in the early 1980s. Since then numerous studies have confirmed the association of HPV with AIN causation. In a recent study involving HPV genotyping it was found that high-risk HPV types were present in 56% of low-grade AINs and in 88% of high-grade AINs. HPV type 16 can be found in one-third of low-grade lesions and in about two-thirds of high-grade lesions. Nine out of 10 anal cancers can be associated with type 16 HPV. It is obvious from various studies that AIN3 (high grade) is the true precursor of squamous cancer. AIN2 associated with types 16 and 18 usually continues to progress, whereas that of the non-16 and non-18 subtypes shows more regression. In men with a history of receptive anal intercourse there would seem to be a high risk of the development of anorectal intraepithelial neoplasia. The high incidence of cytologic signs of “dysplasia” of the anorectal epithelium is associated with HPV infection. The risk of developing anal cancer in these men is 33 times that in control subjects with colon cancer. The prevalence of HIV in these men is well known, and the finding of AIN lesions must alert the clinician to the possibility of HIV infection, whether the patient be male or female. Many studies over the last two decades have continued to show the increased risk that not only men but also women with HIV have for the development of neoplastic anal abnormalities, especially if associated with HPV. The increased risk of anal neoplasia is significantly associated with, and almost exclusively seen in, the presence of HPV. Immunodeficiency and HIV positivity increased the risk of disease in HPV-positive subjects. A high level of HPV infection is important for the development of anal squamous intraepithelial lesions in this population. The prevalence of AIN in an HIV-infected male population ranged from 26% to 89%. Low-grade AIN can progress to AIN3 in 60% of HIV-positive cases within 2 years. The progression from AIN to cancer seems to be about 10% over a 5 year period. However, a recent study has purported to show a 13% progression over 5 years of surveillance. It has to be stressed that, as in the case of vulval intraepithelial neoplasia (VIN), pre-existing early cancer may exist in association with AIN. In 9–26% of excised AIN specimens in one study early cancer was detected, whereas AIN was found in about 80% of anal cancers. The high-risk groups besides individuals with HIV/AIDS, such as those with transplants or systemic lupus erythematosus, also have a high progression rate, which is estimated at 50% over 5years compared with a negligible rate in immunocompetent individuals. Smoking is a very major etiologic factor, in relation to neoplasia of the cervix and vulva. The relative risk (RR) for the development of anal cancer is 9.4 in men and 7.7 in women, compared with non-smokers. It has been known for many years that cervical, vaginal, and vulval intraepithelial diseases are linked by the common etiologic factor of HPV infections. This has been alluded to many times in this text. It also seems as though women with these conditions are at increased risk for the development of anal carcinoma. However, there have been few case–control studies determining the prevalence of AIN in females with CIN. Up to one-fifth of women with CIN3 can have similar anal lesions. Interestingly, more than half of the women with multifocal intraepithelial neoplasia (cervix plus vulva, vagina, or both) may also have anal lesions. HPV is common to the etiologies of both anal and cervical neoplasia; the presence of HIV would seem to accentuate this association, which was first highlighted by a study in the late 1990s. Cervical CIN/SIL is associated with a more than threefold increased risk of having, simultaneously, an abnormal anal smear with atypical cells. Many subsequent studies have confirmed this association. Women with a history of CIN3 have an increased RR of 6.94 for developing vaginal cancer, and an increased RR of 2.22 for vulval cancer and of 4.68 for anal cancer. There is also an increasing acute awareness of the risk of extragenital neoplasia in women with lower genital tract precancers. It would seem as though HPV infection is a common factor in all these sites. There are a number of risk factors that have been mentioned above and these must be considered when a suspicion of AIN exists and in the taking of a history by the clinician. First, the association with HPV must mean that enquires in respect of sexual behavior are prudent, especially in reference to sexual preference and a history of genital warts. Indeed, in one study, AIN was present in 28–35% of excised anal condylomata. Second, the high risk inherent in HIV/AIDS must be considered, especially as anal cancer is 30 times more common in HIV-positive individuals; therefore, the estimation of HIV status, especially indications of disease severity such as CD4 counts, must be done. Third, the presence of other genital tract precancers in cervix, vagina, and particularly the vulva must alert the clinician to the possibility of anal disease especially in heavy smokers. Finally, it has to be remembered that patients with AIN may present with two major symptoms, namely pruritus and anal discharge. In the 1990s it became apparent that anal cytology could offer a potential method of detecting anal neoplastic disease. A number of studies purported to highlight the benefits that cytologic screening, particularly of HIV-infected men and women, offered. Since that time controversy has existed as to the efficacy of anal screening cytology. The sensitivity of anal cytology can be as high as 83% with specificity of 38%. These results were comparable with cervical cytology. Anal cytology could be employed in selective centers dealing with high-risk populations (HIV-positive men who have sex with men are at a high risk for the development of anal neoplasia). However, there is much controversy as to the value of this technology in screening for AIN. Scholefield et al. (2011), writing on behalf of a prestigious group of colpoproctologists, stated that “there is probably no place for screening for AIN, even for high risk groups.” They continue that anal cytology is “not a sensitive screening test and requires trained cytopathologists.” An examination of this area involves the use of the same techniques as those employed for the vulva, i.e., vulvoscopy. Anoscopy (use of the colposcope to examine the anal canal area) is extremely valuable in defining the extent and characteristics of intraepithelial disease of the anus, anal canal, and perianal margins. However, it must be realized that this area may be difficult to examine and prove uncomfortable for the patient. The area should be soaked with 5% acetic acid and left for at least 2–3 minutes to allow the tissue to stain. If it produces a burning sensation, it should immediately be washed off with warm water. It will be evident that many areas of acetowhiteness will be visible and, as will be shown below, not all of these will be associated with intraepithelial neoplasia. Anoscopy and proctoscopy are usually associated with the taking of a biopsy using local anesthetic from suspected areas of AIN. When considering these conditions, it is important to remember that the following three distinct sites must be examined: The anus comprises both the anal canal and the anal margin, which extends distal to the anal verge, which is where the junction with the hair-bearing skin lies, to a 5 cm circumferential area from it. The anal canal, which measures approximately 3.5–5 cm in men and slightly less in women, commences where the rectum ends, corresponding to the apex of the anal sphincter complex with the squamous epithelium of the anal canal proceeding to blend with the perianal skin. There are a number of physicals signs that alert the clinician to the presence of AIN. These include raised or scaly white hyperkeratotic plaques. There may also be eczematous or fissured lesions or ones that are pigmented or erythematous. Lesions that have undergone rapid physical changes involving the perineum and anal region, especially if associated with pigmented or ulcerated areas or a suspicious-looking skin tag or condyloma, must be considered as suspicious and worthy of biopsy. The vascular patterns that are seen when colposcopy is used for a cervical examination are not commonly seen on the perianal skin but are certainly evident within the anal canal. Typical presentations are shown in Figures 10.1–10.7. In Figures 10.1 and 10.2, a contiguous intraepithelial neoplastic lesion is seen involving both the perineum (Figure 10.1) and the anus (Figure 10.2); it extends to within the anal canal. Biopsy of the perineal lesion (outlined by arrows in Figure 10.1) shows grade 2 intraepithelial neoplasia in the area at (1). There is also a pigmented hyperkeratotic lesion at (2) that, on biopsy, was shown to be VIN3. Examination of the anal canal shown in Figure 10.2 revealed three distinct morphologic types. A biopsy taken from area (1) showed a papillomavirus infection and a biopsy taken from area (2), an area of dense hyperkeratosis that is visible clinically, showed AIN3. In area (3), the same disease as that found in the perineum was present. Obviously, an examination of the anal canal must be undertaken to determine extension into that area, and this is shown in Figures 10.3–10.7. Figure 10.3 shows an extensive acetowhite area around the anus and extending into the anal canal. The outline of this area is shown by arrows. Biopsies taken from (1) in three locations in this area revealed the presence of AIN3 (Figure 10.4). Examination of the specimen showed complete loss of differentiation with marked acanthosis. A biopsy taken from position (2) in Figure 10.3 revealed the presence of wart viral infection. Figure 10.5 shows a very hyperkeratotic irregularly surfaced lesion that stained after the application of acetic acid. A punch biopsy taken from position (1) revealed AIN3 (Figure 10.6). Abnormal mitoses with occasional “corps ronds” can be seen throughout this thickened and irregular epithelium. In Figure 10.7, a flattish fissured lesion (1) can be seen, which was associated with anoscopic and histologic evidence of wart viral infection in area (2). A biopsy taken from position (1) showed the presence of AIN3. With any examination for AIN a digital rectal examination must be made to eliminate the possibility of anal cancer or a pelvic mass. The need to colposcopically examine the cervix and vagina and the vulva by direct vision or vulvoscopy for any associated precancerous lesions has been alluded to above. A number of lesions masquerade as AIN; the commonest is due to condyloma acuminata. In Figure 10.8, an acetowhite area is evident with patches of hyperkeratosis at (1) and a smooth white epithelium at (2). Biopsies taken from both these areas showed the presence of wart viral infection. In Figure 10.9, a view of the anus and anal canal reveals the presence of what seem like typical condyloma acuminata lesions. Multiple biopsies were taken and in positions (1) and (1) on either side of the anal canal; histologic evidence of condyloma acuminata was obtained. However, surprisingly, in the areas designated (2) and (2), AIN3 was found. This illustrates the difficulty of making a diagnosis without resorting to biopsy. In Figure 10.10, a vesicular-like surface (1) is seen on a small anal tag (2). This patient had previously had recurrent episodes of genital herpes simplex virus (HSV) 2 infection. After 1 week, the lesion had completely resolved. The initial diagnosis was of an early invasive carcinoma of the vulva. If any suspicion of HSV2 exists, then it is prudent to observe and not to interfere. Figures 10.11 and 10.12 show the extension of the disease into the natal cleft, and biopsies taken from areas (1) in Figure 10.11 show the presence of one wart viral infection (arrowed). However, in Figure 10.12, an area of diffuse acetowhiteness covered the perianal area and extended into the natal cleft (arrowed). Although histologic evidence of AIN3 was found in the perianal area, the pattern of disease posteriorly in the natal cleft seemed of a minor grade. Punch biopsies performed at points (1) and (1) revealed the presence of wart viral infection, but biopsies taken from (2) surprisingly showed the presence of AIN2. This again illustrates the need for local excision biopsy and the difficulty of predicting the exact nature of epithelial disease by anoscopy. AIN can involve the anal canal in two ways. First, it may extend from the perianal skin inward to the pectinate line (also called the dentate line) with many of the lesions being contiguous with those in the vulval area. The second way is for it to occur within the anal canal with no obvious connection with vulval or perianal disease. Evidence of any perianal papillomavirus infection must raise the possibility of intraepithelial neoplasia within the anal canal. In Figures 10.13–10.17, these types of lesion can be seen. In Figure 10.13, an anoscope (1) displays the anal canal (2) with the obvious pectinate (dentate) line at (3). Distal to this line is a diffuse area of acetowhite epithelium. A biopsy taken from area (4) shows it to be composed of AIN2 with surrounding areas of papillomavirus infection. Figure 8.14a shows that further examination with a proctoscope revealed a raised papular lesion (1) that is highlighted against the reddish mucosa of the anal canal. A biopsy taken from (1) shows it to be composed of typical condylomatous tissue. In Figure 8.14b, typical condylomata acuminata can be seen at (1), but those on the superior aspects appear more acetowhite than the former and a biopsy of them revealed the presence of AIN1/2. Normal mucosa is visible at (3). In Figure 10.15, a swab stick has been used to apply acetic acid to a normal anal canal. Areas of squamous metaplastic epithelium exist within a transformation zone (2). The pectinate (dentate) line is seen at (1) and large bowel mucosa is seen at (3). Figure 10.16 shows tongues of acetowhite epithelium that extend upward into the anal canal (1); an obvious condylomatous lesion exists at (2) and was proven to be so by biopsy. However, a biopsy taken from area (1) showed it to be composed of AIN1/2. Normal large bowel epithelium is shown at (3). Figure 10.17 shows a thickened acetowhite epithelium with some punctation, mosaicism, and corkscrew vessels occurring within the anal canal (1). At (2), the epithelium has become detached and formed an ulcerative lesion. A biopsy taken from point (1) showed the presence of AIN3, while at (2) an early invasive lesion is present. A review of all these patterns illustrates the difficulty in making a diagnosis through the anoscope and proctoscope. Punch biopsy of any acetowhite lesion or, for that matter, a lesion with abnormal vascularity is recommended. After a biopsy has been taken, pressure applied with a firm swab for at least 3 minutes will stop most bleeding. There are two principles that apply to making a diagnosis of AIN. The first is to have a high index of suspicion in women with high risk factors who present with symptoms as detailed above. Second, the need to have a definitive diagnosis depends on a biopsy of any suspicious areas. The major aims in managing and treating AIN are to reduce symptoms and to prevent the development of anal cancer while being aware of the different strategies that now exist. One set of strategies has recently been defined by Scholefield et al. (2011) on behalf of the Association of Coloproctology of Great Britain and Ireland. There are three stages in the management/treatment algorithm and these are as follows. There are a number of management/treatment options that now exist for biopsy-proven AIN3. These are as follows. Evidence shows that in men and women who are immunocompetent there exist very low rates of conversion of AIN to cancer. It is also well known that a high rate of recurrence occurs when aggressive surgery is employed to treat AIN. With these two facts available, it would seem prudent in cases of biopsy-proven low-grade disease (AIN2/3) that a program of 6 monthly observation should be employed. Four methods of ablation are employed for the treatment of AIN. These are: CO2 laser vaporization, cryotherapy, electrocautery fulguration, and infrared coagulator. Laser vaporization of small and localized areas can be undertaken. As there are no glandular elements involved, the depth of destruction can be taken to the second surgical plane, as described for treating vulval lesions. When treating anal lesions, it is advisable to leave an epithelial skin bridge between the lasered areas so that regeneration can occur. In Figure 10.18, a mixture of condyloma acuminata and biopsy-proven AIN1/2 existed in lesions at the entrance to the anal canal. Laser vaporization was performed. A small epithelial bridge can be seen at (1). An area of intraepithelial neoplasia had existed in the area at (2). Some carbonization is present and accounts for the darkness of the tissues. In area (3), where condyloma had existed, vaporization has been performed only to the first surgical layer and the dermal papillae with their shining surfaces can be clearly seen. Results seem to indicate varied success. Recurrences in some studied are most likely the result of HPV persistence because of the deep involvement of the perianal skin and appendages by AIN and the inability to clear this with ablation. Importantly, the presence of invasive cancer cannot be excluded when employing ablation without prior biopsy. It is therefore a method useful for clearance of localized and small lesions. Many studies of both of these methods showed high recurrence rates and significant morbidity. In one study of HIV-positive men treated with electrocautery the recurrence rate at 12 months was 79% with all patients having recurrence by 1 year. The same reasons for failure as for using CO2 laser vaporization apply with these methods. The IRC200 Infrared Coagulator (IRC) (Redfield Corp., Rochelle Park, NJ) has been used since the late 1990s. Unfortunately, this technique is associated with a high persistence of cases and high morbidity with significant postoperative pain. Around two-thirds of patients develop new or persistent AIN3 after IRC within a year. Multiple treatments may be required to achieve optimum cure. The probability of curing a retreated lesion can be 70–75%. Although appealing as an office/outpatient procedure, further long-term trials are needed to determine its place in ablative treatment of AIN. Although this therapy is employed for invasive anal cancer its place in treating large AIN3 lesions is uncertain and no literature for its use exists. Those areas of AIN3 adjacent to an invasive cancer do regress when the technique is employed. This is a nucleoside analogue of the imidazoquinoline family with potent antitumor and antiviral activity and is proinflammatory, acting through a number of subcellular pathways. Used as a 5% cream it has been shown to induce regression in AIN3 lesions in 60% of patients over a 3 year period in a double-blind randomized trial. A recurrence rate of 34% was reported in this study. Most studies have used HIV-positive individuals but it would seem as though it is a safe and effective topical treatment in HIV patients in whom there is a high recurrence rate. The vaccine against HPV, in the form of a virus-like particle, can be effective against cervical, vaginal, and vulval precancer as well as anogenital warts. The quadrivalent vaccine against HPV types 6, 11, 16, and18 will in the future cause a dramatic reduction in AIN3 in vaccinated individuals. The efficacy of primary prevention has been shown in a recent quadrivalent vaccine study in which there was a 77% reduction in incident high-grade AIN among vaccinated HIV-uninfected men who have sex with men compared with a placebo group and more than a 90% reduction in persistent anal HPV infection with vaccine HPV types. A photosensitizer is employed prior to photodynamic therapy being given. It is a painful method and requires multiple treatments. There is no evidence from larger series with long-term follow-up on its effectiveness. As can be seen from the algorithm above, local excision can be used for lesions that constitute less than 30% of the circumference of the perianal skin or of the anal canal occupied by the AIN3 lesion. Any defects can be closed as a primary procedure or left to heal under secondary intention. Preoperative mapping before the procedure is recommended, although this does not preclude recurrence. Mapping requires eight to 12 biopsies to be obtained from around the anal margin and canal. A written or visual record of the biopsied sites should be made. Wide local excision of larger AIN3 lesions may be performed because if the worst area is removed it will reduce its bulk, allowing the adjacent areas to be kept more easily under observation Wide local excision with flaps and reconstruction carries significant morbidity and is probably overtreatment as the natural history of AIN3 is still uncertain. However, as some AIN3 lesions are associated with extensive premalignant vulval disease (as part of a multifocal intraepithelial neoplastic process) then the need for more radical surgery is called for and this is described below. Localized surgical excision can be undertaken for lesions that extend into the perianal and anal areas from the vulva and perineum. In Figures 10.19 and 10.20, a contiguous lesion, involving both the posterior fourchette and perineum, has extended to the anal verge. Biopsy in multiple sites showed it to be AIN3. The patient was extremely symptomatic. The technique described in the previous chapter and which involves wide excision with undercutting of the vagina and its advancement has been employed. Figure 10.19 shows the outline (arrowed) of the abnormal area, and in Figure 10.20 the final operative result is presented. The vagina has been advanced to point (1), just distal to the site of the original posterior fourchette. A longitudinal wound (2) unites the edges of an elliptically excised area. The incision was taken to the anal verge (3). The anal canal and an accompanying hemorrhoid have been grasped in an Allis forceps and retracted downward. The labial wounds can be seen at (4). Figures 10.21–10.23 show a very extensive lesion that involved both the labia minora and the inner part of the labia majora; it has extended downward, involving the posterior fourchette and the perineum with a further extension caudally to the anal verge. The technique of a wide excision with vaginal advancement has also been employed in this case. In Figure 10.21, the defect is obvious, and the clitoris and urethra can be seen at (1) and (2), respectively. The posterior perineal excision is obvious and the incision has been carried to the anal verge (4). In Figure 10.22, the periclitoral and labial areas have been united, two layers having been used for this procedure. At this stage, the vagina is undercut, advanced to the perineum, and retracted downward. In Figure 10.23, the final result can be seen with the vagina attached at point (1); a longitudinal incision at (2) unites the edges of the excised perineal region. The anal verge is at (3). There existed small areas of AIN3 within the anal canal but these were treated by a combination of local excision and laser vaporization. When this 26-year-old patient was seen 6 months later, there was no intraepithelial neoplasia within the genital tract and no interference with sexual function. Pathology of the excised portion showed large areas of the vulva, perineum, and perianal area to be composed of AIN3. When an extensive but localized area extends onto the perineum, it can be widely excised using a knife, a fine diathermy needle, or the CO2 laser. In Figure 10.24, such a lesion has been cleanly excised using the CO2 laser. The edges of the wound can be undermined to allow primary closure. However, if this is not possible, a bilateral rhomboid flap or local skin flaps can be used to cover the defect. Any localized excision of lesions at the anus or in the anal canal should not necessitate resuturing of the epithelial defect. A tulle-gras swab or a hemostatic gelatin sponge (Spongostan anal; Johnson and Johnson) should be inserted into the anal canal postoperatively. Lesions within the anal canal, such as those shown in Figures 8.14 and 10.16, can be treated by the CO2 laser after appropriate confirmation biopsy. It must be remembered that metal instruments need to be inserted in many of these cases for exposure purposes. For lesions lower down in the anal canal vigorous retraction of the anus can provide just as adequate exposure as the metal instrument; obviously the patient should be anesthetized. There is a theoretic risk of ignition of some lower bowel gases by the laser beam but, although anecdotal reports are forthcoming, no substantiated case has been reported. However, it is the author’s (A.S.) practice to place a swab soaked in saline above the area to be lasered. In view of the reflection of the beam when it contacts metal instruments, it is suggested that only matted-surface instruments be used and that glasses be worn by all members of the operating team. Intraepithelial lesions on hemorrhoids can be treated by hemorrhoidectomy. Likewise, lesions within the natal cleft are best removed by excision rather than by vaporization using the CO2 laser. Extended follow-up is mandatory as the natural history of AIN is uncertain. In some women with multifocal intraepithelial neoplasia that involves both the perineum and anus, it is difficult to achieve removal with a localized surgical excision. This can lead to more extensive surgery being performed in which vulvoperineal resection is combined with a simultaneous vulval reconstruction using meshed split skin grafts or a combination of skin grafts and local flaps. In such procedures an initial colostomy needs to be performed with closure only after achieving local control of the disease. Many surgeons believe that such a staged approach achieves the objectives of eliminating disease and alleviating symptoms while preserving function and attempts to reconstruct normal anatomy without compromising the principles of surgical oncology. Such a procedure is seen in Figures 10.25–10.28. In Figure 10.25, a large area of multifocal intraepithelial neoplasia is seen, involving not only the vulva and perineum but also the perianal and anal area. A colostomy has been performed and the area to be radically removed has been outlined in blue marking ink. In Figure 10.26, the area to be covered is now exposed with complete hemostasis. Two flaps will be developed in areas (1) and (2) by incisions along the dotted line that extends upwards and outwards: points A eventually forming the upper part of the central band of tissue formed by the joining of these two flaps, and point B the lower point. The incision will produce two flaps that will be brought together and united in front of the anus (3). In Figure 10.27 this has been done and the two flaps (1) and (2) united at points B1 and B2. Flap 1 (1) overlaps its fellow (2) and forms the anterior margin of the anus, which is at (3). The areas to be covered by the skin graft are at (4) and (5). The colostomy is closed after 10 weeks. In Figure 10.28 the vulva and perineum/perianal area is seen at 10 weeks; the area covered by the posterior skin graft is at (5).
Perianal and anal intraepithelial neoplasia
10.1 Epidemiology
10.2 Etiology
10.3 Association with other genital intraepithelial neoplastic diseases
10.4 Examination
The importance of history
Screening: any role in detection of anal intraepithelial neoplasia?
Anoscopy (colposcopy of the anal area)
10.5 Presentation
10.6 Lesions masquerading as intraepithelial neoplasia
10.7 Anal canal involvement
10.8 Management and treatment
How is the diagnosis made?
Management algorithm
Management/treatment options
Observation only
Treatment options
Ablation
CO2 laser vaporization
Cryotherapy and electrocauterization
Infrared coagulator
Chemoradiotherapy
Immunomodulation therapies
Imiquimod 5%
Human papillomavirus vaccine
Photodynamic therapy
Surgery
Localized surgical excision
Extensive surgical excision
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


