Cutaneous mastocytosis
(a) Solitary mastocytoma
(b) Maculopapular cutaneous mastocytosis/urticaria pigmentosa
(c) Telangiectasia macularis eruptiva perstans
(d) Diffuse cutaneous mastocytosis
Indolent systemic mastocytosis (ISM)
Systemic mastocytosis with associated clonal hematologic disease (SM-AHNMD)
Aggressive systemic mastocytosis (ASM)
Mast cell leukemia (MCL)
Mast cell sarcoma (MCS)
Extracutaneous mastocytoma
Mast cells originate from hematopoietic progenitor cells in the bone marrow and migrate in a precursor state to connective tissue where they acquire their mature phenotype [9]. Mast cell precursors mature via activation of the transmembrane receptor CD117 with intrinsic tyrosine kinase activity, also known as KIT. Human c-KIT is a proto-oncogene that encodes for KIT. Coupling of KIT with the soluble KIT ligand, also known as stem cell factor (SCF), induces mast cells to express the high-affinity IgE receptors (FCERI) and proliferate [10]. The KIT receptor contains five domains: the extracellular domain, the transmembrane domain, the juxtamembrane domain, and two tyrosine kinase domains (tyrosine kinase 1 and tyrosine kinase 2, also known as the activation loop) [10].
Mutations in c-KIT activate tyrosine kinase activity independent of SCF binding. These are termed “self-activating mutations” and are in turn capable of inducing hyperproliferation of KIT-expressing cells [10]. Two types of c-KIT mutations have been described: regulatory type, which are located within the extracellular and juxtamembrane intracellular part of KIT, and enzymatic site type, which are located in the tyrosine kinase domain. Therapeutic implications are implied as the former is sensitive to the tyrosine kinase inhibitor imatinib mesylate while the latter is insensitive (see “Treatment” below) [11].
Adult mastocytosis has clearly been shown to be related to mutations in c-KIT resulting in clonal proliferation of mast cells [12, 13]. Majority of adult patients with SM have mutations in D816V (exon 17) on the activation loop domain [14]. In contrast, pediatric mastocytosis was formerly regarded as a reactive disease due to hyperplasia of mast cells to abnormal stimuli [15]. However, recent evidence reveals that pediatric mastocytosis is in fact, similar to adults: a clonal disease due to self-activating mutations in the c-KIT gene [7]. Analysis of the entire c-KIT sequence from cutaneous biopsies of 50 children with mastocytosis by Bodemer et al. demonstrated somatic mutations in exon 17 in 42 % of cases, with 36 % harboring mutations in D816V; mutations outside exon 17 were observed in 44 %, namely in exons 8, 9, and 11; all mutations constitutively activated c-KIT [16]. A study of 12 Japanese children similarly found D816V mutations in 10 of the patients [17] while a series of 9 cases of solitary mastocytomas identified c-KIT mutations in six cases (67 %), including 3 with the D816V mutation and 3 with an internal tandem duplication (p.A502_Y503dup) in exon 9 [18].
Familial forms of diffuse cutaneous mastocytosis have been reported. Germline mutations in c-KIT including mutations in the transmembrane domain of KIT A533D in members of 3 generations of a Scottish family and mutations in the extracellular domain of KIT S451C in 2 generations of a Han Chinese family have been described [19, 20]. Members of an Austrian family were found to have somatic mutations in c-KIT S849I and M835K (both exon 18) which was associated with a condition suspicious of mast cell activation syndrome and a tendency to incomplete resolution in adulthood [21]. Autosomal dominant inheritance of familial diffuse cutaneous mastocytosis has been suggested in a Japanese mother and her 3 daughters though no genetic studies were performed [22].
In essence, mastocytosis in children, like in adults, is a clonal disease due to self-activating mutations in the c-KIT gene.
Epidemiology
Key points
Most lesions of cutaneous mastocytosis in childhood appear in the first 2 years of life.
Pediatric mastocytosis is more common in the Caucasian population compared to Asians or Africans.
Pediatric mastocytosis is more common in males with skin of color.
Age
Most of the lesions of pediatric mastocytosis (55–97 %) appear during the first 2 years of life [2, 23–26]. Indeed, series from Mexico and Poland found that 92 and 94 % of cutaneous lesions occurred during the first year of life [23, 26]. The occurrence of different subtypes of cutaneous mastocytosis at different ages has been described. 40 % of mastocytomas occurred at birth and almost all by the age of 1 year in a series of Israeli children [2]. In contrast, only 20 % of the patients with urticaria pigmentosa had lesions present at birth while 80 % did so by the age of 9 months. Conversely, Kiszewski et al. found no correlation between the type of mastocytosis and the age of onset in a Mexican cohort.
Sex
Race
A genetic factor could explain these differences amongst various ethnic groups. Pediatric mastocytosis seems to be more common in Caucasians [27]. Reports of Asian or African children exist only as scattered case reports in the literature.
Clinical Presentation
Key points
Pediatric cutaneous mastocytosis is typically divided into mastocytoma, urticaria pigmentosa, and diffuse cutaneous mastocytosis.
Cutaneous mastocytosis in children can be associated with systemic symptoms due to mast cell degranulation and release of mast cell mediators, even when there is no systemic infiltration. Many stimuli can trigger mast cell degranulation.
Elevated serum total tryptase levels indicate extensive skin involvement and higher risk for severe mast cell activation symptoms. Extent of skin involvement also parallels symptom severity.
The typical presentation of pediatric mastocytosis consists of cutaneous lesions. In comparison to adults, bone marrow biopsies are not routinely performed or indicated and when they are, technical difficulties make it difficult to obtain significant results. Consequently, a definitive status of cutaneous or systemic involvement in children is usually not feasible (Table 37.2). Therefore, a working classification of pediatric mastocytosis based on the WHO criteria and the morphological appearance of lesions is adopted [7]:
1.
Mastocytoma
2.
Urticaria pigmentosa
3.
Diffuse cutaneous mastocytosis
Major |
Multifocal dense infiltrates of mast cells (MC) (MC aggregates of 15 or more cells) in bone marrow and/or other extracutaneous organs, confirmed by special stains |
Minor |
(a) In MC infiltrates in bone marrow or other extracutaneous organs, 25 % or more of MC are spindle shaped; or in bone marrow, 25 % or more of MC are atypical |
(b) c–kit point mutation at codon 816 in bone marrow or blood or other extracutaneous organs |
(c) Immunophenotyping of MCs in bone marrow, blood, or other extracutaneous organs that co-express CD2 or/and CD25 |
(d) Serum total tryptase levels persistently over 20 ng/ml (not valid if patient has associated clonal hematological non-MC disease; AHNMD)a |
If one major and one minor or three minor criteria are fulfilled, then the diagnosis is systemic mastocytosis |
Urticaria pigmentosa (UP) is the most common form of CM in children and represents 70–90 % of all cases [29]. It is sometimes referred to as maculopapular CM. The lesions range from red to brown to yellow in color, are occasionally telangiectatic, and present as multiple macules, papules, plaques, or small nodules (Fig. 37.1). Stroking a lesion leads to wheal and flare formation. This response is known as Darier’s sign and is considered clinically diagnostic [30]. Blister formation can also occur (Fig. 37.2).
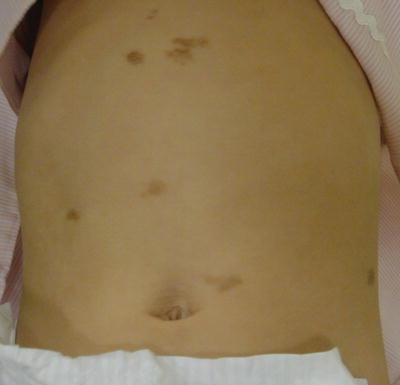
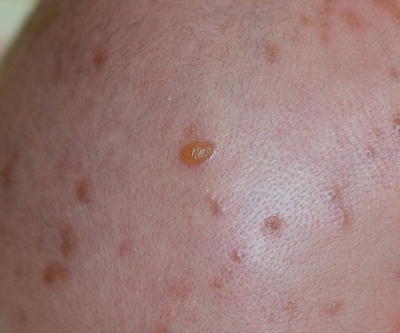

Fig. 37.1
Urticaria pigmentosa: red-brown macules and plaques (courtesy of Dr Mark Koh, MD)

Fig. 37.2
Blister formation in urticaria pigmentosa (courtesy of Dr Mark Koh, MD)
Mastocytoma is defined as a solitary round-to-oval elevated lesion, usually present at birth (Fig. 37.3). It represents 10–30 % of cases of pediatric mastocytosis. Such lesions can vesiculate and blister especially with application of mast cell degranulators such as polymyxin B. Most lesions resolve by puberty, but persistent lesions have been described in adults [31].
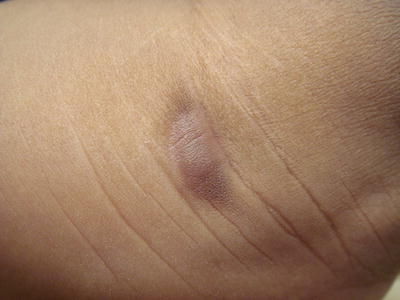

Fig. 37.3
Mastocytoma: solitary red-brown oval nodule (courtesy of Dr Mark Koh, MD)
Diffuse cutaneous mastocytosis (DCM) is the rarest form of CM, accounting for 1–3 % of cases. It generally presents in the neonatal period. Large areas of skin are infiltrated by mast cells. Two variants of DCM have been described. The first type describes extensive blistering and erythroderma early in life which can mimic staphylococcal scalded skin syndrome [32, 33]. Patients with the second type have extensive yellow-orange smooth-to-leathery skin with minimal blistering [34–36].
In reality, the WHO classification may be an oversimplification of the various forms of CM. Descriptions of patients in the literature show overlapping features that do not fit neatly into a particular subtype, based on traditional classification. For example, Hartmann et al. have argued that UP or maculopapular CM has varied clinical appearances and disease courses. Maculopapular CM with small lesions that occurs in children and in adults rarely resolves spontaneously and is often eventually categorized as ISM. In contrast, plaque-type CM, which is often grouped under maculopapular CM, as well as nodular CM or mastocytomas (single or multiple), has a tendency to resolution [37]. The authors have proposed a modified classification of cutaneous mastocytosis (Table 37.3). Torrelo et al. have argued that the term DCM is very ambiguous: very extensive forms of UP with ISM and extensive large mastocytoma-like nodular lesions with WDSM have also been included [7]. The same authors have also argued that different clinical manifestations classified as UP are known to fade away, develop into ISM, or evolve into WDSM. A case series by Azana et al. reported spontaneous resolution in all 67 cases of pediatric UP in a Spanish population, while Ben-Amitai et al. reported complete resolution in only 56.4 % of 180 Israeli children with UP, again highlighting different variations within the same subset [2, 38].
Table 37.3
Proposed modified classification of cutaneous mastocytosis
Classification of cutaneous mastocytosis |
1. Maculopapular cutaneous mastocytosis |
2. Plaque-type cutaneous mastocytosis |
3. Nodular cutaneous mastocytosis/mastocytoma (i.e., solitary or multiple) |
4. Diffuse cutaneous mastocytosis |
5. Telangiectatic cutaneous mastocytosis |
Mastocytosis can involve unusual anatomical locations and associations. Vulval mastocytosis was reported in two Spanish females aged 5 and 8 years [39]. Intertriginous CM involving the axillae and groins occurred in a 16-year-old Chinese male patient [40]. DCM was associated with a large bathing trunk nevus in a 4-year-old Indian male while maculopapular CM was associated with the epidermal nevus syndrome in a 2-year-old Japanese male [41, 42]. Congenital scarring alopecia was reported in a 3-year-old Korean female with UP and a mastocytoma [43].
Dermoscopy has recently been promoted as a feasible method for diagnosis and subclassification of cutaneous mastocytosis. Vano-Galvan et al. described four dermoscopic patterns in a large Spanish cohort of 127 patients (61 children and 66 adults): yellow-orange blot, pigment network, reticular vascular pattern, and (most frequently) light-brown blot. The light-brown blot pattern accounted for most of the maculopapular CM and plaque-type mastocytosis, while the reticular vascular pattern was found in all patients with telangiectasia macularis eruptive perstans and a small proportion with maculopapular CM. The yellow-orange blot pattern was described in all children with mastocytoma and half of all patients with nodular mastocytosis. These dermoscopic patterns also showed histological correlation. Interestingly, the need for daily antimediator therapy was higher among patients with a reticular vascular pattern compared to other dermoscopic patterns [44].
Symptoms
CM in children can be associated with systemic symptoms due to mast cell degranulation and release of mast cell mediators, even when there is no systemic infiltration. These cutaneous symptoms include flushing, dermographism, and pruritus, which may occur spontaneously or in response to specific stimuli. Gastrointestinal symptoms include nausea, vomiting, abdominal pain, and diarrhea; hyperacidity and peptic ulcer disease are rare in children. Other symptoms include dyspnea, headache, fatigue, lethargy, or neuropsychiatric symptoms. Children with extensive forms of cutaneous mastocytosis are at risk of pseudoanaphylactic reactions [7].
A variety of stimuli and agents are known to activate/trigger mast cells (Table 37.4). In children, the main trigger is a change in temperature, followed by irritability, fever, and teething [46].
Physical stimuli |
Heat |
Cold |
Sudden changes of temperature |
Mechanical friction or pressure |
Sunlight |
Emotional factors |
Stress |
Anxiety |
Sleep deprivation |
Infectious diseases with fever |
Viral (upper respiratory tract infection) |
Bacterial (bronchitis, pneumonia) |
Drugs |
NSAIDs |
Alcohol |
Narcotics (morphine, codeine, and derivatives) |
Cough medication (dextromethorphan, dimemorfan) |
Acetylsalicylic acid |
Procaine |
Polymyxin B |
Amphotericin B |
Vancomycin |
Atropine |
Thiamine |
D-tubocurarine |
Quinine |
Radiographic contrast media containing iodine |
Scopolamine |
Gallamine |
Decamethonium |
Reserpine |
Foods |
Aged cheese |
Alcohol |
Chocolate |
Strawberries |
Miscellaneous |
Dentition, e.g., teething and dental procedures |
Vaccinations |
Surgery |
Endoscopic procedures |
Various noninvasive methods have been studied to estimate the risk of severe systemic symptoms, including anaphylaxis, and the risk of disease progression in children with CM.
Tryptase Parallels Extensive Skin Involvement and Symptom Severity
The most reliable marker for severity of mastocytosis is serum tryptase [4, 47, 48]. In adults, total serum tryptase levels correlate with the type and severity of mastocytosis. Levels below 20 ng/ml usually indicate cutaneous mastocytosis without systemic involvement while levels above 20 ng/ml are associated with ISM; even higher levels indicate ASM. Serum tryptase levels permanently over 20 ng/ml are a minor criterion for systemic mastocytosis. However, up to 25 % of adults with proven systemic mastocytosis have serum tryptase levels below 20 ng/ml [49].
In children, the relationship between elevated serum tryptase levels and systemic mastocytosis has not been established. Levels are, however, useful to predict symptom severity. Serum tryptase levels correlated with a mastocytosis severity scoring system (SCORMA) in a group of 64 Dutch patients (31 children and 33 adults) [47]. A large Spanish study of 111 children with CM showed a strong correlation between serum baseline total tryptase levels and extent of skin involvement as well as symptom severity [7]. Unlike adults, a serum tryptase higher than 20 ng/ml in children does not translate to a diagnosis of systemic mastocytosis, which requires bone marrow studies that are not routinely performed. Rather, an elevated serum baseline total tryptase level reflects children with extensive skin disease and higher risk of severe, even life-threatening mast cell activation symptoms. Brockow et al. further highlighted that extensive blistering in children with mastocytosis, instead of extensive flushing or pruritus, should be regarded as a predictor for severe complications in children with mastocytosis [48].
It is worth noting that despite evidence for correlation between serum tryptase and cutaneous extent of mastocytosis, there are a small number of children with minor forms of CM who have persistently elevated levels; the significance of elevated serum tryptase in the outcomes of these children is unknown [7]. Other mediators such as histamine and N-methyl histamine have been correlated with increased mast cell numbers in the skin, but levels vary with the age of the child [50].
Skin Severity Parallels Symptom Severity
Serum tryptase is not the only marker that can predict symptom severity. The severity of skin disease itself has been shown to parallel symptom severity. In a cohort of 67 American children (61 % Caucasian, 10 % African-American, 10 % Asian, and 3 % Hispanic) with maculopapular CM, the maximum number of skin lesions and the number of skin symptoms were significant predictors for the number of systemic symptoms [51]. In contrast, an earlier study by Brockow et al. [10] revealed a correlation between the extent of cutaneous involvement with symptom extent and severity in adults with maculopapular CM but not in the pediatric cohort [52]. Due to the extent of skin symptoms in DCM, severe systemic symptoms including whole body flushing, gastrointestinal bleeding, hypotension, hypovolemic shock, and even death have been reported in a 17-month-old Irish male [53].
Treatment
Key points
Treatment of pediatric mastocytosis is aimed at preventing and controlling skin and systemic mast cell activation symptoms
Therapy can be divided into topical and systemic forms, as well as antimediator and cytoreductive therapies; these should be tailored according to the grade of symptom severity in the individual patient
Anesthesia is generally safe in the pediatric mastocytosis population; however meticulous planning should be ensured to manage potential cardiovascular decompensation
General Concepts
The treatment of pediatric mastocytosis is not curative but instead aimed at preventing or controlling skin and systemic mast cell degranulation symptoms with the anticipation that skin lesions will fade as the child grows.
A four-grade scale for mastocytosis severity from mast cell degranulation, which is also applicable to children, has been established by consensus guidelines [4, 45]. This grading severity can be used to determine suitable treatments.
1.
Grade 0: no symptoms
2.
Grade 1: mild symptoms, no therapy required
3.
Grade 2: moderate symptoms, kept under control with antimediator-type drugs
4.
Grade 3: severe symptoms, not sufficiently controlled with therapy
5.
Grade 4: severe adverse events that require emergency therapy and hospitalization
Avoidance of Triggering Factors
A variety of stimuli and agents are known to activate mast cells (Table 37.4). Measures should be undertaken by parents and patients to recognize and avoid or control these. For example, an ideal ambient temperature can be adjusted based on the child’s response. Castells et al. recommend a trial of lukewarm water for bathing (20–23 °C) and air-conditioning at 26 °C [29].
Topical Therapy
Cutaneous lesions with pruritus and flare can be treated with water-soluble sodium cromoglycate cream and lotion [54]. Topical corticosteroids can prevent blistering, while topical zinc sulfate and antibacterials are useful for denuded skin [55]. Topical therapy can be used for all cutaneous lesions of all grades of mastocytosis severity.
Systemic Therapy
The cornerstone of treatment of all categories of mastocytosis is symptom control. Antimediator therapy takes the form of drugs used for symptom control or receptor signaling for these mediators, and on occasion by reducing the production of mast cell mediators or preventing the release of mediators from mast cells [56].
Both sedating and non-sedating H1 antihistamines have been shown to be useful in decreasing pruritus, flushing, urticaria, tachycardia, and symptom severity of anaphylaxis [57, 58]. In particular, diphenhydramine, hydroxyzine, and cetirizine have proven to be useful in children. Most experts do not regard the H1 antihistamine ketotifen as a medication unique in its class, despite its ostensible mast cell stabilizing properties [58, 59]. H2 antihistamines such as ranitidine and famotidine can be used to treat gastrointestinal symptoms associated with mastocytosis [7, 60]. H2 antihistamines can be combined with H1 antihistamines to control severe pruritus and flare [56, 56].
Oral cromolyn sodium can alleviate a variety of symptoms associated with mast cell degranulation in patients with systemic mastocytosis: gastrointestinal (diarrhea, abdominal pain, nausea, and vomiting), cutaneous (pruritus, whealing, and flushing), and central nervous (cognition) [63, 64]. In particular, studies of pediatric patients with cutaneous mastocytosis (urticaria pigmentosa and bullous mastocytosis) have reported efficacy of oral cromolyn sodium [65, 66]. Introduction with small incremental doses instead of the full dose reduces adverse effects of abdominal cramping and diarrhea [56].
Oral corticosteroids have been used for various indications in mastocytosis, albeit supported by only anecdotal evidence or case reports. In children, they are indicated for anaphylaxis or abdominal pain (with or without diarrhea) that is unresponsive to oral cromolyn sodium [29].
Leukotriene antagonists may be employed when symptoms are refractory to standard therapy. A 2-month-old male infant with systemic mastocytosis (skin and bone marrow biopsy done), prominent bullous skin lesions, and wheezing showed improvement of symptoms when monteleukast was added on to high-dose oral prednisolone, cromolyn sodium and, H1 and H2 antihistamines [67]. An 8-year-old female diagnosed with ISM (based on skin lesions of urticaria pigmentosa, symptoms of flushing, diarrhea, abdominal pain and urinary incontinence, and elevated tryptase level of 199 μg/l (normal <20 μg/l)), similarly demonstrated good response with addition of monteleukast as adjuvant therapy [68].
Oral psoralen with UVA (PUVA) has been reported to be effective in patients with bullous diffuse cutaneous mastocytosis [69, 70], and modestly useful in urticaria pigmentosa [71, 72]. Bath PUVA is not as effective. Adverse effects of PUVA include photofibrosis, non-melanoma skin cancers, malignant melanoma, and direct ocular damage [73, 74].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


