EPIDEMIOLOGY
Sexually transmitted infections (STIs) have been reported at epidemic proportions in the United States, with the Centers for Disease Control and Prevention (CDC) estimating almost 20 million new infections each year at a cost of $6 billion each year. The incidence of
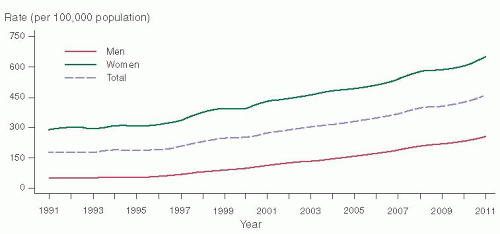
Chlamydia trachomatis infections has increased 8% since 2010, to a rate of 458 per 100,000 people, with a total of 1,412,791 cases reported in 2011 (
Fig. 30.1).
Neisseria gonorrhoeae incidence has also increased to 4% since 2010 with a rate of 104 per 100,000 people, with more than 320,000 cases reported in 2011 in the United States. For women, acute PID is the most common and important complication of STIs. Bell and Holmes in the 1980s estimated that 1 million women a year were treated for acute salpingitis in the United States, but recent estimates by Sutton and colleagues estimated a decrease in cases of PID to approximately 770,000 per year and a 68% decrease in hospitalized PID from 1995 to 2001.
The CDC estimates that of the 750,000 to 1 million women per year who experience an episode of PID, up to 15% will become infertile as a result of the infection. Hospitalization rates have been declining over the past decade. However, it is uncertain if this is due to decreasing incidence of the disease or a change in clinical management to treat more patients as outpatients. A consequence of the latter scenario is that despite
the long-term trend of decreased hospitalization, as confirmed by Sørbye and colleagues (35% reduction in hospitalized cases of PID), they also found that there was a 65% increase (
P = 0.013) of tuboovarian abscesses in these patients.
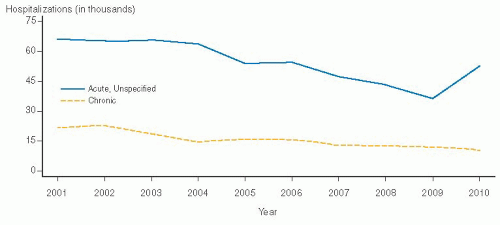
However, after hitting an all-time low in hospitalizations for PID in 2009, there was a marked increase in 2009 to 2010, and we are anxious to see if this will become a long-term trend (
Fig. 30.2).
In addition to the 200,000 women who are hospitalized each year with a diagnosis of salpingitis or PID, the disease also generates nearly 2.5 million visits to physicians and an estimated 150,000 surgical procedures for complications every year. In terms of overall incidence, acute PID occurs in 10% to 15% of women during their lifetime, with a diagnosis in 1% to 2% of young, sexually active women each year. Therefore, PID is the most common serious bacterial infection in women aged 16 to 25 years, and the resultant morbidity exceeds that produced by all other infections combined for this age group.
ETIOLOGY
Within certain geographic areas or populations, N. gonorrhoeae is a common cause of PID. However, most cases of acute PID are the result of a polymicrobial infection caused by organisms ascending from the vagina and cervix to infect the lining of the endometrium and fallopian tubes. Approximately 85% of cases are spontaneous, noniatrogenic infections that occur in sexually active women of reproductive age. The remaining 15% of infections occur after procedures that break the cervical mucous barrier, such as with the placement of an intrauterine device (IUD), endometrial biopsy, or uterine curettage, which allow vaginal flora to infect the upper genital tract.
In the United States, nontuberculous PID was traditionally separated into gonococcal and nongonococcal disease, depending on the isolation of N. gonorrhoeae from the endocervix. However, a variety of organisms can be isolated from the endocervix. Therefore, it is difficult to determine which of these organisms cause PID and which are coexistent cervicovaginal flora representing vaginal colonizers in the upper genital tract at time of diagnosis. While upper genital tract organisms are probably more indicative of the causative organisms, they are often difficult to obtain without suspecting endocervical contamination from the diagnostic procedure. However, studies by Martens and colleagues of transcervical cultures of the infected uterine cavities did not find significant contamination with this approach with either protected catheters or suction curettes. Bacterial organisms cultured directly from tubal fluid may commonly include N. gonorrhoeae, C. trachomatis, endogenous aerobic and anaerobic bacteria, and genital Mycoplasma species. Laparoscopic studies have demonstrated a correlation of no more than 50% between endocervical and tubal cultures, but the presence of N. gonorrhoeae is almost always considered an important causative factor. Even in the presence of N. gonorrhoeae, direct fallopian tube cultures have demonstrated that tubal infections are often polymicrobial. The type and number of species vary depending on the stage of the disease. Gonorrhea, for example, is often cultured from the cervix during the first 24 to 48 hours of the disease but is often absent later. Similarly, fewer organisms are cultured late in the disease, and anaerobic bacteria such as Prevotella, Bacteroides, Peptococcus, and Peptostreptococcus species tend to predominate. Whether these anaerobes play a causative role or increase in number and frequency as a result of the inflammatory response is uncertain. Sweet has summarized the literature by stating that in approximately one third of women with PID, N. gonorrhoeae is the only organism recovered by direct tubal or cul-de-sac culture, one third have a culture positive for N. gonorrhoeae plus a mixture of endogenous aerobic and anaerobic flora, and the remaining third have only aerobic and anaerobic organisms. Chow and colleagues and Monif and colleagues have postulated that the intense inflammatory nature of gonococcus may initiate acute PID and produce tissue damage. This damage changes the local environment, which in turn allows anaerobic and aerobic organisms from the vaginal and cervical flora to invade the upper genital tract. Both Eschenbach and Sweet have suggested that not all PID follows gonococcal infection and that acute PID initially may also have a polymicrobial etiology.
According to Sweet and Gibbs, approximately 20% of all women with salpingitis have tubal cultures positive for C. trachomatis. Also, both N. gonorrhoeae and C. trachomatis are found in the same individual 25% to 40% of the time. Scandinavian studies by Eilard and coworkers have reported the recovery of C. trachomatis from the cervix in 22% to 47% of women with acute PID. C. trachomatis by itself produces a mild form of salpingitis with an insidious onset. In contrast to gonorrhea, Chlamydia can remain in the fallopian tubes for months or years after initial colonization of the upper genital tract. Svensson and colleagues found that women with C. trachomatis infection at laparoscopy had the most severe fallopian tube involvement, probably because of its clinically silent or minimally symptomatic nature, which results in difficult or delayed diagnosis and therefore delayed or absent treatment. The two major sequelae of acute PID are tubal infertility and ectopic pregnancy. These have been strongly associated with prior chlamydial infection as a consequence of intratubal and peritubal adhesions.
Although C. trachomatis is generally believed to be one of the most common causes of PID, its etiologic role is very different when compared to N. gonorrhoeae. N. gonorrhoeae is a Gram-negative diplococcus with rapid growth that is due to a short reproductive cycle of about 20 to 40 minutes to divide. This results in a logarithmic increase in the number of organisms once N. gonorrhoeae reaches a relatively sterile area such as the endometrium or fallopian tube, where growth is relatively unimpeded. This rapid increase in the number of Gram-negative bacteria usually results in a rapid and intense inflammatory response by the woman’s host defenses. The response to this rapid bacterial growth is proliferation and aggregation of white blood cells (WBCs) and their inflammatory products. Migration of this bacterial and leukocytic mixture in two directions, both through the fallopian tube to the ovary and peritoneal cavity and back to the cervix and vagina, causes the symptoms that are pathognomonic of acute PID— abdominal/pelvic pain and cervicovaginal discharge.
For years, N. gonorrhoeae was thought to be the primary PID pathogen. Its often severe immunologic response, mostly due to its release of lipopolysaccharide, resulted in acute and severe pain, high fevers, and a marked WBC response. This triad resulted in an easily recognized clinical course, a prompt diagnosis and often early hospitalization, and initiation of antibiotic treatment.
Chlamydia trachomatis, however, is a slow-growing intracellular organism. Its lack of mitochondria results in its obligatory intracellular existence and also causes its growth cycle to be extremely slow compared with N. gonorrhoeae and other nonintracellular microorganisms. The growth cycle of Chlamydia is 48 to 72 hours; therefore, several weeks to months are required for the growth to reach numbers sufficient to cause clinical symptoms, if at all. Its slow growth does not induce a rapid or violent inflammatory response. This explains the slow and insidious nature of the symptoms of acute C. trachomatis infections. However, because of its intracellular growth cycle, the release of elementary bodies (its infectious vehicle) occurs by rupture of the cell that it has invaded. In addition, Linhares and Witkin have eloquently documented the serious immunopathologic consequences of the 60-kDa heat shock protein (hsp60) from Chlamydia on the fallopian tube.
Thus, the repeated occurrence of elementary body infection of susceptible cells—and their subsequent destruction by rupture—along with a chronic inflammatory response are the major mechanisms by which C. trachomatis causes disease in acute and chronic pelvic infections. Also, because the slow growth and the chronic inflammatory response often result in subtle clinical symptoms, treatment is often delayed or not started at all, adding to the extended tissue destruction and PID sequelae.
The lack of acute symptoms does not lessen the importance of Chlamydia as a PID pathogen. Not only does the tissue destruction result in severe complications such as ectopic pregnancy and infertility, but also the tissue damage provides fertile ground for the growth of secondarily infecting aerobic and anaerobic bacteria. This necrotic tissue is an excellent growth medium, and the epithelial damage enhances the breakdown of the surface defense mechanisms. The importance of Chlamydia was documented during the 1980s when treatment of acute PID during new antibiotic research trials was initially believed to be successfully accomplished with regimens not active against C. trachomatis. However, although success was evident at short-term follow-up, long-term follow-up demonstrated that treatment of C. trachomatis was necessary. PID regimens without Chlamydia coverage resulted in an increased incidence of recurrent episodes and long-term complications such as abscesses and chronic pelvic pain, with resultant increased surgical intervention. Therefore, current treatment of PID includes C. trachomatis coverage, even though this organism may not be the cause of the acute symptoms. Therefore, despite the lack of immediate and acute symptoms, Chlamydia remains an important pathogen in PID, as subtle or absent symptoms are more difficult to diagnose and treat early, resulting in much of the serious long-term sequelae found with PID. This is supported by the high incidence of Chlamydia antibodies in patients with acute PID, ectopic pregnancy, and infertility in several studies.
Despite the focus on these two STIs, nongonococcal and nonchlamydial pathogens cause more than 60% of PID infections. Besides N. gonorrhoeae and C. trachomatis, aerobic and anaerobic bacteria and other microorganisms, particularly other mollicutes, have been implicated as etiologic agents in acute salpingitis.
Haggerty et al., Cohen et al., Short et al., and others have all demonstrated the clinical significance of Mycoplasma genitalium in PID. Other novel pathogens are certain to be identified as the Human Microbiome Project identifies new, difficult-to-culture organisms found to be associated with PID.
However, M. genitalium is the current pathogen generating much attention after recent studies by Clausen et al. and Svenstrup and colleagues have found an association with M. genitalium and tubal factor infertility. More importantly, current PID treatment regimens may not adequately treat M. genitalium. Since it is a mollicute similar to Chlamydia, it lacks a cell wall and thus is resistant to cell wall-inhibiting antibiotics such as the penicillins and cephalosporins.
Also, studies by Björnelius and Falk and their colleagues in men and women with M. genitalium have found persistence of the organism despite treatment with levofloxacin and tetracyclines. However, these same researchers have found azithromycin to have better activity against M. genitalium. Moxifloxacin, a newer quinolone, also appears to have good in vitro activity.
In the large National Institutes of Health (NIH) PID study called the PID Evaluation and Clinical Health trial, Haggerty reported that 41% of M. genitalium-positive women experience microbiologic failure to eradicate the organism with standard Centers for Disease Control (CDC) PID treatment regimens, and more importantly, 44% experienced clinical failure. These results will most certainly be addressed at the next CDC review of PID treatment guidelines scheduled for 2015.
Bacterial vaginosis (BV)-related organisms such as Gardnerella, Mycoplasma hominis, and Ureaplasma urealyticum have also been suggested as causal agents in acute salpingitis by Ness and colleagues. However, their role remains controversial, as other studies have not found an association with PID. Cervical cultures positive for both M. hominis and U. urealyticum have been recovered from women with PID. However, the rate of isolation can be as high as 75%, which is not statistically different from that of women who are sexually active but without PID (baseline rate of about 50%), as found by Lemeke and Lsonka. Also, Clarke et al. have published evidence that Cytomegalovirus (CMV) may be associated with PID, but additional confirmatory investigations are limited.
Group B streptococcus, Escherichia coli, and other facultative anaerobic agents are also associated with PID, but fortunately are generally adequately covered by current CDC guidelines. In light of all the new data, the main point to emphasize is that although N. gonorrhoeae and C. trachomatis are significant pathogens, they are still only associated with 30% to 40% of all PID episodes. Thus, while current standard of care requires testing for both these organisms, negative results do not mean the patient does not have PID, and treatment should be initiated or completed based on the patient clinical course or additional microbiologic testing if possible.
RISK FACTORS
Several factors that predispose to the development of acute PID have been identified. Risk factors are important considerations in both the clinical management and prevention of UGTIs. First and foremost, there is a strong correlation between exposure to STIs and PID. In the United States, recent studies have confirmed this association with the recovery of N. gonorrhoeae or C. trachomatis in 40% to 50% of patients hospitalized with acute PID. Age at first intercourse, frequency of intercourse, number of sexual partners, and marital status are all associated with the frequency of exposure to STIs and thus are associated with PID. Women with multiple partners have an increased risk (four to six times normal) for development of PID, compared with women who have monogamous sexual relations. Additional studies by Ness and colleagues have found significant factors including age at first sex, cervicitis, history of PID, family income, smoking, medroxyprogesterone use, and sex with menses.
The incidence of acute PID decreases with advancing age. Adolescent girls are at significant risk for development of acute salpingitis. Westrom reported that nearly 70% of women with PID were younger than 25 years of age, 33% experienced their first infection before the age of 19, and 75% were nulliparous. The risk for development of acute PID in a sexually active adolescent female patient was 1:8, whereas the risk was 1:80 for a sexually active woman 24 years of age or older. Several reasons have been suggested for this increased risk. The two microorganisms most commonly considered to be the inciting agents in cases of PID, N. gonorrhoeae and C. trachomatis, have a predilection for columnar epithelium. As suggested by Schaefer and by Sweet and colleagues, cervical columnar epithelium is exposed to a greater extent in younger individuals and recedes into the cervical canal with increasing age.
Clinical and laboratory studies have documented that the use of contraceptives change the relative risk for development of PID. Multiple case-control studies have shown an increased risk of acute PID in women who use an IUD. It has been estimated that IUD users have a threefold to fivefold increased risk for development of acute PID, with the greatest risk the first 20 days after insertion. The incidence of infection has recently been described by Sufrin and colleagues in over 57,000 IUD insertions in California from 2005 to 2009. They reported an overall rate of approximately 1 in 200 patients.
Barrier methods of contraception (condoms, diaphragms, and spermicidal preparations) are effective both as mechanical obstructive devices and as chemical barriers. A nearly 60% decrease in the risk of PID has been demonstrated among women using a barrier method of contraception. Ness and colleagues found a 30% to 60% decrease in recurrent PID in women using condoms consistently.
Oral contraceptives have also been shown to reduce the risk and severity of acute PID. The mechanism for such protection remains speculative. The thicker cervical mucus produced by the progestin component of oral contraceptives is believed to inhibit sperm and accompanying bacteria penetration into the upper genital tract. The decrease in duration of menstrual flow accompanying oral contraceptive use theoretically creates a shorter interval for bacterial colonization. Svensson and coworkers reported that in addition to protecting against PID, the use of oral contraceptive pills was associated with a better prognosis for future fertility than was seen in women with acute PID using other contraceptive methods or no contraceptive methods. The pill’s ability to inhibit ovulation also helps prevent an open nidus for tuboovarian abscess formation.
Surgical procedures of the female genital tract also place the patient at risk for PID. About 15% of pelvic infections occur after procedures that break the cervical mucous barrier, allowing for colonization of the upper genital tract. Eschenbach and Holmes reported that these procedures include endometrial biopsy, curettage, IUD insertion, hysteroscopy, and hysterosalpingography. The incidence of UGTI associated with first-trimester abortions is approximately 1 in 200 cases. Recent practice has emphasized the use of prophylactic antibiotics in high-risk cases to attempt to decrease the incidence of iatrogenic acute PID. A randomized trial by Jackson and a randomized trial by the Luton and Dunstable Hospital Study Group have indicated that the treatment of BV with metronidazole substantially reduced postabortion PID.
Acute salpingitis occurring in a woman with a previous tubal ligation was once believed to be rare. However, Phillips and D’Abling reported that acute PID developed in the proximal stump of previously ligated fallopian tubes in 1 of 450 women hospitalized for acute salpingitis. In addition, it is suspected that many cases may be undiagnosed because of the absence of peritoneal signs from the prevention of retrograde spillage of bacteria and inflammatory exudate in the pelvic cavity.
Previous acute PID is also a risk factor for future episodes of the disease. Another acute tubal infection develops in approximately 25% of women who have had acute PID. The exact mechanism for this increased susceptibility has not been determined, but it may be loss of the natural protective mechanisms of the fallopian tube lining against microorganisms. This increased risk may also be related to the sexual habits of the woman involved, such as reinfection from an untreated male partner. Eschenbach has documented that more than 80% of male contacts are not treated.
Genetic factors for PID have been further delineated recently. Paavonen and Taylor et al. have demonstrated that variants in the genes that regulate toll-like receptors can interfere with the innate immune system and are associated with an increased progression of infection, especially with C. trachomatis.