Parvovirus B19 Infection
INTRODUCTION
Human parvovirus B19, a single-stranded DNA virus, is a member of the erythrovirus genus within the Parvoviridae family and the only erythrovirus that is a pathogen in humans.1 Human parvovirus was first identified by electron microscopy in 1975 and was associated with clinical disease approximately a decade later.2 It is the etiologic organism that manifests clinically as erythema infectiosum (fifth disease), a common childhood viral exanthem. In immunocompetent adults, infection with B19 is generally asymptomatic or mild. It is most commonly transmitted through respiratory droplets, and primary B19 infection in pregnant women can also result in vertical transmission to the fetus. Although most commonly asymptomatic for both mother and fetus, in some cases primary or acute infection in pregnancy may lead to adverse perinatal outcomes, including spontaneous abortion, hydrops fetalis, and fetal demise.
EPIDEMIOLOGY
Parvovirus B19 infection occurs worldwide, and cases may be sporadic or may occur in clustered outbreaks and even as epidemics.3,4 The infection is easily transmitted from person to person via the respiratory route. In the United States, B19 infection occurs more frequently between late winter and early summer. Cycles of local epidemics have also been reported, with case numbers peaking approximately every 4 years.5,6 Hematogenous transmission can also occur from administration of blood or blood products containing B19. Individuals requiring regular infusions of blood products that are made from large plasma pools are at greater risk for acquiring the virus compared to those individuals receiving single units.7
Seroprevalence rates of parvovirus B19 vary based on age and geographic location. The percentage of people with measurable levels of B19-specific immunoglobulin (Ig) G increases with increasing age, with most individuals becoming infected during their school years. Seroprevalence is approximately 15% of preschool children, 50% in adults, and 85% in the elderly3,8 and is high (82%–89%) in women who live or work with young children.9 The secondary attack rate for household contacts may be as high as 50%.3 Immunity is lifelong in immunocompetent individuals.10
Nonimmune pregnant women have the same susceptibility to B19 infection as other immunocompetent adults. In the United States, 50%–75% of women in the reproductive age group are immune and demonstrate antibodies to human parvovirus B19.11–13 Therefore, approximately 30%–50% of pregnant women are susceptible to B19 infection, which then places their fetus at risk of infection. The incidence of acute B19 infection in pregnancy may range from 1% to 3.8% and may be as high as 10% during epidemics.14–17
PATHOPHYSIOLOGY
Parvoviridae are small, nonenveloped DNA viruses that infect a variety of animals, usually in a species-specific fashion; only parvovirus B19 is known to cause disease in humans. The virus replicates in erythroid progenitor cells (late erythroid cell precursors and burst-forming erythroid progenitors) of the bone marrow and blood leading to inhibition of erythropoiesis, which can result in symptoms of anemia. B19 also may stimulate a cellular process initiating apoptosis; this characteristic may attribute to the minimal inflammatory response noted in infected tissues.18
The pathophysiology of adverse fetal effects is primarily a result of the anemia caused by destruction of red blood cell precursors.19 Cardiac failure leading to fetal hydrops and fetal death may result from severe fetal anemia but can also be due to hypoalbuminemia, hepatitis, myocarditis, and placental inflammation.20,21 Risks to the fetus are higher in the second trimester than the third for a number of reasons. Transmission across the placenta is higher in the second trimester because of the far greater second vs third trimester expression of the globoside receptor in the trophoblast that allows transplacental transfer of B19.22 In addition, in the second trimester the fetus undergoes a rapid increase in red cell mass while experiencing a significant reduction in red cell life span. These factors combine to make the second-trimester fetus particularly vulnerable to the impact of B19 infection on erythropoiesis.3
The humoral immune system is responsible for the development of the antibody response, which results in viral clearance and subsequent protection from disease. Parvovirus B19 infection also elicits an inflammatory cell-mediated immune response, including the production of tumor necrosis factor (TNF) α, interferon (IFN) γ, and interleukin (IL) 2 or IL-6. CD8 T cells may also play a role in the control of parvovirus, as a significant CD8 T-cell response may be maintained and eventually increased over several months after the resolution of illness.23
Clinical Features
Parvovirus may be transmitted via respiratory, hematogenous, and vertical routes, with respiratory transmission the most common. Although B19 is primarily associated with nonrespiratory symptoms, it is transmitted through close person-to-person contact, fomites, and respiratory secretions or saliva. Young children are the main source of respiratory acquired B19. Therefore, individuals at highest risk for acquiring the virus include household contacts, day care workers, and those in a crowded environment. Nonimmune pregnant women with young children at home are therefore at high risk of becoming infected. Health care workers may also be at increased risk of transmission depending on the degree of contact that they have with infected patients.
The incubation period for human parvovirus B19 infection usually ranges from 4 to 14 days but may last up to 3 weeks. Infected individuals are most contagious during the phase of active viral replication. Viremia occurs approximately 7 to 10 days after the exposure and usually lasts for 5 days.24 Parvovirus B19-specific IgM antibodies are detected at days 10 through 12 and can persist for 3–4 months or longer; specific IgG antibodies are detectable approximately 2 weeks following infection and persist lifelong.
The clinical presentation associated with B19 infection may vary. Manifestations may depend on the infected individual’s age, as well as hematologic and immunologic status. Most immunocompetent individuals are asymptomatic or only mildly ill. Approximately 25% of infected individuals will be completely asymptomatic during their infection; 50% will have only nonspecific flu-like symptoms of malaise, muscle pain, and fever. The remaining 25% of infected individuals will present with the classic symptoms of B19 infection, including rash, arthralgia, or edema.
In the first week after exposure, intense viremia may be accompanied by a nonspecific flu-like illness, including fever, malaise, myalgia, coryza, headache, and pruritus. Hematologic abnormalities, including reticulocytopenia, reduced hemoglobin concentration, leukopenia, or thrombocytopenia may also be present. In the following week, the more specific symptoms of rash or arthralgia occur. The rash in adults is less characteristic than the rash seen in children. During the second phase of parvovirus infection, which generally occurs 2 weeks after infection, a person may develop additional symptoms or signs (eg, arthralgia, arthritis, or an exanthem) of B19 infection. This is also the phase in which B19-specific antibody production and B19 antigen-antibody immune complex formations occur. Individuals are generally no longer infectious when exhibiting these clinical characteristics.
Maternal Infection and Fetal Disease
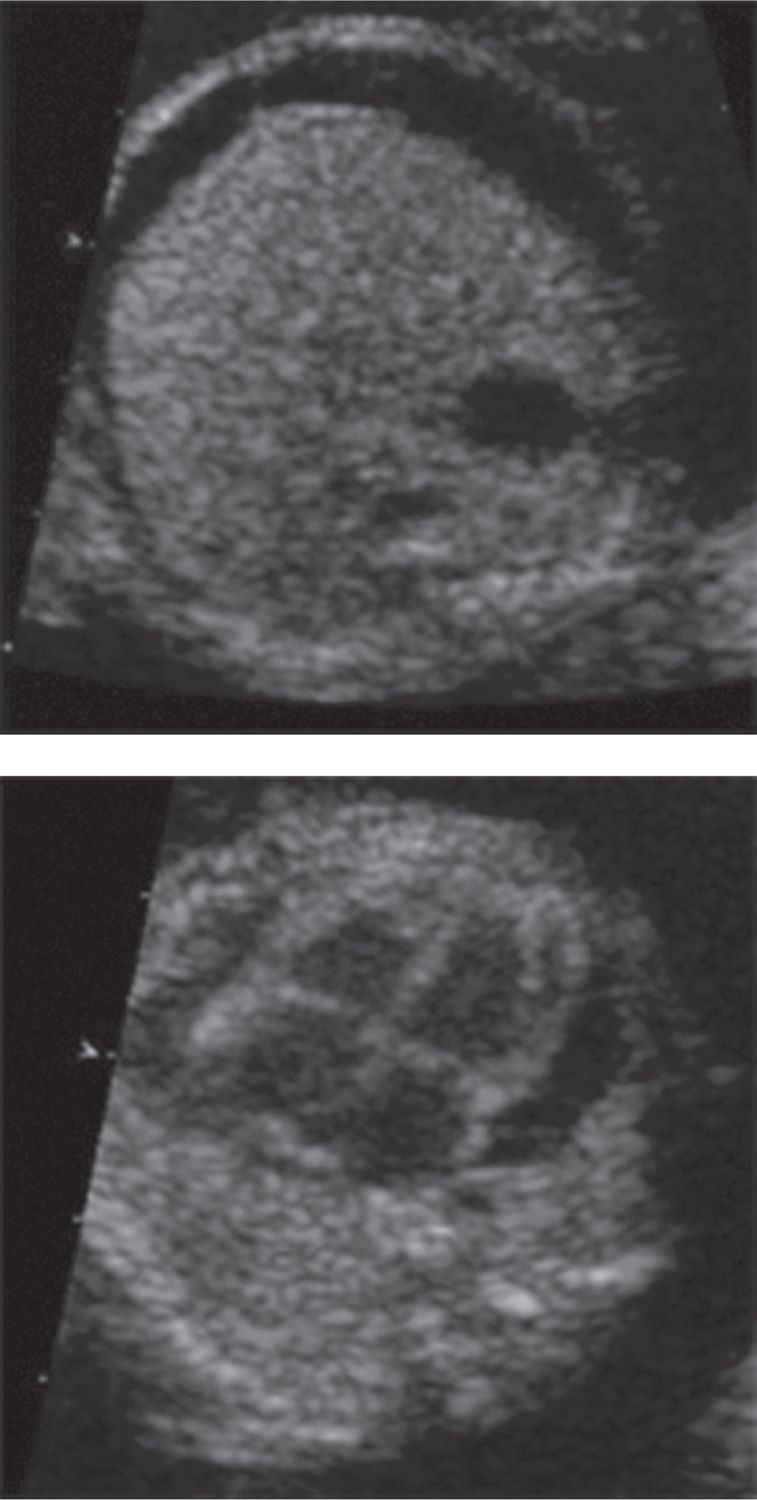
Vertical transmission to the fetus can occur if a nonimmune woman becomes infected during her pregnancy. Although 30%–50% of pregnant women are susceptible, a relatively small percentage of them will become infected with the virus.11–13 Of those that do become infected, as many as half will be asymptomatic, so infection may not be recognized.21 If maternal infection does occur, there is a reported 30% rate of transmission to the fetus,25,26 although in the majority of cases the fetus is unaffected. The chance of fetal loss in cases of fetal infection is reported to be about 5%–10%.15–17 Overall, B19 infection is thought to account for 8%–20% of fetal nonimmune hydrops (Figure 56-1).22,26
FIGURE 56-1 Ultrasound images of fetus with hydrops fetalis due to parvovirus B19 infections. A, Cross section through the fetal abdomen demonstrating fetal ascites. B, Cross section through the fetal chest demonstrating a pericardial effusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree