Fig. 1.1
Progesterone itself produces and plays an important role in the myoma growth and development
Table 1.1
Role of progesterone in the pathogenesis of uterine myomas: new theory
Progesterone contributes to regulation of mitotic activity |
Estrogens stimulate progesterone receptors |
There is increased mitotic activity in the secretory phase |
Progesterone appears to inhibit GnRH-analogues induced hypo-estrogenism and shrinkage of myomas |
Progesterone down regulates estrogen receptors |
Progesterone induces up-regulation of the Ki-67 cell proliferation index |
Progesterone up-regulates Bcl-2 protein expression, an apoptosis inhibitor in myoma cells |
Growth Factors
Of the many growth factors that play a role in the myoma growth through synergistic actions with estrogens and progesterone, there are three that need mentioning: epidermal growth factor (EGF), vascular endothelial growth factor (VEG-F) and insulin-like growth factor (IGFs I-II). Also the extracellular matrix (ECM), a reservoir of growth factors that could promote leiomyoma growth, is an important factor to consider. All are responsible in the growth and development of myomas. EGF increases DNA synthesis in myoma cells. IGFs increase cell proliferation in myomas by activation of the MAPK (mitogen activated protein kinase) pathway involved in the proliferation of myoma cells. It also up-regulates Bcl-2 proliferation expression in myoma cells. VEG-F promotes angiogenesis in myomas [6, 7]. Finally, the ECM composed of collagen, fibronectin and proteoglycans, all involved in remodeling and growth of myomas. There is 50 % more ECM component in myomas than in the corresponding host myometrium. All these factors play a significant role along sex steroids in the development of myomas (Table 1.2) [7].
Table 1.2
Growth factors in myoma development
Epidermal growth factors (EGFs) |
Insulin-like growth factors (IGFs-I, II) |
Transforming growth factor-B |
Heparin binding growth factors (HBGFs) |
PDGF (platelet-derived GF) |
BFGF (basic fibroblast GF) |
VEGF (vascular endothelial GF) |
HBEGF (heparin-binding epidermal GF) |
Extracellular matrix |
Cytogenetics
Forty percent of women affected with uterine myomas have cytogenetic abnormalities mainly comprised of rearrangements in C-12q14-q15, C-6p21 and C-10q, deletions in C-7q [7q22] and C-3, structural aberrations in C-6, and translocations in C-12 [8, 9]. About 50 % of myomas show clonal abnormalities involving chromosomes 1,7,12, and translocation (12;14). Whole-genome sequencing of myomas also show frequent fragmentation and random rearrangements similar to the chromothripsis phenomenon seen in malignant tumors (Table 1.3) [10, 11].
Table 1.3
Most frequent Cytogenetic alterations in Myomas
C-12q14-q15, C-6p21 and C-10q rearrangements |
C-7q (7q22) and C-3 deletions |
C-6 structural aberrations |
C-12 translocations (14) |
High Mobility Group 1 Proteins
These proteins have been found mainly in malignant and embryonic cells; however myomatous cells may also express these proteins, particularly HMG1, HMG1-C and HMG1 (Y), encoded in specific chromosomes that may have a role in the myoma growth. Normal myometrium does not harbor these proteins [12, 13].
Uterine Inner and Outer Myometrium
While the uterine myometrium looks anatomically uniform, two distinct zones have been described by Brosens et al. [14], showing that the junctional or inner myometrial zone and the outer myometrial zone are two distinct zones with different pathophysiology. Myomas originating in each of these two zones respond differently to the ovarian hormones and their surrounding host myometrium is biochemically abnormal with increased cellular concentration of estrogen receptors, compared with normal myometrium. The junctional myometrium mimics the endometrium in its response to estrogen and progesterone and active contractions occur in the junctional myometrium throughout the menstrual cycle in contrast to the outer myometrium. Submucous myomas have less karyotypic aberrations than outer myometrial myomas and karyotypically abnormal myomas seem to be less hormone-dependent than myomas without chromosomal rearrangements. Also, GnRH-analogue therapy is more effective in size reduction of submucous myomas than outer myometrial layer myomas [15]. The arterial visualization in submucous myomas with Doppler ultrasonography is more markedly apparent (85 %) than in intramural myomas (42 %). These variations represent important factors to be considered in relation to reproduction and symptomatology (Figs. 1.2 and 1.3) [16].
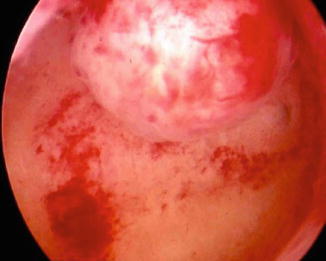
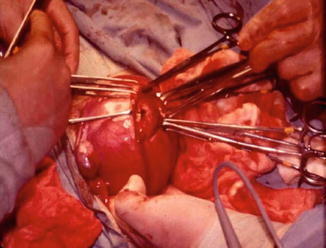

Fig. 1.2
Hysteroscopic view of fundal submucous myoma

Fig. 1.3
Intramural myoma being removed by laparotomy
Vascularization and Location of Uterine Myomas
Uterine myomas are parasitic tumors that borrow vascularization from the surrounding myometrium or other adjacent structures and when present, they may disrupt the delicate network that accompanies vascularization of the normal myometrium. This network originates from the uterine arteries extending to the arcuate and radial arteries and crossing the myometrium to reach the straight and spiral arteries that feed the endometrium. Disruption at any level of this vascular network will result in venous engorgement, dilatation and congestion that will disrupt the endometrium, producing abnormal bleeding and disrupting normal function and receptivity.
With the advancements in ultrasonography, hystero-sonography, 3-D ultrasonography and Doppler flow technology, the proper location and vascularization of myomas can be accurately determined and mapped to obtain information about location, number and size. Additionally, these modalities delineate the relationship of the myomas to the endometrium and uterine cavity, particularly in determining the percentage of uterine wall invasion of submucous myomas and their proximity to the uterine serosal surface (Figs. 1.4 and 1.5) [16].
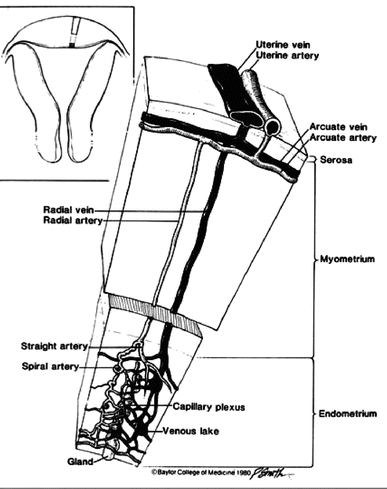
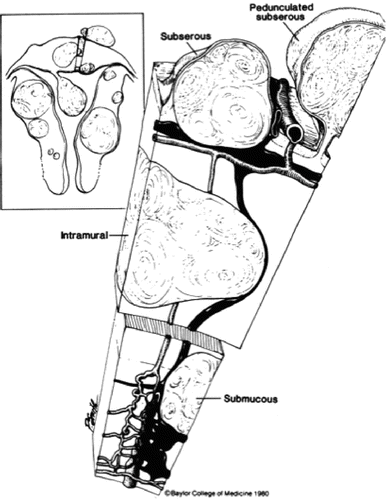

Fig. 1.4
Schematic representation of vascular network of a normal uterus. (Reprinted with permission from Elsevier, Inc.)

Fig. 1.5
Myomas obliterating and distorting the uterine vascularization at various sites of the uterine wall. (Reprinted with permission from Elsevier, Inc.)
These are important factors to consider in planning appropriate surgical treatment. Myomas have been classified according to their location in the uterine body: protruding away from the uterine body (subserosal), encased in the uterine muscle (intramural) and impinging at various degrees into the uterine cavity (submucosal). These locations determine the mode of surgical removal with or without invasion of the uterine muscular body (Figs. 1.6 and 1.7).
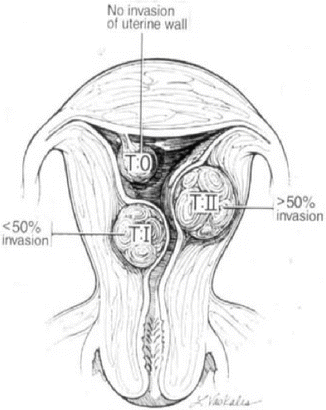
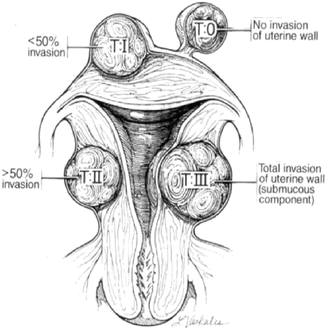

Fig. 1.6
Schematic representation of various invasions of the uterine wall by submucous myomas. (Reprinted with permission from Elsevier, Inc).

Fig. 1.7
Myomas impinging and penetrating the uterine wall from the periphery of the uterus. (Reprinted with permission from Elsevier, Inc).
Symptomatology of Uterine Myomas
The most frequent symptoms associated with myomas are: abnormal uterine bleeding, infertility, pregnancy losses and pelvic pain.
Abnormal Uterine Bleeding
Approximately 30 % of patients harboring uterine myomas present with abnormal uterine bleeding, especially submucous myomas where bleeding can be severe [3]. Many theories have been proposed to explain the pathophysiology of this symptom including coexisting associated anovulation, alteration of uterine contractility, compression of the venous plexi in the adjacent myometrium, increase in endometrial surface to more than 15 cm2, erosion of the surface of submucous myomas and inability of the surrounding endometrium and myometrium to produce hemostasis [17]. However, none of these factors alone can satisfactorily explain the abnormal bleeding and perhaps all play a synergistic role (Figs. 1.8, 1.9, and 1.10) [3, 4].
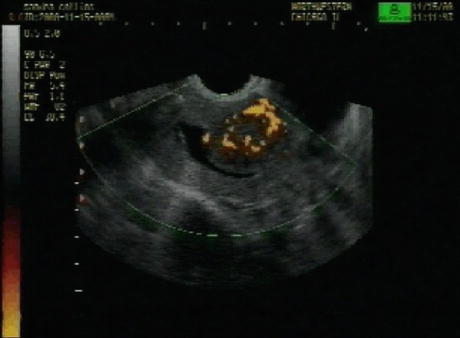
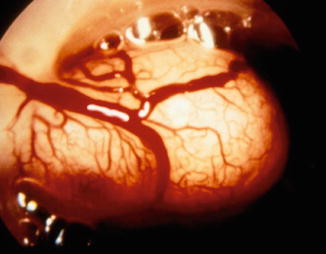
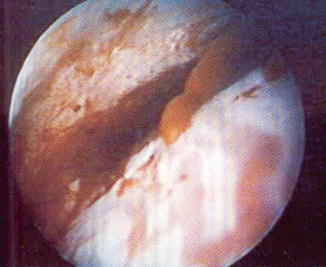

Fig. 1.8
Doppler flow aided ultrasonography demonstrating the peripheral vascularization of a submucous myoma

Fig. 1.9
Hysteroscopic view of submucous myoma showing its rich peripheral vascularization

Fig. 1.10
Hysteroscopic view of active bleeding from a ruptured peripheral vessel of a submucous myoma
Infertility
In an extensive and comprehensive review of the literature, Pritts [18] demonstrated that infertility could only be caused by submucous myomas and rarely by subserous or intramural myomas unless these latter types significantly distort or impinge the uterine cavity. In a retrospective analysis of 249 women with intramural myomas not distorting the uterine cavity who underwent in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI), Yan et al. found no adverse effects in the IVF/ICSI outcomes. However, when the intramural myomas were greater than 2.85 cm in size, there was a significant impairment in delivery rates in these patients compared with controls without myomas [19]. Subserosal and intramural myomas may only be coincidental to the infertility, as no objective evidence in their causal effect has been demonstrated in prospective randomized studies. Submucous myomas, however, may interfere with fertility and following treatment fertility is usually restored. There is evidence that submucous myomas not only produce local irritation and disruption of the intrauterine environment for implantation but also globally reduce intrauterine endometrial receptivity by interfering with specific molecular markers of endometrial receptivity such as HOXA 10 and HOXA 11 gene expressions, LIF (leukemia inhibitor factor) and BTEB1 (basic transcriptional binding protein 1) [20]. Interestingly, when endometrial cells are cultured with fluid removed from hydrosalpinges, these molecular markers are suppressed and normalize when the hydrosalpinges are removed [21, 22]. So, this suggests that not only does the mere presence of the myomas in the uterine cavity impact endometrial receptivity, but also these tumors have a deleterious global effect on the molecular markers of endometrial receptivity (Table 1.4).
Table 1.4




Molecular markers of endometrial receptivity globally decreased by submucous myomas
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


