Operative Vaginal Delivery
James A. Bofill
James N. Martin Jr.
Most likely, there has always been a desire of obstetric attendants to safely grasp the fetal head in order to accelerate delivery of the infant and shorten a woman’s difficult labor. The story of the use of obstetric instruments to facilitate delivery—either forceps or vacuum devices—is both colorful and unique within the history of medicine. Properly used obstetric forceps may have saved more lives, both infant and maternal, than any other instrument devised by physicians. The heyday of obstetrical forceps use was in the early 20th century, when nearly half of deliveries were accomplished by their means. Obstetricians of that time considered that the obstetric forceps, with liberal use of episiotomy, protected the maternal genital tract and prevented more extensive injury. Similarly, the forceps were considered to provide the fetus, especially the preterm fetus, with a “helmet” that prevented rapid changes in cranial pressure during delivery. These notions have been discarded by the rigors of modern clinical studies. Early in the 21st century, the practice of operative vaginal delivery is considered by some obstetricians to be anachronistic, a “dying” art and not something to be mastered or practiced.
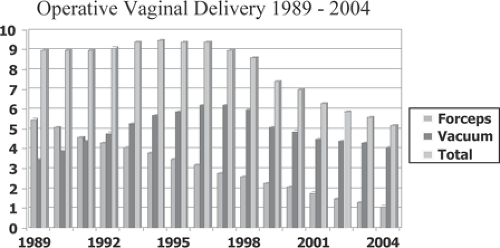
The total rate of operative vaginal delivery in 2004, the last year for which complete data are available, was only 5.2% in the United States. Obstetric forceps were used in 1.1% of deliveries, and 4.1% were delivered via the vacuum extractor (Fig. 26.1). Since 1989, there has been an 80% decrease in the frequency of operative forceps use, steadily falling over 15 years from 5.5% in 1989 to 1.1% in 2004. During this time, the frequency of vacuum extractor procedures peaked in 1997 at 6.2%, thereafter decreasing by one third to a level of 4.1% in 2004.
There are several possible causes that relate to diminishing operative vaginal delivery usage in the United States. Certainly, the crisis of litigation in the field of obstetrics is one cause. Yeomans and Hankins perceive a vicious cycle whereby (a) concern about litigation lessens usage and reduces teaching about obstetric forceps and vacuum in obstetrics and gynecology (OBGYN) residency training programs; (b) physician training and confidence with operative vaginal delivery is diminished; (c) usage of operative vaginal delivery decreases further, increasing the probability of poor clinical outcomes when used; and (d) poor clinical outcomes often lead to litigation in our society.
Utilization of operative vaginal delivery was very important to physicians during times when cesarean delivery was considered to present a significant health risk to an obstetric patient or its low frequency of use was considered to be a quality assurance barometer—both concerns were a driving force in minimizing abdominal delivery while encouraging vaginal delivery. Currently, there is a paradigm shift away from the former line of reasoning—cesarean delivery has become a very safe procedure, and a physician’s personal cesarean delivery rate is rarely used today as a reflector of quality care. Hence, the rate of operative vaginal delivery in the United States has been declining as the frequency of primary and repeat cesarean delivery has been climbing—from 20.7% in 1996 to a record high of 29.1% in 2004. During the same time interval, trial of labor to accomplish vaginal birth after cesarean (VBAC) has decreased 67%. Not only has cesarean delivery become the most common surgical procedure in modern medicine, it has for the first time to any significant degree been considered appropriate for elective reasons when traditional obstetric indicators are absent.
Nevertheless, teaching and clinical experience with some types of operative vaginal delivery are considered an integral part of contemporary residency training in obstetrics. Although very challenging instrumental deliveries are
seldom undertaken when cesarean delivery is the better option, most physicians continue to learn outlet and low-forceps operations with some rotation as well as the proper techniques for vacuum extraction.
seldom undertaken when cesarean delivery is the better option, most physicians continue to learn outlet and low-forceps operations with some rotation as well as the proper techniques for vacuum extraction.
Delivery with the Vacuum Extractor
The vacuum extractor has a history nearly as colorful as the obstetric forceps. Its early development and use have been described previously. Since inception and the earliest stages of development, the vacuum extractor has been considered an easier instrument to use than the obstetric forceps. For example, Arnott in 1829 considered that the vacuum extractor was “a substitute for steel forceps in the hands of men who are deficient in manual dexterity, whether from inexperience or natural ineptitude.” This, of course, is inaccurate in today’s practice. The history of the forceps and vacuum extractor are intertwined, a fact that is best personified by James Young Simpson of Scotland, who performed the first well-documented series of successful vacuum deliveries and also designed the popular obstetric forceps that bear his name. Simpson’s report was met with skepticism and caution. The vacuum instrument was essentially discarded, probably due to lack of appropriate materials for the construction of a durable device.
The modern era of vacuum extraction began in 1954, when Malmstrom introduced a metal cup that he termed the vacuum extractor. The original Malmstrom vacuum extractor consisted of a mushroom-shaped stainless steel cup with a smooth, inverted lip and an outside diameter of 60 mm. Unlike current obstetric practice, smaller Malmstrom devices were also commonly placed prior to full cervical dilatation late in the first stage of labor in order to overcome dystocia and hopefully accelerate delivery. The vacuum extractor was considered to be a simpler instrument as well as one that required less anesthesia than the obstetric forceps.
Vacuum-assisted vaginal delivery did not become popular in the United States in part due the entrenched place of obstetric forceps and reports of fetal complications with vacuum delivery. The vacuum device often was associated with scalp abrasions, and the edematous area of the scalp raised by the device (the chignon) was cosmetically unpleasant. Several reports of life-threatening neonatal complications after vacuum extraction were published. Many of these patients may not have been appropriate candidates for vacuum delivery. Nevertheless, these reports of poor neonatal outcomes led to refinements in technique. Wider’s group refined the vacuum procedure in the 1960s and limited the instrument’s use to the second stage of labor. To minimize neonatal morbidity, it was recommended that vacuum application to the fetal head should not exceed 15 minutes. Using the Bird modification of the Malmstrom device and comparing vacuum with forceps and cesarean delivery, Greis and colleagues demonstrated that neither perinatal mortality nor serious neonatal injury were more likely with vacuum extraction when the procedure time was limited to 15 minutes and/or two sudden disengagements (“pop-offs”). Preferential use of vacuum over forceps was reported for managing challenging deliveries. In that retrospective study, it was noted that 60% of vacuum cases were initiated in the midpelvis compared with only 9% of forceps deliveries. Malpositioning of the fetal head as occiput posterior (OP) or occiput transverse (OT) was seen in 81% of vacuum cases as compared with only 9% of forceps cases.
The utilization of the vacuum for operative vaginal delivery has surpassed the forceps in the United States, which traditionally has been considered a “forceps nation.” Findings from surveys undertaken in the mid 1990s revealed that vacuum delivery techniques were taught in most U.S. OBGYN residency training programs and frequently were practiced by U.S. obstetricians. Another survey found that residency program directors expected their OBGYN residents to be proficient with forceps and vacuum extraction for outlet and low-pelvic deliveries (with or without rotation). However, very challenging operative vaginal deliveries today probably reside more often in the vacuum domain since only 38% of the residency program directors expected their graduates to be proficient with midforceps procedures, while 69% expected proficiency with midpelvic vacuum extractions.
The age of the obstetrician is clearly important and relevant to choice of instrument for operative vaginal delivery. Recently trained obstetricians more commonly choose vacuum extraction over forceps, especially for the challenging case. Geography plays a role as well, since data from the National Center for Health Statistics and the National Hospital Discharge Survey demonstrated that the frequency of vacuum extractor use surpassed that of the forceps in the western United States in 1988, in the Northeast in 1990, and in the Midwest in 1991. Forceps procedures continued to outpace vacuum procedures in the southern United States during the study period until there was near parity between the two instruments in 1994, which was the last year of the study.
With increasing use of operative vacuum delivery, there also was a significant increase in the number of reported neonatal complications. On May 21, 1998, the U.S. Food and Drug Administration (FDA) distributed a public health advisory entitled Need for Caution When using Vacuum-Assisted Delivery Devices. The advisory included reports of 12 neonatal deaths and 9 serious injuries that were received during the preceding 4 years (about 5 events per year) in newborns for whom vacuum devices were used to accomplish delivery. The FDA was concerned that some health care professionals who were using vacuum devices for delivery might not be fully aware of the possibility of life-threatening neonatal complications such as subgaleal hematoma or intracranial hemorrhage. The FDA’s main points were as follows:
The vacuum extractor should be used only when a specific indication exists.
The operator should be versed in its use and aware of indications, contraindications, and precautions.
The operator should follow manufacturer recommendations regarding cup placement, vacuum strength, cumulative duration of applications, and number of extraction attempts.
Rocking movements or torque should not be applied to the device, and only steady traction in the axis line of the birth canal should be used.
Neonatal staff should be educated about the specific complications that can occur in association with the use of vacuum devices to accomplish delivery.
Adverse events and complications associated with vacuum-assisting devices should be reported to the FDA under the auspices of the Safe Medical Devices Act of 1990.
The American College of Obstetricians and Gynecologists (ACOG) responded to the release of the FDA advisory with a committee opinion, which noted that an average of 228,354 vacuum deliveries occurred annually during the period of time covered by the FDA advisory—the equivalent of one adverse event for every 45,455 vacuum deliveries. Despite the infrequent occurrence of fetal injury, the ACOG committee recommended that all clinicians using vacuum devices for delivery should be familiar with the indications for the use of these devices and be properly educated in their use. This committee strongly recommended the continued use of vacuum-assisted vaginal delivery devices in appropriate clinical settings. In the first 6 months following release of the FDA advisory, Ross and colleagues observed a 22-fold increase in the reporting of adverse events associated with vacuum delivery, including 10 neonatal deaths, 30 life-threatening events, 12 nonlife-threatening events, and 3 equipment failures. The increase in reports was ascribed to better compliance with reporting, increased awareness of the potential for fetal injury with vacuum extraction, and increased use of the vacuum device.
In response to four neonatal deaths secondary to subgaleal hemorrhage, the Health Protection Branch of Canada issued their own warning on February 23, 1999. The recommendations issued were directly in line with those of the FDA.
Types of Vacuum Extractors: Stainless Steel Devices
Stainless steel devices are mentioned primarily for their historical significance and because they have served as prototypes for plastic devices in use today. Early in 2000, the labor and delivery unit utilized by the authors attempted to buy new Malmstrom and Bird cups in order to teach our OBGYN residents the proper use of these devices. However, the European manufacturers declined to sell these devices to buyers in the United States. Their stated reservation was due to U.S. product liability laws and their wish to avoid any potential medicolegal entanglements.
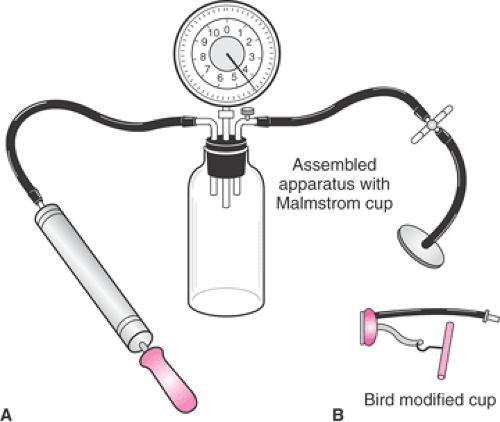
The Malmstrom vacuum extractor (Fig. 26.2) is a shallow, mushroom-shaped stainless steel cup, with two
vacuum hoses, a traction chain and attached metallic disk, a traction handle, and a vacuum source. The center of the cup has a metallic suction port through which the traction chain passes. The traction chain is attached to the cup by a metallic disk that is inserted within the cup to prevent the fetal scalp from being pulled into the vacuum port. The margin of the metallic disk is scalloped at regular intervals such that the vacuum may be developed beyond the underside of the disk and onto the fetal scalp. The Malmstrom vacuum extractor can be assembled in seconds. After its use the device is easily disassembled, and the components are washed, autoclaved, and packaged for future use.
vacuum hoses, a traction chain and attached metallic disk, a traction handle, and a vacuum source. The center of the cup has a metallic suction port through which the traction chain passes. The traction chain is attached to the cup by a metallic disk that is inserted within the cup to prevent the fetal scalp from being pulled into the vacuum port. The margin of the metallic disk is scalloped at regular intervals such that the vacuum may be developed beyond the underside of the disk and onto the fetal scalp. The Malmstrom vacuum extractor can be assembled in seconds. After its use the device is easily disassembled, and the components are washed, autoclaved, and packaged for future use.
The Malmstrom cup has an inverted lip such that the diameter of the opening is smaller than the internal diameter of the cup. When the cup is placed on the fetal scalp and vacuum is established, a small artificial caput forms, termed the chignon. When the fetal scalp fills the internal dimensions of the cup, the scalp underlies the opening of the cup in a “key-in-lock” fashion. This allows a considerable traction force to be applied before cup detachment occurs.
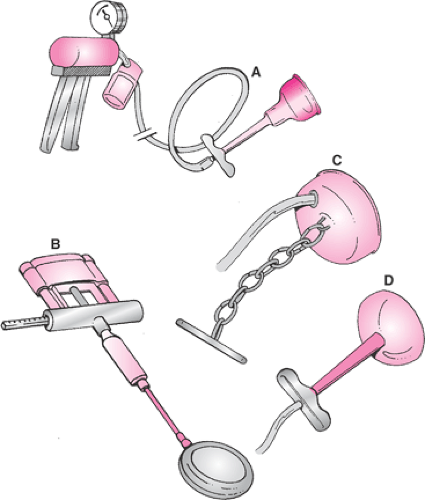
The utility of the Malmstrom cup is limited by a combined vacuum port and traction apparatus in the dome of the cup, which makes appropriate application somewhat difficult in positions of the fetal head other than occiput anterior (OA). In 1969, Bird developed two modifications (anterior and posterior) of the Malmstrom cup to increase the versatility of the vacuum extractor for proper placement with difficult positioning of the fetal head. Bird separated the vacuum port and the traction apparatus (Fig. 26.2). The traction chain remained anchored to the center of the cup, while the vacuum port was attached eccentrically to the dome of the anterior cup. The vacuum port was attached laterally for the posterior cup. With these modifications, the vacuum tubing exited in the same plane as the cup, a change which enabled the physician to better maneuver the cup into its proper location within the maternal pelvis. In even later versions of the Bird cups, the central traction chain was replaced by a nylon cord anchored to the edges of the cup and to a traction bar for physician convenience.
Types of Vacuum Extractors: Soft-Cup Devices
There are several types of plastic or silicone vacuum cups currently in use in the United States (Fig. 26.3). The cups are separated into three groups, depending on the shape of the vacuum cup: funnel, bell, or mushroom. Of these, the funnel-shaped cups are the least used. The prototype for this vacuum extractor was based on the original Kobayashi silastic cup, introduced in 1973. The diameter of this device (65 mm) is the largest of any of the commercially available cups, and therefore its size obviates the need for a chignon to form in order to achieve appropriate traction. This device also has a vacuum release valve on the stem, which allows the operator to reduce vacuum pressure between contractions, if desired. Variations on the original Kobayashi design are available from several vendors. A much lower rate of neonatal scalp trauma was reported with the Kobayashi cup in contrast to experiences with stainless steel cups, a finding that was corroborated by five randomized studies of the silastic cup versus the stainless steel cups. Although the Kobayashi vacuum extractor was noted to effect delivery in less time than stainless steel devices, a higher failure rate occurred, especially when the fetal head was in the OP presentation.
Bell-shaped vacuum cups are widely available from several vendors in the United States. One example is the Mityvac device (Cooper Surgical, Trumbull, CT), which was compared with a silastic funnel-shaped cup and Tucker-McLane forceps in a prospective, randomized study of 118 delivered patients by Dell in 1985. Trauma to the maternal genital tract trauma was noted in 48.9% of the women delivered by forceps as compared with 36.1% and 21.6% of the women delivered by the silastic and bell-shaped cups, respectively. This represented a statistically significant reduction in maternal genital tract trauma with use of the bell-shaped vacuum device. Success rates using the bell-shaped cup (89.2%) versus forceps (93.3%) were not significantly different. However, there was a significantly higher rate of cephalohematoma formation in the neonates delivered with vacuum devices (funnel-shaped cup, 13.9%; bell-shaped cup, 16.2%) than with forceps (2%). Caput and
scalp discoloration was more apparent with the bell-shaped cup than with the funnel-shaped cup. With regard to the utility of the soft-cup vacuum devices, Dell’s group concluded that “the dramatic usefulness of these instruments to quickly deliver multiparas whose infants experienced fetal distress in late labor was far more impressive than their performance as outlet instruments in nulliparas.”
scalp discoloration was more apparent with the bell-shaped cup than with the funnel-shaped cup. With regard to the utility of the soft-cup vacuum devices, Dell’s group concluded that “the dramatic usefulness of these instruments to quickly deliver multiparas whose infants experienced fetal distress in late labor was far more impressive than their performance as outlet instruments in nulliparas.”
The first generation of mushroom-shaped cups represented an apparent attempt to produce a plastic version of the Malmstrom stainless steel cup. Several vendors offer disposable mushroom-shaped vacuum cup devices made of semirigid plastic; one of the more popular devices has been the M-cup (Mityvac). The efficacy of this cup was tested prospectively against the obstetric forceps in a large randomized clinical trial by Bofill in 1996. Both devices were comparably effective as tools to accomplish vaginal delivery, but deliveries with the M-cup were accomplished in less time and with less maternal genital tract trauma. As with nearly all other studies of vacuum and forceps, the clinical diagnosis of neonatal cephalohematoma was made significantly more often after vacuum delivery.
The first generation of mushroom-shaped vacuum cups faced the same problems as the original Malmstrom stainless steel device—limited maneuverability. They were, however, more easily maneuvered than funnel- or bell-shaped cups within the maternal introitus because the traction stem could be bent at a 90-degree angle with respect to the cup. Nevertheless, the combined and centrally located vacuum port and traction stem limited the ability of the physician to place the vacuum cup on the median flexing point, especially in cases of difficult positioning of the fetal head such as OP or OT positions or in the presence of significant asynclitism. Past experience with improvements to the stainless steel cups afforded a design solution to this shortcoming as a plastic equivalent of the Bird posterior cup. In 2001, Vacca tested the OmniCup (Clinical Innovations Inc., Murray, UT) and found that it was quite useful for both nonrotational and rotational vacuum-assisted vaginal deliveries (Fig. 26.3). Vacca was able to achieve a median flexing application in 90% of applications, which resulted in “autorotation” in 32 of 33 cases in which rotation was expected.
Vacuum Pressure and Traction Forces
The disposable plastic or silicone vacuum cups currently in use in the United States are marketed with a pistol grip hand pump that is connected to the cup by vacuum tubing (Fig. 26.3). A filter in the vacuum tubing prevents the suctioning of liquids or other debris into the pump. While it is possible to sterilize this pump, it is most commonly managed by a nurse or other attendant while the physician attends to the delivery. More recent versions of the mushroom vacuum cup are marketed with a disposable vacuum pump attached to the cup by a slender vacuum tube with an internal chain. This pump also contains a traction bar with a vacuum gauge and a vacuum release button. This allows the physician to better control the appropriate timing of vacuum pressure and traction.
The recommended operating vacuum pressures for the majority of cups, whether stainless steel or plastic, is from 0.6 kg/cm2 to 0.8 kg/cm2 (about 500 to 600 mm Hg). The original stainless steel devices used a device that resembled a bicycle hand pump to achieve the required vacuum strength. The vacuum tubing was attached to a stoppered vacuum bottle that had ports for the vacuum pump and a vacuum gauge (Fig. 26.2). Alternatively, a portable electric vacuum pump or the hospital’s wall suction system could be used to generate vacuum pressure. These systems are used rarely, if at all, in the United States for vacuum-assisted delivery.
Newer vacuum devices permit the rapid achievement of required vacuum pressure as a single step over several seconds, much faster than the slow and stepwise increase in pressure required with the original Malmstrom device. There is no difference between the final recommended vacuum pressure to achieve for plastic or silicone cups compared with the original stainless steel cups. With steel, the vacuum pressure is slowly brought to 600 mm Hg and is maintained at that level until delivery is accomplished—there is no reduction between uterine contractions and traction efforts. However, with the plastic cups, some authorities have recommended that the level of vacuum pressure should be reduced from 600 mm Hg during traction and contractions to 100 mm Hg during rest. The rationale for this recommended reduction in vacuum pressure is to expose the fetal scalp to the least amount of time at high vacuum pressures. A randomized trial of the intermittent versus continuous techniques of vacuum pressures during delivery with a mushroom-shaped cup failed to demonstrate any difference in neonatal outcomes. Regardless of the technique, however, the longer a vacuum cup resides on the fetal scalp, the greater is the likelihood of cephalohematoma formation and other cosmetic scalp lesions.
Vacuum Extraction Delivery: Recommended Practice
The following list summarizes the recommended steps for use of a mushroom-shaped cup to accomplish vacuum-assisted vaginal delivery, analogous to the check-offs that a pilot undertakes while readying a plane for flight:
CHECK—Specify the Indication: An indication should exist for the procedure.
CHECK—Get Informed Consent: Obtain either verbally or as a written document.
CHECK—Gestational Age ≥34 Weeks: The pregnancy is at term or near term—in the authors’ practice, vacuum delivery generally is not used at a gestational age <34 weeks.
CHECK—Cervix Dilation and Effacement: The cervix is fully dilated and the fetal head is well engaged, preferably deeply engaged for low-pelvic or outlet-pelvic delivery.
CHECK—Fetal Head Position: The correct position of the fetal head should be determined.
CHECK—Contraindications Absent: Face, brow, or breech presentations are strict contraindications to vacuum use for delivery.
CHECK—Test Vacuum: The vacuum apparatus is tested against the clinician’s gloved hand to assure achievement of adequate but not excessive vacuum pressure.
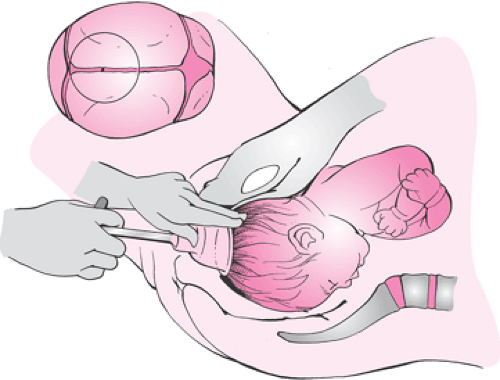
Correct Cup Application: The vacuum cup is applied to the median flexing point of the fetal head (Fig. 26.4).
Partial Vacuum Pressure: Vacuum pressure initially is raised to approximately 100 mm Hg so that the clinician can palpate around the cup rim to be assured that no maternal tissue is trapped under the cup.
Time the Procedure: The procedure is timed from cup placement to completion of newborn delivery.
Full Vacuum Pressure: Vacuum pressure is increased to 600 mm Hg, with subsequent initiation of traction concurrent with uterine contraction and maternal expulsive efforts—vacuum pressures higher than 600 mm Hg are not recommended.
Correct Traction: Traction is undertaken in the direction of the pelvic axis and perpendicular to the cup.
Descent with Traction Rule: The physician should be certain that traction produces descent of the fetal skull as well as that of the fetal scalp.
Traction—Not Torsion: Intentional torsion of the cup is never applied to produce rotation of the fetal head; direct traction can lead to spontaneous rotation of the fetal head with descent (“autorotation”).
STOP—Three Pop-Offs: The procedure should be terminated if there are three pop-offs.
STOP—Fetal Scalp Trauma: The procedure should be terminated if there is evidence of fetal scalp trauma after a pop-off.
STOP—20 Minutes: The procedure should be terminated if delivery has not occurred within 20 minutes.
STOP—No Descent: The procedure should be abandoned if there is no descent with correct application and appropriate traction (without waiting for three pop-offs or 20 minutes).
NEWBORN—Inspect the Scalp: After delivery, the scalp should be inspected for trauma and position of the chignon.
NEWBORN—Communicate Vacuum Use: Nursery personnel should be alerted that the delivery was accomplished via vacuum.
Document: The procedure should be documented thoroughly in the medical record.
The indications for vacuum-assisted delivery are nearly identical to those for obstetric forceps. The most common indications include a prolonged second stage of labor or nonreassuring fetal status. Other indications include maternal exhaustion and maternal illnesses in which maternal expulsive efforts should be limited. The indication for vacuum delivery should be included in the delivery note portion of the medical record.
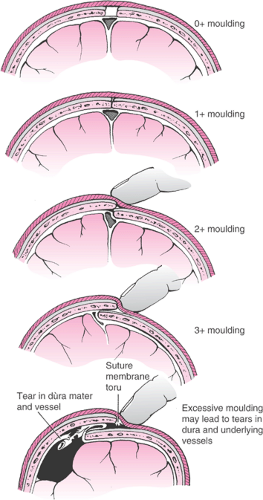
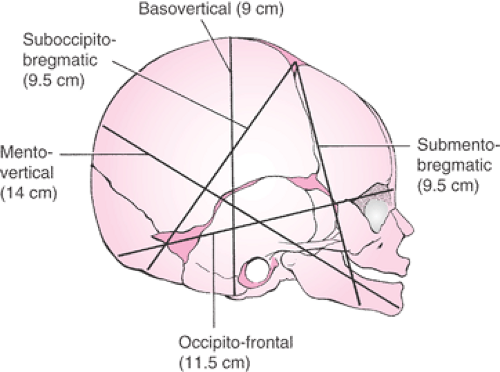
It is important for the physician to perform a careful assessment of the fetal head with regard to the size and position of the caput succedaneum and the amount of molding. Severe molding of the fetal head may indicate cephalopelvic disproportion. The method of Stewart is used to describe the amount of molding by examination of the parietal bones (Fig. 26.5). Safe and successful vacuum-assisted delivery is contingent on an appropriate application of the cup onto the fetal head. It is very important that the cup be placed on the median flexing point, as defined by Vacca (Fig. 26.4). To achieve the correct median flexing application, the center of a 60-mm cup should be placed 3 cm from the posterior fontanelle and symmetrically astride the sagittal suture. In this application, one edge of the cup will encroach on the posterior fontanelle, while the opposite edge will be about 3 cm from the anterior fontanelle. The median flexing point is located at the emergence of the mentovertical diameter and is about 3 cm anterior to the posterior fontanelle and in the midline (Fig. 26.6). The fetal head is considered to be optimally flexed when the mentovertical diameter points in the direction of the pelvic axis. The center of the vacuum cup should be placed over the median flexing point so that it is symmetrically astride the sagittal suture. Correct application of the vacuum cup as described is important because traction in the direction of the pelvic axis will maintain flexion of the fetal head and minimize or eliminate any asynclitism. Thus, the maternal pelvis is presented with the most favorable diameter of the fetal head, and atraumatic delivery is optimized.
There are several potential consequences of suboptimal cup placement. Asymmetric placement of the vacuum cup astride the sagittal suture (a paramedian application) will
produce asynclitism when traction is applied. If the center of the cup is placed too far anteriorly, subsequent traction will produce deflexion of the fetal head (deflexing application). If the placement of the cup is both paramedian and deflexing, successful and safe delivery is unlikely.
produce asynclitism when traction is applied. If the center of the cup is placed too far anteriorly, subsequent traction will produce deflexion of the fetal head (deflexing application). If the placement of the cup is both paramedian and deflexing, successful and safe delivery is unlikely.
The least resistance to delivery of the fetal head occurs when the direction of vacuum traction is parallel with the pelvic axis. Traction should be perpendicular to the vacuum cup. Angular (off-center) traction commonly produces lift at the far edge of the cup, which can lead to disconnects or “pop-offs.” If traction at an angle cannot be avoided secondary to asynclitism or deflection of the fetal head, the physician may be able to compensate for this off-center traction by using the thumb or index finger of the nontraction hand to apply counterpressure on the far side of the cup. This is the so-called three-finger grip, which also assures that descent of the fetal skull occurs with vacuum traction and maternal expulsive efforts.
Physicians should never apply torsion to the vacuum cup when it is used to deliver an infant from an OP or OT presentation, as this can result in fetal scalp injury. Straight axis traction of a properly placed cup will often produce rotation to the OA position when the fetal head encounters resistance from the maternal levator musculature in a pelvis of adequate size for vaginal delivery. This is termed autorotation, a phenomenon that is commonly seen as the fetal head descends in both spontaneous as well as vacuum-assisted delivery. Vacuum traction should be applied only in concert with maternal expulsive efforts.
The physician should be willing to abandon an attempt at vacuum-assisted delivery if it does not go well and complications are noted. If fetal scalp trauma is observed with the vacuum cup, the cup should be removed and delivery accomplished otherwise. As the length of time a vacuum cup is on the fetal head increases, so does the potential for development of a cosmetic scalp lesion and cephalohematoma. Manufacturers of vacuum delivery devices frequently recommend that the fetal scalp be subjected to no more than 10 minutes at high vacuum pressures. Because recording of the cumulative time that the fetal scalp has been at high vacuum pressures is a cumbersome and frequently inaccurate procedure, we recommend that the length of time that a cup spends on the fetal scalp be limited to 20 minutes. It is the authors’ opinion that if a vacuum cup pops off three times, it is wise to terminate any further attempt at vacuum delivery. Pop-offs may indicate the presence of a nonmedian or deflexing cup application, angular traction, or cephalopelvic disproportion. Perhaps the most important tenet of vacuum delivery is that if a proper application and adequate traction does not produce descent of the fetal head, the procedure should be terminated without waiting for three pop-offs or for 20 minutes to elapse. These deliveries are an excellent exercise in physician judgment and discretion.
After each vacuum delivery, it is recommended that the fetal scalp be examined to assess the position of cup placement and to document scalp trauma. Nursery physicians should be informed that the infant was delivered by vacuum so that attention can be focused on the fetal scalp after delivery. Documentation of the vacuum delivery is of obvious importance, with a detailed written, dictated, or recorded-by-computer entry in the medical record. Details of importance to record include the indication(s) for the procedure, the instrument used, the station and position of the fetal head at the time the cup was applied, identification of the vacuum procedure as midpelvic or low or outlet in type, the number of pop-offs, and the length of time the cup was on the fetal head. Caput, molding, asynclitism, and autorotation, if encountered or noted during delivery, are documented as well. The type of analgesia provided and any maternal or fetal trauma also should be documented.
Unsuccessful Vacuum Delivery
Any physician who performs a substantial number of vacuum-assisted deliveries will occasionally need to abandon a vacuum procedure for one or more of reasons noted previously. In this situation, the physician has three options to consider. First, spontaneous delivery may be possible if there are good maternal expulsive efforts and the fetal head is on the pelvic floor. Second, cesarean delivery is clearly indicated if the fetal head is in the midpelvis or at low pelvic station with suspicion of cephalopelvic disproportion. Third, obstetric forceps can be used to accomplish vaginal delivery after a failed vacuum attempt, especially if the fetal head is deep within the pelvis. These “sequential operative vaginal deliveries” (vacuum first and then forceps or forceps first and then vacuum) are controversial. Some physicians consider initial use of a midpelvic vacuum extractor to be appropriate in order to convert what would have been a difficult midforceps procedure into a much easier low- or outlet-forceps procedure.
Small studies by two groups of investigators were unable to demonstrate any additional neonatal complications associated with “sequential” operative vaginal deliveries. The frequency of this sequential operative vaginal delivery ranged from 4% to 27% in nine studies of vacuum and forceps deliveries. However, a population-based study of fetal intracranial hemorrhage demonstrated that the risk of fetal intracranial hemorrhage clearly was increased after the sequential use of instruments as compared with spontaneous delivery, cesarean delivery, or the single use of either forceps or vacuum.
Another large study of the sequential use of instruments was produced by Gardella. In this study, the sequential use of instruments was associated with significantly higher rates of neonatal intracranial hemorrhage, brachial plexus injury, facial nerve injury, neonatal seizures, and depressed 5-minute Apgar scores. Thus, the sequential use of instruments for operative vaginal delivery is not recommended except in dire circumstances.
Neonatal Injury—Vacuum Extraction
The prevalence of neonatal scalp injury with vacuum delivery is difficult to ascertain from the literature because uniformity in reporting is lacking. A 1979 summary of outcomes with the Malmstrom vacuum extractor observed that neonatal scalp abrasions or lacerations ranged widely, from 0.8% to 37.6% (mean 12.6%). The relatively frequent occurrence of neonatal scalp injury in part led to the development of the soft plastic cups for vacuum extractors in order to accomplish a more gentle delivery. Most scalp abrasions or lacerations are noted in place(s) where the fetal scalp is pulled under the rigid rims of the stainless steel cups or the mushroom-shaped plastic cups. However, the funnel- and bell-shaped cups mold themselves to the fetal scalp, create less artificial caput, and are associated with lesser degrees of superficial scalp injury. Many investigators have observed a lower prevalence of fetal scalp trauma with the soft cups as opposed to the stainless steel devices. Using logistic regression analysis, Teng and Sayre examined how neonatal scalp trauma occurred during a trial using the M-cup vacuum device. Independent predictors of neonatal scalp trauma included duration of the procedure (>10 minutes), duration of the second stage of labor, and a paramedian cup application. It is difficult, if not impossible, to compare the incidence of scalp trauma in studies of forceps and vacuum deliveries.
Cephalohematoma
A cephalohematoma is a self-limited injury that is caused by the rupture and bleeding of an emissary vein into the potential space just beneath the periosteum of the neonatal parietal bone (Fig. 26.7). The periosteum of the
parietal bone is firmly attached at the periphery, and only a limited amount of fetal blood can collect in this potential space. Cephalohematomas are diagnosed more commonly after vacuum extraction as opposed to forceps delivery. They typically resolve spontaneously over the course of a few days without long-term sequelae, although neonatal hyperbilirubinemia can develop. Most often, a cephalohematoma will not cross the suture lines of the skull, but edematous scalp can render the examination difficult and make the diagnosis challenging. Illustrative of this is the ultrasound confirmation of only three cephalohematomas in 12 neonates thought to have the condition. The other 9 infants had edematous scalps and not cephalohematomas.
parietal bone is firmly attached at the periphery, and only a limited amount of fetal blood can collect in this potential space. Cephalohematomas are diagnosed more commonly after vacuum extraction as opposed to forceps delivery. They typically resolve spontaneously over the course of a few days without long-term sequelae, although neonatal hyperbilirubinemia can develop. Most often, a cephalohematoma will not cross the suture lines of the skull, but edematous scalp can render the examination difficult and make the diagnosis challenging. Illustrative of this is the ultrasound confirmation of only three cephalohematomas in 12 neonates thought to have the condition. The other 9 infants had edematous scalps and not cephalohematomas.
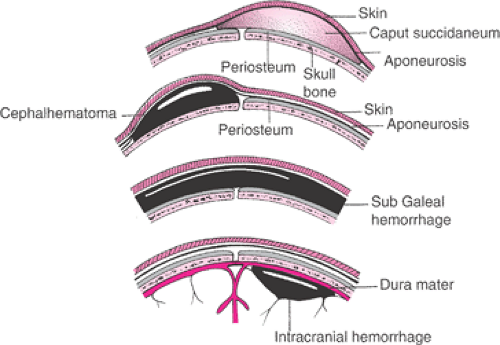 Figure 26.7 Normal caput, cephalohematoma, subgaleal (subaponeurotic) hemorrhage, and intracranial hemorrhage. |
Factors associated with cephalohematoma formation were studied in a Mississippi series of 322 vacuum extraction procedures performed with the semirigid mushroom-shaped cup. The rate of cephalohematoma formation in this study was 11%. Significant contributors to the diagnosis of neonatal cephalohematoma included the duration of the vacuum procedure and the amount of asynclitism at the time of cup placement. Asynclitism easily leads to a paramedian cup application that is productive of the chignon over one parietal bone more so than the other, which enhances the possibility of a false diagnosis of cephalohematoma.
Subgaleal (Subaponeurotic) Hemorrhage
A much more serious and potentially fatal complication of vacuum extraction is a subgaleal (subaponeurotic) hemorrhage, in which a scalp vessel ruptures distal to the level of the periosteum where blood can accumulate in the layer of loose connective tissue known as the subgaleal or subaponeurotic space (Fig. 26.7). This potential space is very large and extends from the orbital ridges anteriorly, to the nape of the neck posteriorly, and to the zygomatic arches laterally. This space can easily accommodate the entire blood volume of a term newborn, even if filled only to a 1 cm depth. The most common physical finding with a subgaleal hemorrhage is a fluctuant mass that extends over the cranial sutures and fontanelles. This usually is accompanied by edema and bruising that extends posteriorly into the neck and laterally around the ears. The fluctuant mass can reach the upper eyelids and the root of the nose. The infant will commonly have evidence of hypovolemic shock. Shock may manifest hours after delivery or even later in the nursery. Subgaleal hemorrhage also has been associated with midforceps delivery, leading at least one investigator to conclude that instrumental delivery is the principal predisposing factor for this lesion. The incidence of subgaleal hemorrhage is estimated to occur in 4 of 10,000 spontaneous vaginal deliveries and in 59 of 10,000 vacuum-assisted deliveries.
The 1998 FDA public health advisory primarily was directed at alerting all physicians who practice vacuum-assisted vaginal delivery to be very aware of subgaleal hemorrhage as a potential complication. Advising nursery personnel that a vacuum-assisted vaginal delivery has been performed also is key to facilitating the early diagnosis of a subgaleal hemorrhage.
Intracranial Hemorrhage
Although intracranial hemorrhage in the term infant often is associated with traumatic operative vaginal delivery, it can occur after spontaneous delivery as well and have profound neonatal consequences (Fig. 26.7). In 1990, Hannigan and coworkers reported three cases of tentorial hemorrhage that were associated with soft-cup vacuum extraction deliveries. They reasoned that vacuum delivery could produce vertical stress in the occipitofrontal diameter, thereby causing tentorial venous hemorrhage. Nine other cases of tentorial subdural hemorrhage in term newborns were reported by another group, with five cases following delivery using the vacuum extractor. Seven of the nine infants were neurologically normal at 13 months of age. One infant delivered by vacuum extraction required a ventriculoperitoneal shunt for progressive hydrocephalus.
Vacuum extraction typically is avoided in the fetus that is very preterm because of the increased risk for intracranial hemorrhage at earlier gestational ages. There is no evidence-based gestational age threshold on which to base practice, but most clinicians avoid vacuum extraction altogether at <34 weeks gestation. Two small retrospective studies have been published that were unable to demonstrate an increased risk of intracranial hemorrhage in preterm infants delivered via the vacuum. Finally, Towner and colleagues utilized a population-based study to show that the rates of intracranial hemorrhage are not statistically significantly different for vacuum-assisted delivery, forceps delivery, and for cesarean delivery performed during labor. However, those deliveries accomplished by the sequential use of instruments (vacuum, then forceps) were associated with a significantly higher rates of intracranial hemorrhage.
Retinal Hemorrhage
Neonatal retinal hemorrhage after vaginal delivery is quite common. In 1974, Ehlers and colleagues noted neonatal retinal hemorrhage in 28% of newborns following spontaneous delivery, 38% with forceps delivery, and 64% after delivery with the Malmstrom vacuum extractor. Others reported the incidence of moderate to severe retinal hemorrhages in infants delivered with the soft plastic bell-shaped cup to be 18% with spontaneous deliveries, 13% with forceps, 28% with vacuum, and 50% in infants delivered using sequential forms of operative vaginal delivery. Multiple logistic regression analysis has been used as well to identify the factors associated with moderate to severe retinal hemorrhage. These have included vacuum delivery of low–birth-weight infants, short second stages of labor, fetal acidemia, and the sequential use of vacuum-then-forceps
for delivery. Neonates delivered by vacuum extraction typically will have higher rates of retinal hemorrhage. Neonatal retinal hemorrhages have not been demonstrated to have long-lasting effects. Following a 5-year period of study, children delivered by random assignment either to vacuum or forceps extraction demonstrated no difference in visual problems.
for delivery. Neonates delivered by vacuum extraction typically will have higher rates of retinal hemorrhage. Neonatal retinal hemorrhages have not been demonstrated to have long-lasting effects. Following a 5-year period of study, children delivered by random assignment either to vacuum or forceps extraction demonstrated no difference in visual problems.
Delivery with Obstetric Forceps
In modern-day obstetric practice, it is not uncommon for an educated and well-read patient to present for her first prenatal care visit with a birth plan that specifically excludes any form of operative vaginal delivery, especially one that might include obstetric forceps. The use of obstetric forceps has always engendered controversy. Points of view tend to cycle, to some extent in relation to the age of the obstetrician and the era during which he or she trained.
Most historians credit the 17th century Chamberlen family as the first well-known purveyors of the use of these instruments that were designed to deliver a living infant. Because the Chamberlens were engaged in an effort to monopolize the midwifery practice of London, family members attempted to keep their instruments secret from their patients and other physicians alike. For some time, these instruments had limited use in the hands of a skilled few, but this ended when a family member in difficult financial straits sold the secret to the Dutch medical community. The reader interested in the convoluted story of the forceps and the generations of the Chamberlen family can easily find a more complete history. With more widespread use of obstetric forceps, particularly by unskilled providers, there was a predictable increase in poor maternal and neonatal outcomes. By the end of the 18th century, the use of obstetric forceps had declined greatly.
The well-known impetus for the rebirth and more frequent use of the obstetric forceps was the “triple obstetric tragedy.” In 1817, after a very protracted second stage of labor, Princess Charlotte delivered without assistance a stillborn who would have been the future king of England. The obstetric management of labor characterized by nonintervention became a contentious topic, as it was considered to account for the poor outcome of the pregnancy. Some considered that the judicious use of forceps could have shortened the labor since the fetal head was reportedly on the perineum for 10 hours. Not only was the infant stillborn, but the princess also died of a postpartum hemorrhage. The triple obstetric tragedy was completed when the royal obstetrician took his own life some weeks after this debacle.
This tragedy and the development of obstetric anesthesia in the mid 19th century helped to initiate an era of liberalized obstetric forceps use. In the first issue of the American Journal of Obstetrics and Gynecology, DeLee discussed his views on the prophylactic use of these instruments. By the 1940s, the rate of obstetric forceps deliveries in the United States approached 70%, but soon thereafter the trend began to reverse for several reasons, including the introduction of the vacuum extractor and expanding, liberal, and increasingly safe use of cesarean delivery. Thus, in recent decades, all but the simplest of obstetric forceps have disappeared from the armamentarium of the recently trained obstetrician. The rate of forceps deliveries in the United States in 2004 was just over 1%.
Types of Obstetrical Forceps
Hundreds of obstetric forceps have been developed for utilization in this country and elsewhere in the world—there are many choices (Fig. 26.8). Most obstetricians, however, comfortably and competently use no more than three or four different types of instruments in their practices.
Anatomy of the Forceps
Obstetric forceps are a paired instrument, fashioned of two branches that articulate with one another by a lock. The branches (left and right) are side specific and named according to the side of the maternal pelvis into which it will be applied. Before using any obstetric forceps, the obstetrician should be certain that the two branches of the pair constitute a matched set that is symmetrical when articulated. Application techniques are described below.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree