BASIC CONCEPTS
Pelvic organ prolapse is the downward displacement of central pelvic organs that are normally located at the level of or adjacent to the vaginal vault. Because these displacements are each associated with defects in integrated connective tissue structures, they may each be considered a pelvic hernia. These conditions are common and affect a progressively larger percentage of women as age advances especially in the postmenopausal years. Whereas mortality from this condition is negligible, significant morbidity or deterioration of lifestyle may be associated with prolapse. Women in developed countries who have access to modern health care can benefit from the advances that have been made in nonsurgical and surgical treatments for pelvic organ prolapse. If the problem is viewed from a worldwide perspective, however, the scope of suffering is much greater. In areas of high parity and little or no access to health care, countless women suffer from problems associated with pelvic organ prolapse with no real possibility of resolution. The direct effect that these conditions have on urinary, gastrointestinal, and sexual functions can only be appreciated by those women burdened with these problems on a daily basis.
Treatment of pelvic organ prolapse and the associated symptoms constitutes a major subject in gynecology. Especially in the advanced state, management of these conditions is one of the most challenging problems a pelvic surgeon can face. Indeed, success in treating prolapse is frequently used to judge the overall skill of those surgeons. Providing permanent relief from this classic malady by restoring normal anatomy and maximum physiologic function always tests the ingenuity of gynecologists. As medical sophistication has progressed, so has the ability to understand more completely and better treat pelvic organ prolapse.
A brief review of the history of treatment of prolapse is helpful in understanding modern treatments and current concepts of these conditions. Because it was mentioned in the writings of Hippocrates and Galen, prolapse was clearly known to the ancients. Early treatments may seem quaint by today’s standards. Yet some of these interventions continue to be used today. Fortunately, others have not survived. Vaginal packing, tampons, massages, and exercises were used with some success. Other patients were suspended from their feet for a period of 24 hours to treat prolapse. Rodericus A. Castro advised that prolapse should be attacked with a red hot iron as if to burn it, “when fright would cause it to recede into the vagina.” Various caustics were used, including silver nitrate, nitric acid, acid nitrate of mercury, hot metal, and sulfuric acid.
Perhaps, the first real advance in treatment was the development of pessaries. These devices functioned as trusses. Their fitting and placement became a desirable skill. They continue to bring relief to a large number of women and seldom do any serious harm. They were especially popular in the middle of the 19th century. Some were held in place by waistbands. In some cases, pessaries were deliberately left in place until erosions of the vaginal epithelium occurred. The subsequent healing
was expected to reduce the caliber of the vagina with scarification adding to support, but serious complications could occur. Reports exist of neglected pessaries being retrieved from the peritoneal cavity and bladder. Fistulae may also occur in various locations, especially the bladder and anorectum.
The earliest surgical attempts to relieve prolapse were relatively simple. These procedures included labial suturing and removing portions of the vaginal epithelium to reduce the caliber of the vagina. Although Heming operated on the anterior vaginal wall in 1831, surgery for uterovaginal prolapse was not common until the advent of anesthesia and antisepsis later in the 19th century. The first vaginal hysterectomy for prolapse was performed by Samuel Choppin of New Orleans in 1861. Many years passed before this surgical technique became common. By the beginning of the 20th century, European and American reports of hysterectomy, colporrhaphy, cervical amputation, transposition/interposition operations (Manchester-Fothergill), cervical ligament plications, colpocleisis (Le Fort), ventral fixation of the uterus to the abdominal wall, and trachelorrhaphy for procidentia were being published. The timing of this ingenious variety of operations is certainly consistent with the development of anesthesia and various surgical techniques in all fields of medicine.
During the 20th century, advances in understanding and treatment of prolapse have progressed at an ever-increasing rate. In 1909, George R. White of Georgia published an account of cystocele repair using a transvaginal paravaginal approach. His correct perspective on the importance of lateral vaginal support took nearly 50 years to be rediscovered by mainstream gynecologic surgeons. The paravaginal repair was not widely known and accepted at the time because it was overshadowed by the work of Howard A. Kelly of Johns Hopkins. This great and influential surgeon popularized the concept of fascial attenuation. Midline anterior and posterior plications were touted as the correct surgical approach to the problem of prolapse. The Kelly-Kennedy anterior plication and levator ani plication of the posterior vaginal wall remain as commonly performed procedures today, despite the fact that more contemporary surgical techniques described in this chapter more effectively correct the anatomic defects of pelvic prolapse and achieve better clinical results with fewer long-term side effects.
In the 1950s, Milton L. McCall of Louisiana developed a culdoplasty technique that emphasized the important suspensory function of the uterosacral ligaments. He believed this operation prevented enteroceles and posthysterectomy vaginal vault prolapse when it was performed at the time of hysterectomy. Currently, the restitution of vaginal vault support at the time of any type of hysterectomy is considered a very important step in prevention of future prolapse.
In the 1960s, Baden and Walker of Texas began to systemize a new defect-specific approach to pelvic organ prolapse repair. Page 1 of their 1992 book, Surgical Repair of Vaginal Defects, stated: “In a sense, the defect approach reverses the prior evolution toward ‘compensatory’ reparative techniques—our goal is to return all vaginal supports to their original anatomic status.” Many other surgeons have contributed to this powerful concept of pelvic reconstructive surgery. A. Cullen Richardson and associates of Georgia developed the concept of classifying fascial defects as proximal, distal, central, and lateral. This observation and the teaching of such master surgeons as David H. Nichols of New England encouraged gynecologists to not only identify and repair each vaginal defect but to return support attachments to their original anatomic location. Emphasis was focused on the hernial nature of prolapse and led to the abandonment of absorbable suture in favor of permanent suture in critical locations in these repairs. In the 1990s, pelvic anatomist, John O.L. DeLancey of Michigan, published a biomechanical analysis of normal vaginal anatomy. His observations have the precision of an engineer’s work and identify specific structural goals for each of three vaginal levels of support. Proximal (apical) vaginal suspension, midvaginal lateral attachment, and distal vaginal fusion to the perineum and urogenital diaphragm are the basic concepts that modern pelvic surgeons must satisfy to successfully complete a prolapse surgery.
In the 1980s, hernia repair literature began to demonstrate better long-term results with the use of bolsters. For that reason, autografts, allografts, xenografts, and various synthetic meshes achieved frequent use in surgical techniques for prolapse in the first decade of the 2000s. Because of an increasing number of reported problems and lack of strong data to support their use, the Food and Drug Administration has issued two statements urging caution in the use of these bolsters, especially polypropylene meshes. Considerable debate exists regarding the indications for their use (if at all) and the type, amount, and best location for pelvic support grafts.
A fusion of anatomic, physiologic, and biomechanical principles has allowed surgeons to offer their patients better treatments for prolapse than have historically been available. This brief and incomplete historical discussion outlines the evolution of our current understanding of a complex topic. Historically ineffective treatments of the presurgical era gave way to surgical approaches for advanced cases. As surgical sophistication has increased, anatomically distorting operations have gradually been replaced by anatomically restoring procedures. Many interesting aspects of the historical development of this subject are described in previous editions of this book and in the bibliographic selections at the end of this section.
A better understanding of a problem always leads to more questions that deserve answers. For example, the process of childbirth is regarded as an important cause of vaginal prolapse. Little or no effort has been made to analyze the forces of childbirth as they relate to specific patterns of damage to the deep endopelvic connective tissue. Delineation of these patterns would assist the surgeon in recognizing defects and undoubtedly improving operative outcome. Precise knowledge of the effects of labor and delivery on the pelvic floor would likely lead to new surgical techniques and create the potential for better obstetrical decisions designed to reduce the potentially damaging effects of childbirth. The concept of prolapse prevention is underused in the practice of gynecology. Teaching patients about physical therapy of the pelvic floor, better lifestyle, and proper evacuation habits should be part of the gynecologist’s job. The long-term benefits of an organized program of prolapse prevention have never been evaluated.
Controversy exists as to the best surgical management for specific cases of female pelvic organ prolapse. In fact, now that reparative options are no longer limited to midline plications, more procedures are available than ever before. Vaginal, abdominal, laparoscopic, robotic, minimally invasive devices, and combined approaches each have their advocates. The necessary randomized trials with matched controls will likely never be done to generate true evidence-based outcomes, especially because two of the most powerful variables are surgical skill and experience. The necessary long-term follow-up is rarely available in the pelvic surgery literature. New concepts and techniques appear before the older ones can properly be compared in studies. Surgical techniques for prolapse must be carefully evaluated so that anatomic and functional results will continue to improve.
The authors of the following chapters of this section describe interventions and operations that gynecologic surgeons should consider when managing pelvic organ prolapse. Each of these surgeons possesses a combination of operative skill, experience,
and special interest in their topics that have led to their selection. The operative techniques presented should be combined with appropriate clinical evaluation and skillful technical performance to obtain maximum benefit for the patients.
ANATOMIC CONSIDERATIONS
The normal position, support, and suspension of the uterus, vagina, bladder, and rectum rely on an interdependent system of bony, muscular, and connective tissue elements. This entire system is three dimensional, and even subtle alterations in one part may lead to stresses in other parts that eventually lead to alteration or failure of normal anatomy. An understanding of normal applied pelvic anatomy is imperative in the repair of pelvic organ prolapse.
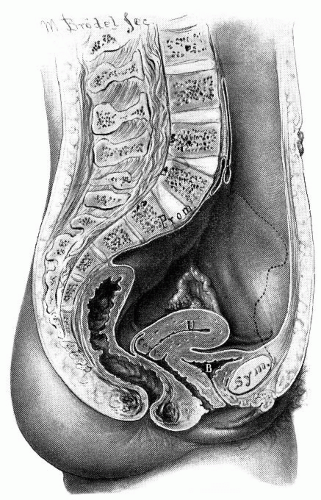
The bony pelvis has a central opening that is necessary for reproductive function. During evolutional transition to upright bipedal posture, the potential for prolapse became more likely because of gravitational stress. In the human female, a lordosis of the lumbosacral portion of the spine places the pelvic inlet in an oblique orientation reminiscent of the pelvic posture of a quadruped. The physical result of this shift is that the posterior aspect of the pelvic inlet is approximately 60 degrees above the anterior aspect with the promontory of the sacrum placed in a vertical plane above the pubic symphysis (
Fig. 36.1). This partially vertical orientation of the pelvic inlet deflects force onto the superior symphysis pubis rather than directly on the pelvic outlet and urogenital hiatus. Consequently, the pelvic outlet is partially shielded from downward stresses in the anatomically normal woman.
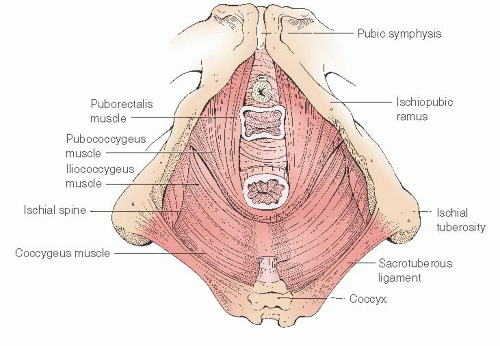
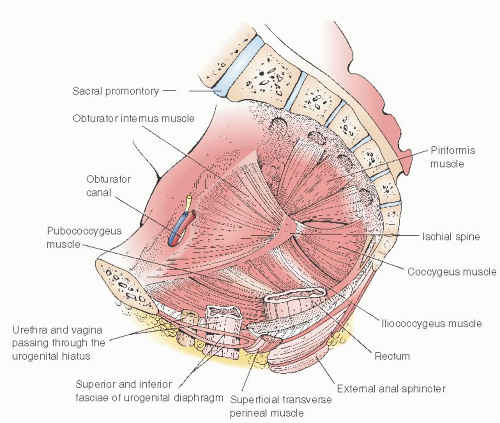
The muscles of the pelvic diaphragm primarily provide pelvic support. These muscles form a basin or covering of the pelvic outlet and are often grouped together as the levator ani or levator sling (
Fig. 36.2). Within this diaphragm is the urogenital hiatus, an opening large enough to allow childbirth. This large central opening in the muscular pelvic floor explains why prolapse is such a significant problem in humans. The most medial portion of the pelvic diaphragm is formed by the puborectalis muscle, the muscular boundary of the urogenital hiatus. The obstetric axis of the pelvis passes through the urogenital hiatus medial to the puborectalis muscle. In the standing patient, the puborectalis muscle is horizontal and is palpable as a 2- to 2.5-cm band of voluntary muscle on each lateral side of the distal one third of the vagina. When well innervated and contracted, the puborectalis muscle closes the distal vagina and displaces the posterior wall of the rectum anteriorly creating the anorectal angle. Forming the bulk of the pelvic diaphragm, the
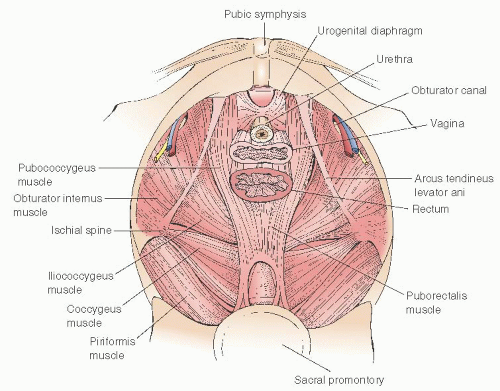
pubococcygeus and iliococcygeus muscles cover the posterior and lateral portions of the pelvic outlet (
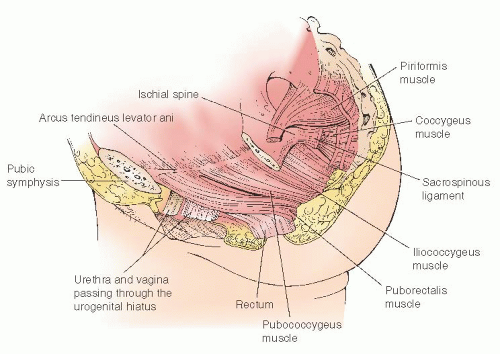
Fig. 36.3). The superior insertion of the iliococcygeus muscles is an important landmark in pelvic support anatomy. These insertions are thickenings of the pelvic sidewall parietal fascia of the obturator internus muscle that extend from the ischial spines posteriorly to points on the pubic bone known as the pubic tubercles. These lines of insertion are known as the arcus tendineus levator ani or muscular arches (
Figs. 36.4 and
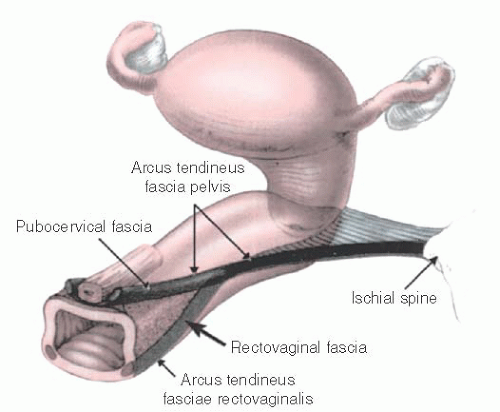
36.5). Immediately inferior to the muscular arches are thickenings of the parietal fascia of the bellies of the iliococcygeus muscles known as the arcus tendineus fasciae pelvis (fascial arches) or white lines. These structures are the lateral attachment points for the pubocervical septum and apical rectovaginal septum. The white line serves the function of midvaginal lateral support. Paravaginal and apical pararectal defects are located immediately medial to the white line. In the standing patient, the white line is nearly horizontal; in the standard lithotomy position, it is nearly vertical.
During paravaginal repair, the white lines are palpable as stringlike structures that extend between the ischial spines and the pubic arch. Another fascial thickening has been described that runs posteriorly from the white line to the structures of the lateral perineal body and serves as lateral support for the distal rectovaginal septum of the posterior vagina. This structure
has been named the arcus tendineus fasciae rectovaginalis (
Fig. 36.6). The lateral supports of the anterior and posterior vaginal septa merge and are not separate in the apical half of the vagina. Superior to the muscular arch is the uppermost portion of the obturator internus muscle and the parietal obturator fascia. The obturator internus muscles qualify as pelvic muscles because they form the lateral borders of the upper portion of the pelvic basin. Posterior to the iliococcygeus, the pelvic floor is covered by the coccygeus muscle and the closely associated sacrospinous ligament (
Fig. 36.5). These structures pass between the ischial spine and the terminal sacrum and coccyx. The most posterior portion of the pelvis is bounded by the piriformis muscle. The midline confluence of the levator muscles forms a particularly strong band of connective tissue between the coccyx and the posterior anus known as the levator plate or sacrococcygeal raphe. This plate is oriented horizontally in the standing patient. The vagina and the rectum are suspended by the endopelvic fascia (primarily the uterosacral ligaments) directly over the levator plate. Myopathies or neuropathies cause weakness of the pubococcygeus and iliococcygeus muscles and may allow the levator plate to sag and descend permanently. This descent causes the genital hiatus to remain open as it does during defecation. This increased opening changes the normal horizontal axis of the apical vagina to a more vertical orientation and predisposes the central pelvic organs to prolapse.
The pudendal nerve is an important motor and sensory nerve of the pelvic floor and perineum. It descends through the pelvic diaphragm between the coccygeus and piriformis muscles in the area posterior to the ischial spine into Alcock canal. Alcock canal is located in the ischiorectal fossa immediately adjacent to the fascia of the obturator internus muscle on the lateral wall of this space. Because of its location, the pudendal nerve is subjected to significant stretch and pressure particularly in the area adjacent to the ischial spines during the descent of a fetus through the pelvis. The muscles of the pelvic diaphragm are also subjected to great pressure and stretch during labor. Magnetic resonance imaging studies have demonstrated atrophy and breaks in the levator muscles of parous women. Neuropathy of the pudendal nerve and myopathy of the levator muscles are believed to be significant contributing factors in the development of pelvic organ prolapse.
Unfortunately, at this time, no treatments are available for myopathies and neuropathies of this type. For that reason, surgical treatment of pelvic organ prolapse is limited largely to manipulation of the deep endopelvic connective tissues.
The connective tissues of the pelvis are collectively known as the endopelvic fascia. This fibroelastic connective tissue matrix contains varying amounts of smooth muscle. It supports and invests all the midline organs and structures of the pelvis. Only the ovaries and fallopian tubes lie outside this investment. At various locations, the endopelvic fascia manifests different characteristics. These forms include loose areolar tissue capable of distention; neurovascular sheaths; septa and ligaments that support, suspend, and separate the pelvic organs; and dense skeletal muscle investments. In the central pelvis, the visceral peritoneum drapes over the midline structures, dipping into recesses but not descending into direct contact with the muscular pelvic floor. The irregular space between the pelvic diaphragm, the muscular pelvic sidewall, and the visceral peritoneum is the location of the endopelvic fascia. The endopelvic fascia may be divided into three parts: parietal fasciae, visceral fasciae, and deep endopelvic connective tissue.
The parietal fasciae of the endopelvic fascia are relatively dense membranes investing the pelvic surface of the skeletal muscles of the pelvic sidewall (
Table 36.1). They are similar in structure, form, and function to other parietal fasciae of the body, for example, the rectus abdominis fasciae. At muscular margins, they blend with the various periostea of the bony pelvis.
The visceral pelvic fasciae are loose, highly elastic, and relatively ill-defined encasements of the central pelvic organs, taking the form of sheaths and sleeves (
Table 36.2). These structures allow for the high degree of physiologic distention necessitated by the intestinal, urinary, and reproductive functions of the pelvic organs. The visceral fasciae blend intimately with the organs that they encase, are not easily separable from those organs, and are not useful for support and suspension purposes in surgery.
The deep endopelvic connective tissue is of central importance in the clinically applied anatomy of the pelvis and is especially significant to the pelvic reconstructive surgeon. This structure is part of a continuum of retroperitoneal connective tissue that extends from the respiratory diaphragm in the upper abdomen to the pelvic diaphragm. Included in this continuum of structures are the mesenteries and ligaments of the upper abdomen. Anatomists debate whether the condensations of this connective tissue should be considered as true ligaments and fasciae or septa. Part of this debate stems from the fact that some of these structures contain a significant muscular component and others serve as neurovascular conduits. Certainly, from a functional standpoint in the pelvis, they meet criteria for being so named. The endopelvic connective tissue is continuous from one part to the other. Separate portions serve different functions, take various forms, and therefore are given different names. The named structures of the deep endopelvic connective tissue include six ligaments, two septa, and one ring. Important anatomic details of these elements are summarized in the following tables.
The six pericervical ligaments form the paracolpium (
Tables 36.3, 36.4, and
36.5). The net effect of these structures is the suspension of the cervix in the posterior pelvis and the consequent placement of the vagina directly over the levator plate and away from direct exposure to the urogenital hiatus. In the normal anatomic position, pressure from above tends to close
the apical vaginal vault by compressing it against the sacrococcygeal raphe with no tendency toward prolapse. This compression of the vagina is a flap valve type of mechanism and is aided by the anchoring of the cervix by the uterosacral ligaments.
Two septa or fasciae (
Tables 36.6 and
36.7) are located within the deep endopelvic connective tissue. These condensations of fibroelastic connective tissue are in close contact with the vaginal epithelium and visceral fasciae of the adjacent organs. Clinically, they are separate from their adjacent structures. When the septa and their supports are intact, the vaginal and rectal axes have a posterior angle of approximately 130 degrees at the anterior point of their suspension over the levator plate due to the action of the puborectalis muscle. Distal to the puborectalis muscle, the vagina is nearly vertical as it
passes through the urogenital hiatus. The apical or proximal two thirds of the vagina is nearly horizontal and is suspended over the levator plate. The normal vaginal axis is oriented posteriorly toward a point just above the center of the fourth sacral vertebra. This area of the anterior sacrum corresponds to the exact area of the origin of the uterosacral ligaments.