Patent Ductus Arteriosus
INTRODUCTION
The ductus arteriosus is a central vascular shunt that interconnects the pulmonary artery and aorta during fetal life. A ductus or similar structure is present in mammals and most other vertebrates and is embryologically derived from the distal portion of the left sixth branchial arch.1,2 As a result of the relatively large size of the ductus and the high resistance of the pulmonary vascular circuit in utero, approximately 90% of right ventricular output flows though the ductus and into the systemic circulation, thus bypassing the unaerated fetal lung.
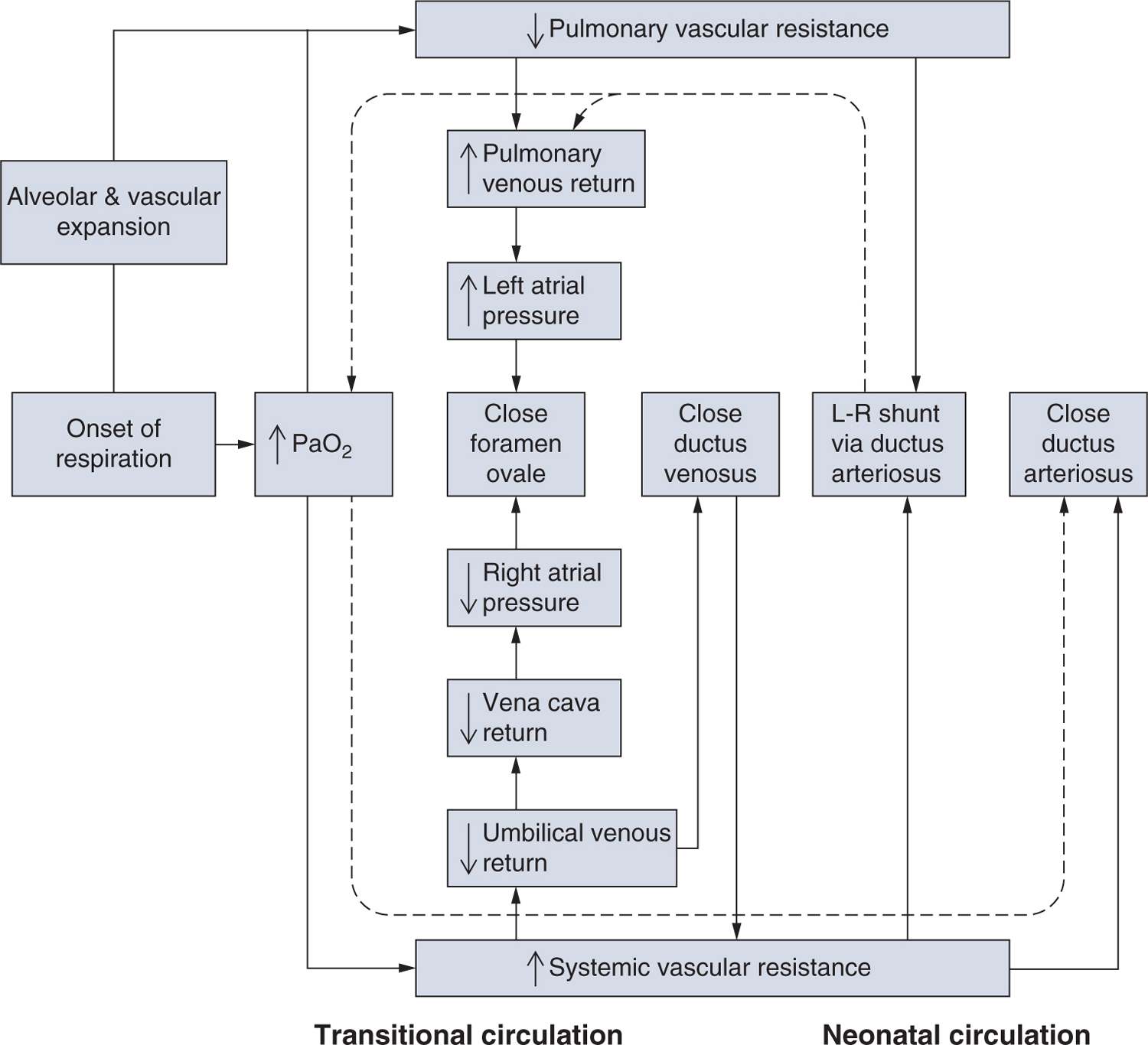
Constriction of the ductus arteriosus is a critical step in postnatal circulatory transition. The normal physiological shift from a placental to a pulmonary respiration pattern is marked by a rapid sequence of events, including loss of umbilical flow and a subsequent fall in right atrial pressures, along with separation from the low-resistance vascular bed of the placenta and an accompanying increase in systemic vascular resistance (SVR)3 (Figure 22-1). The abrupt decrease in pulmonary vascular resistance (PVR) that occurs with the onset of respiration leads to an 8- to 10-fold increase in pulmonary blood flow. As a result, left atrial pressure increases, facilitating closure of the foramen ovale. The combined increase in SVR and arterial blood pressure and decrease in PVR redirects blood flow through the ductus arteriosus in a left-to-right pattern until closure takes place via smooth muscle constriction and obstruction of the lumen. Luminal obstruction is a result of subendothelial thickening, the formation of intimal mounds that protrude into the vessel lumen, and endothelial cell proliferation and crowding with ongoing muscular constriction. The formation of cell-cell adhesions and recruitment of circulating leukocytes or platelets might also contribute to ductus closure.4–7
FIGURE 22-1 Cardiopulmonary transition at birth. The normal physiological shift from a placental to a pulmonary respiration pattern is marked by a rapid sequence of events illustrated by this block diagram of the transitional circulation. (Adapted from Smith and Nelson.3)
Normal closure of the ductus arteriosus involves well-characterized anatomic changes that result in the remodeling of this structure to become the ligamentum arteriosum. Full-term infants with persistent patency of the ductus arteriosus do not undergo this remodeling. The result is a structural defect that occurs in full-term infants and often is associated with other cardiovascular anomalies.
In contrast to the timely closure of the ductus in full-term infants, premature infants, particularly those with lung disease, do not always undergo ductus closure shortly after birth. Unlike the full-term infant, in whom the patent ductus arteriosus (PDA) is a congenital structural defect, the ductus of a premature infant with symptomatic PDA would have closed normally but for the misfortune of the infant being delivered prematurely.
EPIDEMIOLOGY
A large proportion of premature infants will undergo spontaneous closure of the ductus arteriosus sometime during the neonatal period. Other premature infants will not experience ductus closure for weeks or more after birth. Hemodynamic symptoms of PDA are present in 55% to 70% of infants less than 1000 g birth weight or prior to 28 weeks of gestation.8
To avoid ambiguities and inconsistencies, at least within this chapter, the following definitions are used:
• Patent ductus arteriosus (PDA) refers to the anatomic state of the ductus arteriosus.
– Normal in fetal life
– Abnormal when present after birth (sometimes referred to as persistent PDA)
– When present after birth implies some degree of left-to-right shunt
• Hemodynamically significant PDA (hsPDA) is an echocardiographic definition referring to left-to-right ductus shunting with
– diastolic flow reversal in the descending aorta, OR
– evidence of LV failure (eg, LA or LV enlargement)
• Symptomatic PDA involves left-to-right shunting through a persistent PDA associated with clinical evidence of related pulmonary or cardiovascular compromise; some, but not all, infants with persistent PDA will also have symptomatic PDA. All infants with symptomatic PDA also have persistent PDA.
PDA in the Preterm Infant
Efforts to reevaluate the necessity to close a symptomatic PDA have led to renewed interest in the natural history of this disorder and suggest that current predictions of anticipated ductus closure in extremely low birth weight (ELBW) infants may need to be reevaluated. Spontaneous ductus closure may occur in 30% to 35% of immature infants less than 28 weeks’ gestational age9,10; however, interpretation of these rates is difficult because they are based only on infants (by definition) who did not undergo intervention to close the ductus. Spontaneous closure rates are higher in more mature infants and lower when the clinical course is complicated by significant lung disease. In general, there is a significant negative correlation between length of time before spontaneous ductus closure occurs and gestational age, underscoring the strong developmental influence on when the ductus will close if left alone. Most infants with persistent ductus shunting will eventually undergo spontaneous closure. Reports from centers where medical management is emphasized as an alternative to pharmacologic or surgical intervention indicate that final spontaneous closure may not occur for weeks or longer, and that some infants may be discharged home with a persistent PDA and not experience final spontaneous closure until 60–76 weeks postmenstrual age.9–11
The strong influence of gestational immaturity on persistent ductus patency most likely reflects biological immaturity of the developing ductus smooth muscle, the presence of ongoing vasodilatory stimuli,12 or incomplete acquisition of multiple-signaling systems that contribute to ductus closure.13,14 Other factors associated with ductus patency are shown in Table 22-1.
Table 22-1 Factors Associated with Patent Ductus Arteriosus (PDA)a,b
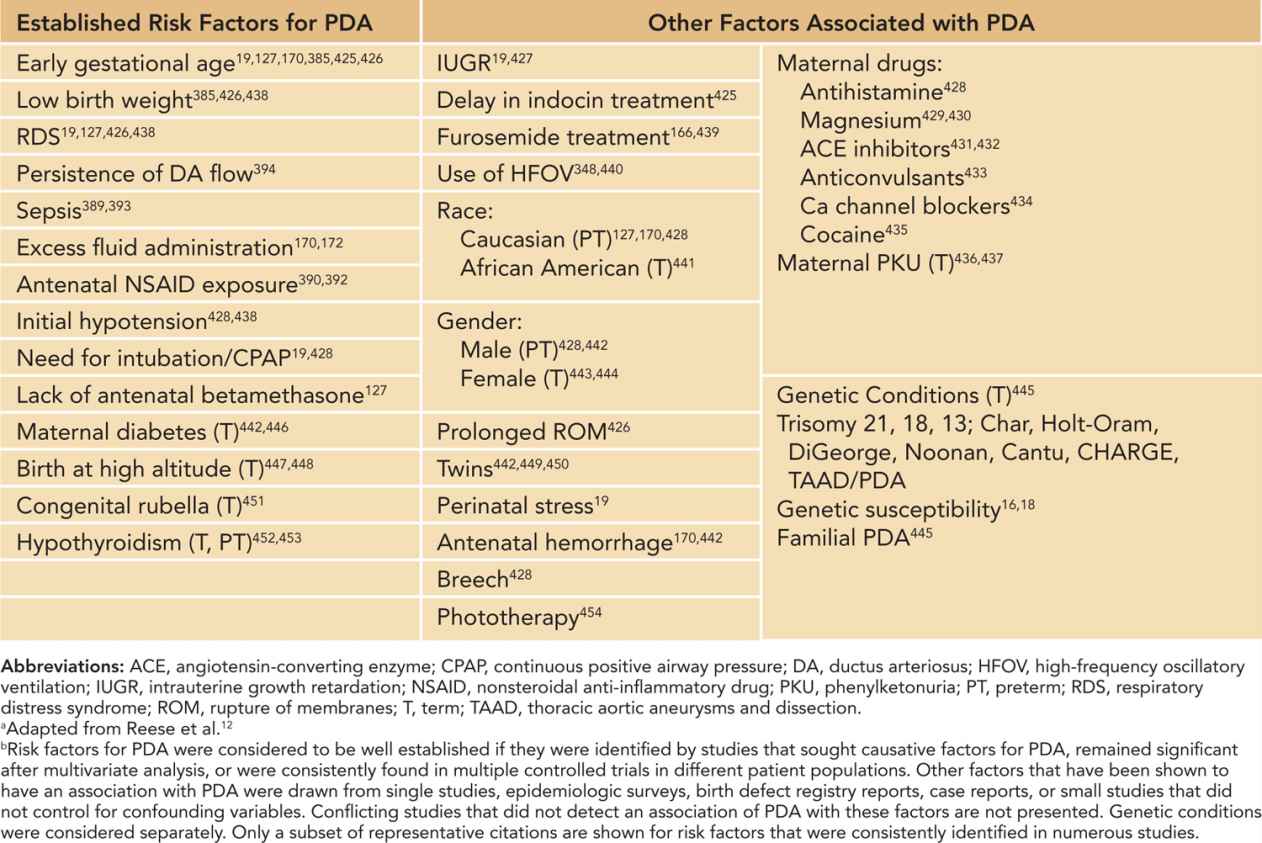
There is growing evidence in preterm as well as full-term infants that hereditary and genetic factors play a role in susceptibility to persistent ductus patency after birth. In 1 study, comparison of monozygotic and dizygotic twin pairs indicated a significant genetic contribution to persistent PDA requiring indomethacin treatment, an effect that remained significant after taking environmental factors into consideration.15 In another study of preterm twin pairs,16 environmental factors rather than genetic factors accounted for a significant hereditary susceptibility to persistent PDA, indicating the inherent complexity of these kinds of studies.17 In a population study designed to determine if genetic risk factors play a role in persistent PDA in preterm infants, 377 single-nucleotide polymorphisms from 130 genes of interest were evaluated in DNA samples collected from 204 infants with a gestational age of less than 32 weeks. The results of this study support a role for genetic variations in transcription factor AP-2β, tumor necrosis factor receptor–associated factor 1, and prostacyclin synthase in the persistent patency of the ductus arteriosus in preterm infants.18 Although it would be premature to assume that these genetic variations reveal a biological etiology, it is reasonable to regard these findings as genetic risk factors for persistent PDA that can be added to the list of established clinical risk factors such as birth weight, gestational age, acute perinatal stress, and hyaline membrane disease. In the absence of genetic risk factors for persistent PDA, the 28-week preterm infant might not have persistent PDA even though the infant had the same clinical risk factors as the 28-week preterm infant who is positive for the genetic risk factors and who develops persistent PDA.19
Persistent PDA in the Term Infant
Persistent PDA in full-term infants is the result of different pathological processes than in preterm infants. Anatomic studies have shown that the ductus in term infants with persistent PDA is abnormally formed.20 The full-term infant with persistent PDA maintains an intact internal elastic lamina rather than undergoing fragmentation to facilitate the migration of underlying smooth muscle cells into the subendothelial space, with a corresponding reduction in the formation of intimal cushions for occlusion of the ductus lumen. The ductus in full-term infants with persistent PDA also has altered formation of elastic lamina, reduced subendothelial swelling and matrix deposition, reduced proliferation of the muscular media, and reduced smooth muscle layers.4,20,21
Prematurity does not protect against the pathophysiology underlying persistent PDA in the full-term infant. If a premature infant happens to have a “full-term-like” persistent PDA, it would probably be thought (mistakenly) to have a preterm persistent PDA that did not close spontaneously, unless it was associated with other anomalies.
The ductus usually closes by 48 hours of age in nearly all term gestation newborns. The prevalence of persistent PDA in term infants is approximately 2 to 8 per 10,000 live births.22 A 2:1 to 3:1 preponderance of PDA cases in female infants is historically based on surveys that include other congenital cardiac anomalies or those that require ductus ligation.23,24 More recent studies showed mixed results for female predominance at term.25–28 Infants who are born at high altitude or experience sustained hypoxia have an increased incidence of PDA. Genetic syndromes and congenital rubella have also been associated with a large proportion of the full-term variety of PDA. Other factors that contribute to PDA in the term infant are given in Table 22-1.
PATHOPHYSIOLOGY
Effects of a Symptomatic PDA on the Cardiopulmonary System
The normal postnatal fall in PVR leads to reversal of blood flow across the ductus in the first few hours after birth.29,30 The adverse effects of a persistent PDA depend on the size of the left-to-right shunt and the capacity for left ventricular compensation. Blood flow across the ductus is primarily influenced by PVR but is also the product of many interacting factors, including the balance between SVR and PVR, myocardial function and systemic blood pressure, the relative size and flow-restrictive characteristics of the PDA, viscosity of the blood, and other factors that regulate the flow of fluids. In the face of falling PVR after birth, freshly oxygenated blood from the aorta is redirected back into the pulmonary bed via the ductus, diverting blood from the systemic circulation and sending excessive blood flow through the lungs into the left atrium and ventricle. Left-to-right shunting through the PDA results in increased blood volume (preload) and excess work for the left heart. In most neonates, maintenance of cardiac output in the face of an increasing left-to-right shunt through the ductus is accomplished by changes in stroke volume rather than heart rate.30–32 The corresponding increase in left ventricular stroke volume is proportional to the size of the shunt. A small PDA may not cause significant hemodynamic effects, and infants with significant or long-standing left-to-right shunt through a large PDA may experience left-sided chamber enlargement that can progress to failure. Right heart or biventricular failure is eventually possible in the presence of pulmonary hypertension due to increased PVR.
The premature heart is more vulnerable to excess volume load from significant left-to-right flow through a PDA, although shunts of up to 4:1 or greater have been documented,33,34 and some studies show no adverse effects on contractility.35 Compared to the adult heart, the fetal or immature myocardium has several features that contribute to enhanced sensitivity to volume overload. Fetal myocardial cells are less compliant than adult cells and have decreased ability to generate active tension. Immature myocardial cells are more dependent on extracellular calcium and the function of L-type calcium channels and have less available calcium via the sacroplasmic reticulum than adult cells. Immature myocytes are disorganized, and their myofibrils are less oriented than adult cells. The shape and dimensions of immature myocytes also contribute to their diminished contractile force and speed. The immature myocardium also has increased water content and an increased mass of noncontractile components (nuclei, mitochondria, cell membranes) compared to adults, which reduce their biomechanical advantage. The immature ventricle is thus less distensible with less-contractile force-generating ability, although individual myofilaments within fetal and adult myocardial cells have similar contractile potential when normalized for these differences.36,37
Increased flow through the preterm PDA distends the pulmonary arteries, with enlargement of the pulmonary veins, left atrium, and left ventricle due to volume overload and the less-favorable characteristics of the immature myocardium. Left heart failure and associated pulmonary edema may ensue. The immature lung is more susceptible to transmural fluid shifts caused by low plasma oncotic pressures in premature infants and increased microvascular pressures from an hsPDA. Increased capillary permeability may be present because of structural impairments and hormonal influences in the immature lung, combined with adverse effects on endothelial integrity in the presence of respiratory distress syndrome (RDS), mechanical ventilation, and inflammatory mediators in the local and systemic circulations. Fluid displacement or leak of plasma proteins into the interstitial and alveolar spaces can interfere with respiratory function and inhibit surfactant function. Lymphatic function in the preterm lung can temporarily compensate for the presence of increased interstitial fluid and protein. Pulmonary edema may be a presenting sign of symptomatic PDA and is common in infants with longer exposure to left-to-right ductus shunting. Improvements in pulmonary function occur following pharmacologic or surgical closure of the PDA. Pulmonary hemorrhage is another possible consequence of pulmonary microvascular fragility, reduced myocardial compliance, and increased back pressure in the presence of an hsPDA. The relationship between surfactant administration and pulmonary hemorrhage is partially explained by the presence of an increasing left-to-right ductus shunt consequent to improved alveolar PaO2 and decreasing PVR, resulting in increasing pulmonary microvascular pressure. The pulmonary response to left-to-right shunting varies according to the degree of shunt and the host response but may be reflected by an increased oxygen requirement, diminished ventilation, and worsening pulmonary compliance, leading to an increased need for respiratory support. Closure of the ductus under these conditions improves pulmonary mechanical properties and reduces the risks for pulmonary hemorrhage, chronic lung disease, and the need for ventilatory support.14,38–42
Effects of Left-to-Right Ductus Shunting on Other Organs
Redistribution of blood flow away from the systemic circulation by a left-to-right ductus shunt also affects the perfusion of other organ systems. Hypotension is a common early manifestation of excess flow through a symptomatic PDA. Diastolic runoff and the compensatory increase in stroke volume are typically able to maintain systolic blood pressure while diastolic pressures decrease, leading to widened pulse pressure.
Preservation of cerebral perfusion and oxygenation in the presence of lower blood pressures are of paramount importance in the perinatal period. PDA has been associated with intraventricular hemorrhage (IVH) and adverse neurodevelopment.43–45 Indomethacin given as prophylaxis (see the section discussing this specifically) decreases the incidence and severity of IVH and as well as any left-to-right ductus shunt that may be present. However, long-term outcomes are not necessarily improved.46–48 Most studies indicated that the presence of an hsPDA has some form of deleterious effect on cerebral blood flow, perfusion, or oxygenation.49–53
The effects of a large PDA on systemic blood flow may be greater for the renal, mesenteric, and other postductal vascular beds than for preductal cerebral perfusion, consistent with a ductal steal phenomenon.54–56 Reversal of blood flow during diastole in the descending aorta is a distinguishing characteristic of an hsPDA, with corresponding hypoperfusion of distal tissues.51,54,57,58 Decreased blood flow or tissue perfusion because of significant left-to-right shunt from an hsPDA is associated with abnormalities of the gut, including feeding intolerance and necrotizing enterocolitis (NEC).55,57,59–65 Spontaneous intestinal perforation (SIP) is distinct from NEC.66–69 The presence of a PDA may be a risk factor for SIP,70–73 but this observation requires further study. Efforts to close or significantly constrict the hsPDA are generally beneficial for the gut.
Reversal of diastolic blood flow in the renal vasculature is a common feature of an hsPDA.54,55,74,75 Oliguria is considered a reflection of hypoperfusion and prerenal insufficiency in association with left-to-right ductus shunting. Treatment with indomethacin poses an additional risk to the underperfused kidney unless systemic flow is restored by closing the ductus. Attempts to negate the renal side effects of indomethacin with furosemide, dopamine, and other agents have been advocated76,77 but with limited success.78–83
Mechanisms Responsible for Normal Postnatal Ductus Closure
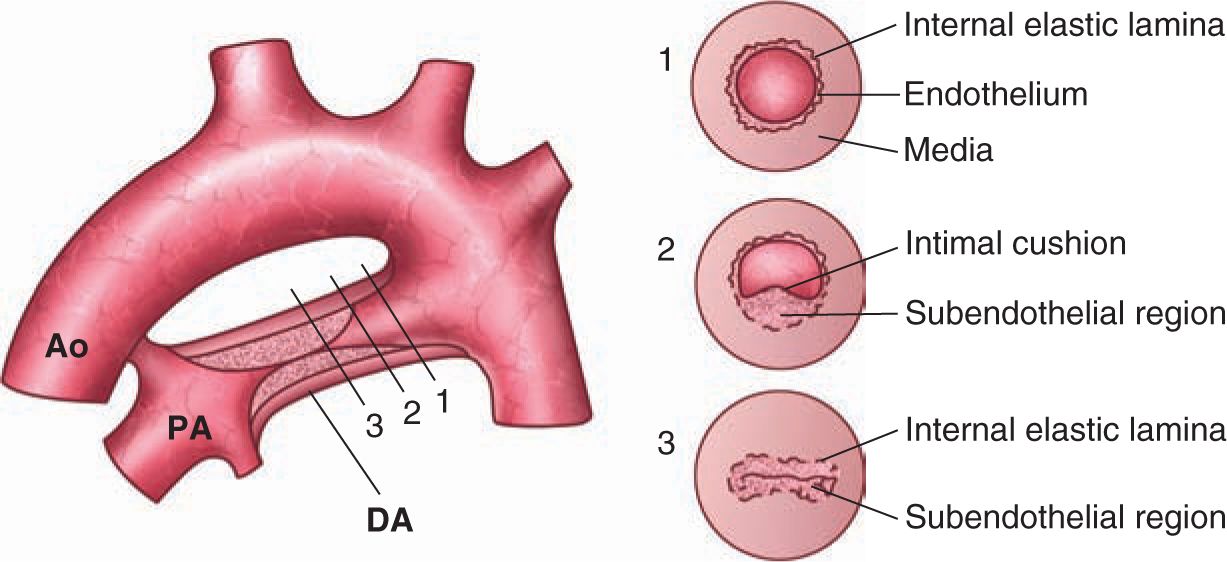
Constriction and permanent closure of the ductus arteriosus is a complex process involving alterations in ductus blood flow, endothelial–smooth muscle interactions, cell trafficking, apoptosis and cell turnover, and unique responses to changes in oxygen and other endocrine and paracrine molecular signals.13,14 Preparation for postnatal ductus constriction begins several weeks prior to birth. Localized areas of subintimal thickening eventually form large mounds, or intimal cushions, that protrude into the ductus lumen4,84–86 (Figure 22-2). Delivery at premature gestation or disruption of this process in large-animal models can result in PDA.87,88
FIGURE 22-2 Schematic structure of the closing ductus arteriosus (DA). In a canine model of DA closure, the following occur: (1) The aortic end of the DA (near the aortic isthmus) still lacks intimal thickening. (2) Intimal thickening starts at the “bottom” of the DA; later stages of this process can be observed toward the pulmonary end. (3) Luminal closure is observed from approximately the middle of the DA to the pulmonary end. Ao, aorta; PA, pulmonary artery. (Adapted from de Reeder et al.84)
After birth, the abrupt increase in oxygen tension with the onset of respiration triggers initial constriction of the postnatal ductus arteriosus (Figure 22-1). Although closure of the ductus is a continuous event that begins with precocious maturation in utero, the postnatal steps that contribute to closure are considered to occur in 2 phases: (1) a functional closure that occurs within hours of birth and is the product of muscular constriction and luminal obstruction and (2) a permanent anatomic occlusion that involves fibromuscular transformation of the ductus into a persisting vascular remnant, the ligamentum arteriosum.14
The initial functional phase is characterized by potent vasoconstriction in response to oxygen. The mechanisms for oxygen-induced ductus constriction are still under investigation but may include the actions of cytochrome P450 (CYP) enzymes and endothelin-1 (ET-1), which acts as a downstream effector of ductus constriction.89–91 A second mechanism involves the presence of oxygen-sensitive, voltage-gated potassium channels.92–94 Here, oxygen exposure triggers a redox cascade in the mitochondria of sensitive cells95 with the production of peroxide or other diffusible mediators, leading to inhibition of Kv channels and disruption of the normal resting potential of the cell membrane. Stimulation of membrane depolarization by alterations in resting membrane potential triggers entry of calcium from the sarcoplasmic reticulum and extracellular sources.96 Calcium influx into ductus smooth muscle cells is primarily mediated through voltage-dependent L-type calcium channels, resulting in vessel constriction,92,97–99 although other calcium channels contribute.100–104
Functional closure also results from prompt withdrawal of the vasodilatory effects of prostaglandins. Although prostaglandins are locally synthesized by both cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2) isoforms in the ductus wall,105–107 the acute decrease in circulating prostaglandin E2 (PGE2) is primarily due to loss of the placenta as a source and increased PGE catabolism by upregulation of prostaglandin dehydrogenase (PGDH) in the lung and peripheral tissues. A postnatal decrease in ductus PGE receptors may also contribute to reduced prostaglandin sensitivity after birth.108–110
In the second phase, permanent anatomic closure of the ductus takes place over a period of several weeks. At term gestation, initial ductus constriction is accompanied by impairment of vasa vasorum perfusion of the thick muscular media, producing circumferential watershed-like regions of hypoxic and ischemic injury in the vessel wall.111,112 Decreased local production of vasodilatory prostaglandins and nitric oxide (NO) permit ongoing contractile forces to maintain ductus closure. Hypoxic signals stimulate local production of growth factors such as vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF-β)113–115 that contribute to a fibrous transformation of the vessel into a ligamentous cord. Permanent remodeling involves localized hypoglycemia and depletion of adenosine triphosphate (ATP) and other nutrients with corresponding energy failure and cell death.116,117 Apoptosis is a prerequisite for permanent ductus remodeling118 and is accompanied by changes in hyaluronic acid, chondroitin sulfate, fibronectin, and other extracellular matrix constituents.85 The presence of a persistent PDA in preterm infants may be due to failure of initial ductus closure or reopening of a previously constricted ductus and failure of vessel remodeling. If the preterm ductus is able to close, the normal process of involution and cell turnover can proceed. However, the preterm ductus with incomplete luminal constriction fails to undergo ATP depletion, nutrient restriction, and energy failure, preventing the necessary cell death and turnover that is required for fibrous transformation.119 Permanent closure of the ductus also involves the recruitment of circulating leukocytes that invade the ductus wall to promote intimal cushion formation.5,7 A role for platelet adhesion in permanent ductus constriction has been proposed, although this observation has not been confirmed in other patient populations.6,120–123
Pharmacologic agents that promote maturation of the ductus also contribute to its postnatal closure. Antenatal steroids are consistently identified as contributing to successful ductus closure.124–127 Postnatal glucocorticoid treatment of BPD is also associated with reduction in the incidence of PDA.128–130 Routine use of postnatal glucocorticoids to promote sensitivity of the ductus to contractile stimuli is not yet supported by clinical trials, although improved use of antenatal steroids should be encouraged. Retinoids have important roles in cardiovascular development and are linked to ductus arteriosus formation and maturation.131–133 However, despite the large number of infants exposed to vitamin A in clinical trials,134 evidence has not emerged linking vitamin A status or treatment with PDA.135,136 Maturation of the ductus may also be enhanced by intrauterine stress and exposure to thyroid hormones or progesterone,137–140 but there are no current therapies to exploit these advantages. Avoidance of drugs and other pharmacologic compounds that contribute to ductus relaxation should also be considered as a conservative measure to prevent symptomatic PDA.12
CLINICAL PRESENTATION AND DIFFERENTIAL DIAGNOSIS
Clinical Presentation of Symptomatic PDA
A persistent PDA becomes symptomatic when left-to-right shunting through the ductus arteriosus becomes of sufficient magnitude to compromise pulmonary or cardiovascular function. For the most part, this occurs in premature infants, not full-term infants. The “symptoms” of a symptomatic PDA relate to pulmonary, cardiac, or systemic findings. Pulmonary symptoms include increasing oxygen requirement, episodes of apnea and bradycardia, ventilator dependence, and pulmonary edema. The pulmonary edema may be severe, resulting in a “white-out” on chest x-ray and requiring substantial increases in ventilatory support. Cardiac symptoms include congestive heart failure; tachycardia, sometimes with a gallop rhythm; increasing heart size; and deterioration of cardiac function. Systemic symptoms reflect inadequate systemic circulatory perfusion due to the diversion of left ventricular output away from the systemic circulation and include feeding intolerance with abdominal distention or NEC, compromised caloric intake, metabolic acidosis, renal insufficiency and even central nervous system depression.141
Infants with symptomatic PDA have heterogeneous findings on physical examination.141 The presenting signs are proportional to the size of the left-to-right shunt (see the section on pathophysiology). Typical features evolve over the first few days of life. The physical findings of symptomatic PDA include a hyperactive precordium, increased pulses and pulse pressure (usually > 30 or 40 mm Hg), a low diastolic pressure (usually lower than 30 mm Hg), loud heart sounds, murmur, decreased perfusion of skin with a capillary refill time longer than 3 s, rales, and diminished air entry into the lungs, causing diminished breath sounds.
The classic “machinery” murmur described in 1898 by Gibson142 occurs due to turbulent flow across the ductus throughout the cardiac cycle. The continuous murmur of a persistent PDA is not always present. A holosystolic murmur is frequently described, although the murmur of a persistent PDA can take many forms.142–144 A “silent” ductus in the presence of a widely patent ductus has been recognized for over 50 years.145–148 Burnard (1958) first recognized that the association between a systolic murmur and cardiomegaly on the chest radiograph of newborn premature infants represented a persistent PDA that sometimes evolved into an overt symptomatic PDA.143
While echocardiography is immensely useful in documenting cardiovascular structure and function, it has not made the physical examination obsolete. Findings such as rales and diminished breath sounds, poor capillary perfusion, decreased level of consciousness, and abdominal distention may help provide clinical evidence that a premature infant with persistent PDA has become symptomatic, requiring consideration of different treatment options.
DIFFERENTIAL DIAGNOSIS
The physical findings of a persistent PDA may be similar to other cardiovascular lesions with a continuous murmur or features of pulmonary congestion and diastolic runoff related to left-to-right shunt. These defects include aortico-pulmonary window; arteriovenous fistulae in coronary, pulmonary, or systemic systems; systemic-to-pulmonary collaterals; absent pulmonary valve syndrome; truncus arteriosus; hemitruncus; ruptured sinus of Valsalva aneurysm; and ventricular septal defect with aortic regurgitation. An innocent venous hum or peripheral pulmonary artery stenosis may have auscultory findings similar to a persistent PDA. Differentiation of these conditions from persistent PDA must be made by Doppler echocardiography.
DIAGNOSTIC TESTS
Radiographic findings include cardiomegaly, presenting as an increase or relative increase in cardiothoracic ratio over time. Pulmonary overcirculation is reflected by increased vascular markings or fullness of the outflow tract on anterior-posterior (A-P) views. Pulmonary infiltrates may obscure these findings. The overall diagnostic accuracy of radiographic findings is poor.149
The electrocardiogram is usually normal. Long-standing persistent PDAs or those with large shunts may show signs of left-sided strain, left ventricular hypertrophy, or left atrial enlargement, but these findings are nondiagnostic. Tachycardia may be present but is not specific for left-to-right ductus shunting.
Echocardiography is the definitive method for diagnosis of persistent PDA. Combined with color Doppler, information can be obtained on the size of the ductus, magnitude and direction of shunt, and assessment of diastolic blood flow disturbance. Effects on cardiac chamber size and flow in other regions can be measured. Two-dimensional echocardiography permits detection of associated abnormalities, including aneurysm of the ductus, coarctation, or right-sided aortic arch. In the presence of high PVR, detection of ductus flow by color Doppler may be more difficult.
Consensus criteria for the echocardiographic diagnosis of an hsPDA are lacking.29,150,151 The functional significance of a persistent PDA is indirectly assessed by values that reflect the degree of left-to-right shunt. Retrograde flow in the descending aorta during diastole represents a large-volume shunt.29,56 However, the use of echocardiography to determine whether a persistent PDA is hemodynamically significant requires more than 1 echocardiographic measurement of a single parameter.29,150,152 Commonly used schemes include combinations of the following criteria to define a moderate-to-large ductus: ductus diameter 1.5 mm or greater, left atrial-to-aortic root (LA/Ao) ratio 1.5 or greater, the presence of reversed diastolic flow in the descending aorta, or left pulmonary artery end-diastolic flow 2.0 m/s or greater.29,153 Cutoff values may be indexed for birth weight (eg, ductus diameter ≥ 1.4 mm/kg).153 Echocardiographic criteria should be used in combination with clinical signs of persistent PDA and interpreted with respect to each individual’s clinical condition, postnatal age, and potential for spontaneous closure. The development of uniform staging criteria that incorporate both clinical and echocardiographic parameters will be beneficial.154
Additional criteria have been developed to assess the physiological impact of a persistent PDA. Serum levels of atrial natriuretic peptide (ANP), brain-type natriuretic peptide (BNP), troponin, endothelin, or combinations of these measurements reflect the degree of cardiac distension or myocardial injury in the presence of an hsPDA.155–161 However, changes in BNP levels are poorly predictive of changes in ductus shunt or response to treatment,162,163 while other serological measurements remain incompletely characterized.
MANAGEMENT
Medical Management (Conservative Approach)
All premature infants who develop a symptomatic PDA should have certain anticongestive measures implemented to control pulmonary edema and to maintain adequate systemic perfusion. These measures can be regarded as intervention to “hold the fort” while waiting for the ductus to close, whether spontaneously (weeks or longer), pharmacologically (hours), or with surgery (until tomorrow). Some refer to this regimen as medical management of symptomatic PDA, and others regard it as less-aggressive or conservative management. These approaches may reduce the risk that a persistent PDA will become symptomatic.
Medical management of symptomatic PDA is based on an understanding of the pathophysiology of this condition (see the section on this topic). Management of a symptomatic PDA is a balancing act that attempts to promote adequate systemic flow without causing excessive pulmonary blood flow. Measures to limit pulmonary blood flow and control pulmonary edema may result in inadequate systemic perfusion. On the other hand, measures to achieve adequate systemic perfusion may result in increased pulmonary blood flow and pulmonary edema.
Pulmonary edema can be controlled by regulating fluid balance, which can be achieved by fluid restriction (as low as 60 mL/kg/d and no more than 130 mL/kg/d) and the judicious use of a diuretic. The effects of diuretic therapy need to be assessed by monitoring body weight, serum sodium, urine output, and physical findings such as rales and peripheral edema. When diuretic therapy is used, it should be given on a “one-time” basis to adjust for prior excessive fluid intake rather than being given as a scheduled dose. Choice of diuretic should take into consideration the potentially beneficial extrarenal effects of furosemide164,165 as well as the possibility that loop diuretics may increase the risks for PDA.166,167
Exposure to excess fluid administration, either through early treatment with volume expanders or by increased daily fluid intake, is associated with development of PDA.168–170 An increased risk for PDA exists for fluid intake of more than 170 mL/kg/d, even after controlling for gestational age and illness severity. For small preterm infants, every 10 mL/kg increase in fluid administration rate on the second or third day of life adds additional risk.171 In nurseries where PDA is an ongoing problem, a restrictive fluid management strategy is likely to result in lower risks for PDA.172
The quickest way to control pulmonary edema is to increase distending airway pressure. This can be done with nasal continuous positive airway pressure (CPAP) or with mechanical ventilation. A periodic “sigh” may also help reclaim lung volume previously lost to pulmonary edema. The efficacy of these efforts to control pulmonary edema will result in improved blood gases and the physical findings of improved air entry and decreased rales. Positive end-expiratory pressure (PEEP) and other forms of distending airway pressure decrease pulmonary edema by increasing intraluminal hydrostatic pressure in the terminal airways. In addition, the use of PEEP decreases the degree of left-to-right shunting through the ductus and improves systemic blood flow.173,174 Improved ventilation and reduced work of breathing help improve tissue perfusion and systemic oxygen delivery and relieve increased demands on left ventricular output that is already burdened by much of the left ventricular output being shunted through the pulmonary circulation.
Adequate oxygenation is important to stimulate and maintain postnatal ductus constriction. Recommendations to lower the oxygen saturation target range in small preterm infants have successfully reduced the frequency and severity of retinopathy of prematurity (ROP) but may adversely affect ductus constriction. Noori et al found a 1.7-fold increased risk for hsPDA after chronic exposure to reduced saturation ranges. The need for ligation of the ductus was unchanged, however, suggesting that pharmacological therapy or spontaneous ductus closure was still possible despite lower levels of oxygenation.175 Increased incidence of symptomatic PDA has not been reported in other studies that target lower saturation limits.176 It is also unclear whether resuscitation of preterm infants with room air or reduced oxygen concentrations will have an impact on ductus closure rates. Any protocol to target the risks of oxygen injury will need to be balanced against the risks for symptomatic PDA and other neonatal morbidities.
Systemic perfusion must be supported in patients with symptomatic PDA. An increase in the capacity of the left heart and pulmonary vascular bed that occurs in symptomatic PDA may require blood transfusions with packed red cells to maintain an adequate blood volume and an adequate hematocrit for oxygen transport to the systemic circulation. Increasing the hematocrit increases PVR more than SVR and as a result reduces left-to-right shunt177 and provides improved forward systemic flow and oxygen-carrying capacity to peripheral tissues. The left ventricle must supply an adequate output to perfuse the systemic circulation in the face of the additional left ventricular output diverted back across the ductus and into the pulmonary circulation. For this reason, care should be taken to reduce other demands on the left ventricle. Under these circumstances, it may become necessary to support myocardial performance with inotropes such as dobutamine. Drugs such as dopamine, which increase SVR, may shift cardiac output from the systemic circulation and into the lungs, thereby aggravating pulmonary edema.
Less-Aggressive Intervention (to Close or Not to Close)
Measures that optimize fluid and respiratory management, hemodynamic status, and hematologic parameters may minimize the complications of symptomatic PDA, but they do not necessarily obviate the need for closure of the ductus. Recommendations to pursue ductus closure aggressively have been traditionally based on evidence that prolonged exposure to the complications of a symptomatic PDA leads to substantial morbidity, if not mortality.38,39,178–180 The comorbidities associated with a symptomatic PDA include an increased need for prolonged respiratory support related to ventilatory failure and BPD, as well as complications such as recurrent sepsis, NEC, feeding intolerance requiring prolonged use of total parenteral nutrition (TPN), metabolic bone disease, and others.
On the other hand, recent studies questioned whether the association between these complications and prolonged ductus shunting is an independent effect that can be decreased by ductus closure. For example, a systematic review of randomized trials181,182 cautioned that infants undergoing ductus closure were no better off than their declared controls with regard to survival, BPD, NEC, or ROP. In addition, outcomes have been no better in infants undergoing ductus closure prior to development of symptomatic PDA.182–184 However, these kinds of studies must be carefully interpreted because some of the “control” infants also underwent backup intervention (surgical ligation or nonsteroidal anti-inflammatory drug [NSAID] treatment) to close the ductus.
Routine closure of the symptomatic PDA has been challenged as the standard of care184,185 for several reasons. First, it is becoming more widely accepted that the vast majority of infants with symptomatic PDA will eventually experience spontaneous closure of the ductus, even when there has been a recurrence of symptomatic PDA following unsuccessful treatment with NSAIDs. Second, the complications related to either surgical ligation or treatment with NSAIDs may outweigh any beneficial effect of attaining ductus closure soon after the PDA of an infant becomes symptomatic. These reasons are the basis of an increased interest in managing infants with symptomatic PDA medically while waiting for spontaneous closure of the ductus, a paradigm that is already practiced in some nurseries, as reflected by the content of surveys and large registries of premature infants.186–192 It has even been suggested that active interventions to close the ductus should be limited to clinical trials.185,193–195 In fact, the lack of information comparing the outcome of this practice of withholding intervention to close the ductus underscores the need for a properly controlled randomized trial to address this question.196
There is accumulating evidence that a patent ductus will eventually close spontaneously even in very immature infants. In a retrospective assessment of medical management without intervention to close the ductus, Herrman et al observed spontaneous ductus closure in the majority of very low birth weight (VLBW) infants who had left-to-right ductus shunting at the time of discharge. Spontaneous closure of small, persistent PDA occurred as late as 60–76 weeks post-menstrual age (PMA).11 Other investigators also have noted spontaneous ductus closure in small preterm infants.9,10,185,187
Until comparisons of long-term outcomes have been made between medical management with and without intervention to close the ductus, it will be difficult, if not impossible, to identify the group of infants who are most likely to benefit from active intervention to close the ductus without incurring unacceptable complications, short or long term. In the meantime, withholding intervention to close a symptomatic PDA should be limited to patients whose systemic circulation is adequately perfused and whose ductus is predicted to close spontaneously sooner rather than later, keeping in mind that even these patients may benefit from ductus closure.
Prophylactic (Preemptive) Indomethacin
Prophylactic indomethacin has consistently been shown to reduce the incidence of symptomatic PDA and the need for ductus ligation, along with a reduction in severe IVH (grades 3 and 4) and pulmonary hemorrhage.42,46,197,198 A prophylactic approach (<24 hours of age) to preempt symptomatic PDA with the administration of indomethacin within the first 24 hours after birth199–201 came into consideration at the time when indomethacin was found to be an effective approach for prevention of IVH.202–208 The beneficial effects of indomethacin for IVH prevention do not appear to be related to ductus closure.203,204 However, the observation that prophylactic indomethacin treatment reduced the incidence of severe IVH legitimized its widespread adoption as a management strategy of symptomatic PDA as well as a means to prevent severe IVH.46,209
Even though prophylactic indomethacin has been shown to prevent symptomatic PDA, reduce the need for ductus ligation, and prevent severe IVH, it is disappointing that its use does not reduce the incidence of BPD or improve survival without neurosensory impairment at 18 months of age or improve other long-term outcomes of interest for preterm infants.46,181,182,198,210 On the other hand, common complications of extreme prematurity such as NEC or gastrointestinal perforation, ROP, chronic lung disease, or cerebral white matter injury were not observed. In addition, it was estimated that only 20 infants would need to be treated with prophylactic indomethacin to avert the need to ligate the ductus of 1 high-risk infant. Given the favorable benefit-to-risk ratio of prophylactic indomethacin, its use would be appropriate in patient populations that experience higher-than-expected rates of severe IVH, pulmonary hemorrhage, or need for surgical ligation.42,197,211 Another beneficial feature of prophylactic indomethacin is that, given the prolonged clearance in the first few days of life, it is possible that a single 0.2-mg/kg dose of indomethacin in the first 24 hours of life can be used as an effective prophylaxis strategy with limited exposure to indomethacin toxicities.201
Ibuprofen has potential benefits for closure of a persistent PDA and prevention of ligation212 but is no longer considered an alternative for prophylaxis of a symptomatic PDA due to unforeseen risks of treatment.213–217 A meta-analysis of 7 trials (931 infants) concluded that prophylactic ibuprofen effectively reduced the incidence of symptomatic PDA, the need for rescue treatment with NSAIDs, and the need for surgical ligation, but exposed many infants to a drug with concerning renal and gastrointestinal side effects without conferring important short-term benefits.218
Pharmacologic Ductus Closure with NSAIDs
Current pharmacological approaches to stimulate ductus closure are based on inhibition of vasodilatory forces (PGE2) rather than triggers for vascular smooth muscle contraction because vasoconstrictive agents (other than oxygen) that specifically target the ductus are not yet available. An important role for prostaglandins in ductus relaxation was suspected when fetal ductus constriction occurred in pregnant women who were treated with salicylates or indomethacin.219,220 Prostaglandins or their inhibitors were concurrently found to have potent effects on ductus tone in various animal models.221–224 In 1975, prolongation of human ductus patency by PGE infusion was noted.225 In 1976, two clinical trials established the effectiveness of indomethacin for ductus closure in premature infants.226, 227 Ibuprofen was found to have similar effects on ductus closure soon thereafter,228,229 but until 1995, clinical trials in preterm infants were not reported.230–232
Indomethacin and ibuprofen, the most commonly used NSAIDs for pharmacologic constriction of the ductus, inhibit the prostaglandin synthase (or cyclooxygenase) enzymes, COX-1 and COX-2. NSAIDs exert their effects by binding inside the cyclooxygenase channel to prevent arachidonic acid catabolism and formation of prostaglandins.233 Indomethacin inhibits both COX isoforms in a competitive, time-dependent, slowly reversible manner. The COX-1 and COX-2 enzymes are both present in the human ductus,107 although inhibition of prostaglandin synthesis in peripheral tissues might also be important for ductus closure.234–236 Indomethacin and ibuprofen inhibit COX-1-derived prostaglandins, which are essential for homeostatic functions in the gut, kidney, brain, and elsewhere, leading to the potential toxicities of these drugs and other nonselective NSAIDs.
There are several recommendations for the timing of initial indomethacin treatment. Optimal dosing remains the subject of ongoing debate.237,238,239–241 Clinical trials designed to answer questions about the timing of pharmacologic treatment all suffer from lack of true controls because these trials have used crossover designs and provision for backup treatment.242 A meta-analysis showed that early treatment of a symptomatic PDA (1–3 days of age) compared to treatment at a later stage (7–10 days of age) was effective for ductus closure and reduced the need for ligation, along with less pulmonary morbidity and reduced association with NEC.197 A subsequent prospective trial of early (day 3) vs late (day 7) indomethacin treatment in ventilator-dependent infants also found improved ductus closure rates but did not confirm the pulmonary benefits while raising concerns for renal compromise and more severe adverse outcomes.243 A delay in treatment to close the ductus is a reasonable choice because early treatment of a symptomatic PDA might unnecessarily expose infants to NSAIDs who would otherwise undergo spontaneous ductus closure without the drug. On the other hand, the beneficial effects realized with prophylactic ductus closure may be a justification for early treatment of symptomatic PDA. Moreover, NSAIDs lose their effectiveness with advancing postnatal age.244–248 Taking all of these factors into consideration, the benefit/risk ratio of early treatment appears to justify beginning a pharmacologic agent to close the ductus as soon as the diagnosis of symptomatic PDA is made. Medical management measures discussed previously in this chapter should also be considered as soon as the diagnosis of left-to-right ductus shunting is established.
The FDA-approved package insert for indomethacin calls for the intravenous administration of 3 doses at 12-to 24-hour intervals with an initial dose of 0.2 mg/kg and second and third doses of 0.1, 0.2, or 0.25 mg/kg, depending on the postnatal age (<48 hours, 2–7 days, or > 7days) that the first dose is administered. Administration over a minimum of 20 to 30 minutes is recommended. Another commonly used regimen is to administer 0.2 mg/kg as the initial dose; then, for infants younger than 28 weeks, 0.1-mg/kg doses are given at 12 and 36 hours after the first dose; for infants older than 28 weeks, 0.2 mg/kg is given for each dose.8
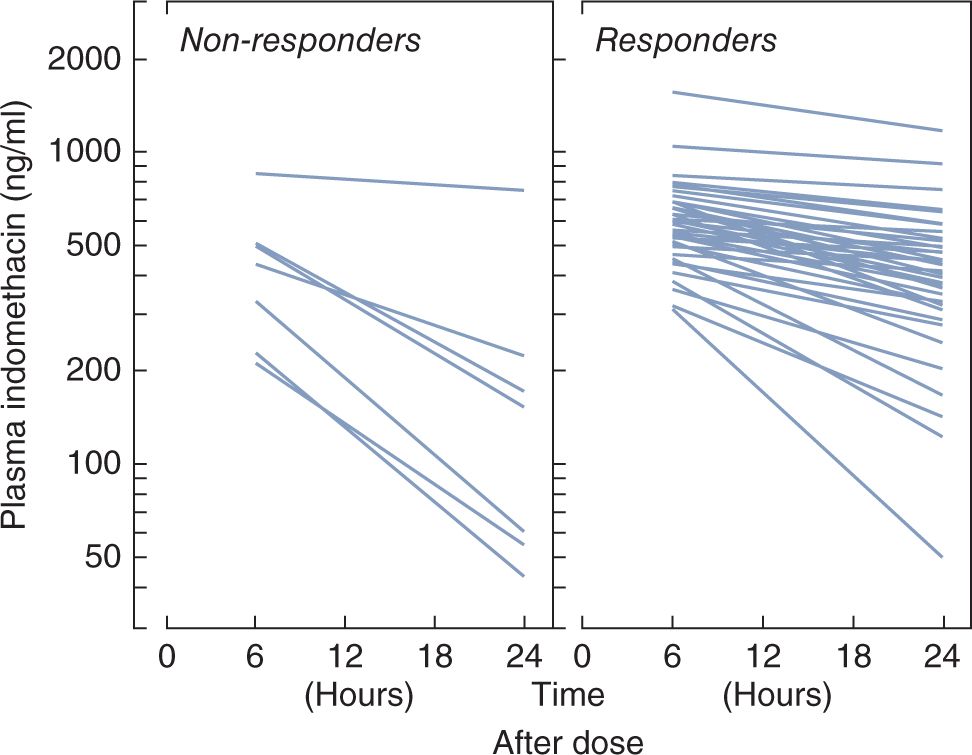
Plasma clearance is dependent on postnatal age, resulting in a long half-life on the first day after birth but more rapid elimination by the end of the first week.249–253 Pharmacokinetic studies have shown that efficacy of indomethacin to close the ductus requires a threshold plasma level of 200 ng/mL at 24 hours after infusion251 (Figure 22-3). Although achievement of threshold indomethacin concentrations has been emphasized,248,253–255 a sufficiently quick turnaround time for assays is generally not available on a routine basis to adjust dosing based on plasma levels.
FIGURE 22-3 Concentration-dependent response of the patent ductus arteriosus (PDA) to indomethacin. Plasma indomethacin levels between 6 and 24 hours after the administration of 0.2 mg/kg of the drug intravenously to premature infants with symptomatic PDA. Each line is derived from the regression analysis of drug levels from 6 hours to as long as 5 days after administration. In 6 of 7 studies not associated with a major constrictive effect (panel on left), the plasma level of indomethacin at 24 hours was less than 250 ng/mL. The corresponding level was greater than 250 ng/mL in 28 of 32 studies that were accompanied by a major constrictive effect. (From Cotton RB. Patency of the ductus arteriosus—its etiologic and pathogenetic relationship in the respiratory distress syndrome. In: Stern L, ed. Respiratory Distress Syndrome. New York, NY: Grune & Stratton; 1984.)
Reopening of the ductus after successful closure with indomethacin may occur and is partially related to immaturity and incomplete luminal constriction.256,257 For this reason, additional doses of the drug may be given based on mid- or posttreatment echocardiographic Doppler examination because ongoing flow through the ductus lumen is associated with treatment failure.8,257–260
A variety of dosing protocols have been studied in an effort to reduce treatment failure and late recurrence of symptomatic PDA. Improved efficacy has been reported with prolonged low-dose administration,259,261–263 repeated short (3-day) courses,264 stepwise advancement up to a 1-mg/kg dose,265 and continuous infusion over 36 hours.266,267 These studies showed no excess adverse effects over standard dosing. The continuous infusion protocol was noteworthy in that abnormalities in cerebral, renal, and mesenteric perfusion were alleviated. Although a continuous infusion of indomethacin over 36 hours is an appealing protocol in view of the amelioration of peripheral vascular effects, additional studies and long-term follow-up are required before this approach can be recommended for routine clinical practice.237,268,269 Moreover, protocols incorporating prolonged indomethacin dosing or more than 2 repeat courses may be harmful.238,264,270,271 One multicenter study of low- vs high-dosage strategies was associated with severe ROP without affecting ductus closure rates.272
In addition to recommending the dose of indomethacin to treat symptomatic PDA, the package insert also strongly advises consideration of ductus ligation when there is failure to achieve ductus closure after a second course of the drug. This is reasonable advice in view of the increasing risk of adverse effects, such as renal failure and continued feeding intolerance, that will only be prolonged by the likely failure of subsequent courses to result in ductus closure. In this scenario, the most vexing side effect of indomethacin in the treatment of symptomatic PDA is postponement of a decision regarding ductus ligation.
Ibuprofen is another effective NSAID to constrict a persistent PDA.230–232,273 Ibuprofen is a competitive, rapidly reversible inhibitor that nonselectively blocks both COX-1 and COX-2. Ibuprofen-lysine is preferable to formulations containing tromethamine (THAM).274 Standard ibuprofen dosing is 10 mg/kg on day 1, followed by 5-mg/kg doses 24 and 48 hours later.231,232 Doses up to 20, 10, and 10 mg/kg on the same schedule have been given with similar success.275 Taking maturation into account, pharmacology studies suggested that drug dosing can be increased with advancing postnatal age (10-5-5 mg/kg at less than 70 hours; 14-7-7 mg/kg from 70 to 108 hours; and 18-9-9 mg/kg from 108 to 180 hours of age).276 Similar to indomethacin,277 successful ductus closure is less likely when a subsequent course of ibuprofen is required.278–280 Two separate meta-analyses came to different conclusions on whether the risks and benefits significantly favored ibuprofen over indomethacin.281,282 A more recent systematic review that evaluated 20 randomized controlled trials (n = 1092 infants) comparing ibuprofen to indomethacin found that both drugs were equally efficacious for ductus closure. There were no statistically significant differences in mortality, reopening of the ductus arteriosus, surgical ligation, duration of ventilator support, IVH, cerebral white matter injury, time to full enteral feeds, gastrointestinal bleeding, ROP, sepsis, or duration of hospital stay. Ibuprofen was associated with a lower incidence of NEC and had fewer adverse effects on renal function (urine output, creatinine levels).283 The modest, at best, therapeutic advantage of ibuprofen is tempered by rare reports of pulmonary hypertensive crises that were originally attributed to ibuprofen-THAM but have also occurred with the lysine formulation.217 Because ibuprofen is 95% to 99% protein bound, concerns also exist regarding bilirubin displacement and the potential for kernicterus.284,285 Retrospective studies showed an association of ibuprofen with higher serum levels or longer need for phototherapy,286,287 but most clinical studies showed that these risks are limited.274,288–290
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree