Obtaining Biologic Specimens
Louis M. Bell
Nicholas Tsarouhas
Introduction
Biologic specimens can be an important adjunct to the history and physical examination. Poorly obtained specimens not only may lead to erroneous diagnoses but also may cause undue morbidity. This chapter discusses the indications, materials, procedures, and complications involved in the collection of biologic specimens. The interpretation of these results is also addressed.
Blood Cultures
Blood cultures are the cornerstone to the diagnosis of bacteremia and sepsis. The Gram stain is of controversial benefit (1) and may have no value unless bacteremia is overwhelming (2). Consequently, most institutions do not routinely Gram stain blood specimens. For this reason, it is important to optimize the procedure for obtaining a proper blood culture. Isolation of the offending micro-organism is crucial to diagnosis and management of patients with bloodborne bacterial disease.
Anatomy and Physiology
Peripheral veins are the most common vessels from which blood cultures are drawn. The veins used most often in pediatric blood draws are those in the antecubital fossa. However, because of the occasional difficulty in obtaining blood specimens from young children and infants, other sites also are frequently used (Chapter 73). Using the femoral vein, located medial to the femoral arterial pulsation and below the inguinal ligament, is sometimes discouraged because of the possible difficulty in appropriately cleaning the site. This difficulty is more of a problem in the adult patient, and therefore this site need not be excluded in the child.
Arteries are equally acceptable sites for blood cultures. The radial artery is most commonly used, but the ulnar, dorsalis pedis, and posterior tibial arteries and even the temporal artery are occasionally used (Chapter 72). In the newborn, the umbilical artery or vein is often used as a site from which to draw blood cultures. These cultures are most helpful if drawn at placement of the line (Chapter 38). Capillary collections are not acceptable as blood culture specimens.
Indications
The obvious indication for drawing a blood culture is a toxic-appearing child. Such patients should always have a blood culture drawn to rule out sepsis. Many additional scenarios, however, deserve special note.
Children with immunodeficiencies obviously are more susceptible to the development of serious bacterial infections. Examples include children with oncologic disease, acquired immunodeficiency syndrome, chronic renal failure, and sickle cell anemia. Patients on immunosuppressive medication also are at higher risk for sepsis. Neonates are immunocompromised by virtue of the immaturity of their immune systems. All febrile infants less than 2 months of age warrant a full
sepsis evaluation. Additionally, other signs of illness, such as irritability, lethargy, poor feeding, and apnea, may indicate sepsis. Although fever often is the presenting symptom, it should be noted that some of these children may not have the ability to mount a febrile response. All of these scenarios require increased vigilance with respect to possible sepsis.
sepsis evaluation. Additionally, other signs of illness, such as irritability, lethargy, poor feeding, and apnea, may indicate sepsis. Although fever often is the presenting symptom, it should be noted that some of these children may not have the ability to mount a febrile response. All of these scenarios require increased vigilance with respect to possible sepsis.
Much has been written in the pediatric literature regarding occult bacteremia. It is commonly described as the presence of bacteremia in a well-appearing child (usually less than 2 years of age) with a high fever (usually greater than 39.0°C to 39.4°C). Since routine immunization has been instituted against Haemophilus influenzae type b and, more recently, Streptococcus pneumoniae, the incidence of occult bacteremia has declined considerably. It is currently estimated to be 1.5% to 2%.
The optimal time to obtain a blood culture is when the micro-organisms are most likely to be recovered from the bloodstream. Although this may occur just before the onset of symptoms (fever, chills), it is obviously impossible to obtain a blood culture before the patient becomes symptomatic. Ideally, the blood culture should be obtained as close to the onset of symptoms as possible (3).
How many cultures are necessary? Li et al. found no significant difference in the yield of blood culture results in studies performed with two sets drawn simultaneously versus those done with an interval of time between them (4). In older infants and children, a single blood culture suffices for the majority of clinical scenarios. In neonates, however, two blood cultures may be beneficial, especially to distinguish between true coagulase-negative staphylococcus sepsis and simple contamination (5).
Endocarditis merits special consideration. In most cases, the bacteremia is continuous and the timing not so important. However, serial cultures may increase the yield and make the diagnosis easier (3). The micro-organisms usually implicated include Streptococcus viridans, Staphylococcus aureus, Staphylococcus epidermidis, and enterococci. Children with congenital heart disease, prosthetic valves, or a history of rheumatic fever are at highest risk. A high degree of suspicion should be maintained in any child with prolonged fever, especially in the presence of a new heart murmur.
Children who have long-term venous access catheters (Broviac or Hickman) and develop fever are commonly admitted to the hospital to rule out “line” infection. Most clinicians prefer peripheral and central venous catheter cultures to help in the management of line infections as well as to distinguish contaminants from true pathogens. Additionally, in immunocompromised patients, such as those with oncologic diseases, many recommend that cultures be drawn from all lumens of multilumen catheters. It is believed that this increases the sensitivity of the blood culture (6).
Because of the potential seriousness of a line infection, these children are started on presumptive antibiotic therapy until culture results are known. The most common micro-organisms implicated in these infections are the Gram-positives S. aureus and S. epidermidis, although Gram-negative infections also occur. Any febrile child who has a central line and presents with chills, change in mental status, or hypotension should be presumed to have line sepsis. Oncology patients receiving long-term chemotherapy and children with gastrointestinal or nutritional disorders make up a large part of this population.
Immunocompetent children rarely develop pure anaerobic infections. When anaerobes are involved, the infection often is polymicrobial. Examples include dental infections, human bites, and abscesses. Some authorities believe that if an anaerobic infection is strongly suspected, a separate anaerobic blood culture should be obtained. However, there is no routine need for anaerobic cultures (3).
Fungi often are difficult to grow in routine blood cultures. In addition to sometimes taking several weeks to grow, special media and techniques often are required. For instance, Malassezia furfur, a lipid-dependent yeast, grows poorly in standard culture but well in media overlaid with olive oil (7). This species is known to cause systemic infection in babies with central venous catheters in neonatal intensive care units. The laboratory should be consulted when fungi are suspected.
Equipment
Peripheral Blood Culture
Iodine swabs
Alcohol swabs
Chlorhexidine
Blood culture tube or bottle
Tourniquet
Butterfly needle
Syringe, 3 to 5 mL
Gauze
Band-Aids
Sterile gloves
If Vacutainer system is used:
Vacutainer needle and holder or
Vacutainer butterfly and holder
If newly placed intravenous catheter is used:
Peripheral intravenous catheter
T-connector
Central Line Blood Culture
Steel clamp
Iodine swabs
Blood culture tube or bottle
Syringes, 5 mL
Sterile transfer needle
Heparin or saline flush
Sterile gloves
Sterile drape
Needleless Central Line Blood Culture
Steel clamp
Iodine swabs
Vacutainer tube (for discarded blood)
Vacutainer needle and holder
Needleless cannula
Vacutainer tube (for blood culture)
Heparin or saline flush
Sterile gloves
Sterile drape
Totally Implanted Venous Access System Blood Culture
Huber needle with a 90-degree bend
Extension tubing with side clamp
Gauze to stabilize needle
Equipment listed for central line blood culture
Poor skin preparation is the most common cause of blood culture contamination (3). The most frequently used disinfectants contain some combination of iodine, alcohol, and chlorhexidine. Many institutions use disposable swabs of povidone-iodine (Betadine). This preparation yields 1% available iodine. One large, randomized, crossover, investigator-blinded, prospective study compared four antiseptic solutions: 10% povidone-iodine, 70% isopropyl alcohol, tincture of iodine (2% iodine and 2.4% sodium iodide, diluted in ethanol), and povidone-iodine combined with 70% ethyl alcohol (a product called “Persist”) (8). Of the 12,692 blood cultures drawn, 333 (2.6%) were contaminated. These investigators detected no significant differences in the contamination rate among the four disinfectants, although there was some suggestion that those antiseptics that contained alcohol might be slightly more efficacious. Another randomized, controlled study found alcoholic chlorhexidine to be more efficacious in reducing blood culture contamination rates than skin preparation with aqueous povidone-iodine (9). Similarly, another adult study compared 1% iodine pads with iodine tincture pads (2% iodine–47% ethanol) and found less contamination with the latter alcohol-containing pad (10).
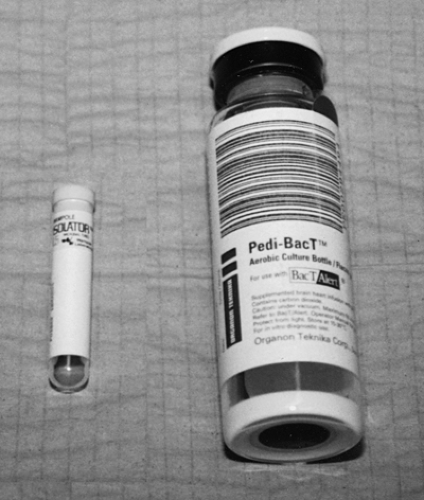
Tubes and bottles are the two main types of receptacles used for blood cultures (Fig. 122.1). Tubes are used with media systems, whereas bottles are used with broth systems. A common media system uses the Isolator 1.5 microbial tubes. This system basically involves extracting the collected blood from the tube and then directly plating the collected blood onto media. With this technique, independent colonies can be counted and identified, which is one advantage of media systems over broth systems.
With broth systems (BACTEC or Pedi-BacT), the blood is inoculated into the bottle, and this culture is not disturbed unless bacterial growth is sensed by automated systems. The broths are enriched with mixtures to enhance bacterial growth (soybean-casein digest, brain heart infusion, etc.). Additionally, antibiotic-binding resins often are added. Sodium polyanethol sulfonate (SPS) usually is added for its anticoagulant properties and its ability to counteract antibacterial factors in the blood (1).
Many authorities believe that the broth system is superior for a variety of reasons. Most important is that there are fewer contaminated specimens. The media system involves several processing steps that expose the culture, whereas the broth system is closed until a micro-organism is detected. Additionally, the broth system is more sensitive and quicker in the detection of a potential pathogen. Finally, the broth system, because it is automated, involves far less labor and therefore less cost.
Procedure
Peripheral Blood Culture
As with all pediatric procedures, the first step involves establishing a relationship with the child and parent. When possible (and when age appropriate), the procedure is explained to both child and parent, and the proper restraint technique is chosen (Chapter 3). The desired site is selected, and the tourniquet is applied. It is important to observe “universal precautions” regarding potential “exposure to bloodborne pathogens” as outlined by the Occupational Safety and Health Administration (OSHA) “to prevent contact with blood or other potentially infectious materials” (11). While it is optimal to use sterile gloves, this is often not practical, so care must be exercised not to recontaminate a sterilized area with palpation by a nonsterile glove (12).
The site should be sterilized by scrubbing with an alcohol wipe for at least 60 seconds. Next, a Betadine swab is used to wipe in concentric circles outward from the anticipated point of venipuncture. This is repeated with two more Betadine swabs. The clinician must now allow the Betadine to dry for 1 to 2 minutes. The most common breach of technique is not to wait at least 60 seconds for this important step (13). Alternatively, some clinicians reverse the order of the Betadine and the alcohol preparation. Again, it is important not to contaminate the area by repalpating the vein once the site has been sterilized.
With the site now prepared, the vein is punctured and the blood withdrawn (Chapter 73). The appropriate volume for blood culture depends on the institution’s culture system. Many broth systems recommend up to 5 mL of blood. Of course, this is neither practical, nor prudent, in the majority of pediatric patients. In pediatric patients, the magnitude of the bacteremia may be greater than in adults; therefore, a lesser volume is sufficient (14). While it is possible to recover organisms from volumes less than 0.5 mL (15), most experts agree that 0.5 mL of blood is the minimum necessary for a good result.
Only one blood culture tube or bottle is sufficient for the vast majority of pediatric situations. Notable exceptions may include endocarditis, central line infections, and strong suspicion of anaerobic infections. The adult literature supports obtaining more than one blood culture in the majority of clinical circumstances (16).
The unclotted blood should be inoculated into the tube or bottle as quickly as possible after the draw. Studies done in children (13) and adults (17,18,19) have shown no benefit from changing needles between the one used for the blood draw and the one used to inoculate the tube or bottle. The former practice of changing needles is now agreed to be a “no-benefit, high-risk procedure” (20). Similarly, needles should not be recapped; they should be discarded immediately into a sharps container. It is estimated that one third of all needlestick injuries occur during recapping (21).
A common practice in many institutions involves drawing blood specimens through a newly placed peripheral intravenous catheter. While there is literature support for this practice (19,22), a larger, more recent study found blood contamination rates were lower when specimens were drawn from a separate site than when they were drawn through a newly inserted intravenous catheter (23). Nevertheless, if a blood culture is to be drawn through the catheter, the same technique to sterilize the site with three Betadine pads and one alcohol pad should be performed before the intravenous catheter is inserted. An assistant attaches a T-connector with syringe and then withdraws the blood. The T-connector is removed and a sterile needle is attached to the blood-filled syringe. It is important to dispense the first inoculum of blood into the blood culture receptacle.
Central Line Blood Culture
The technique for drawing blood is described in Chapter 74. The most important step is sterilizing the site. Betadine swabs are used to disinfect the connection between the extension tubing or cap and the hub. The tubing or cap is then disconnected, and the hub itself again is disinfected. A sterile syringe is attached, and 0.5 mL (infant) to 1 mL (child) is withdrawn for discard. Interestingly, while the “discard” step is generally recommended, a recent study found no benefit to this common practice (24). Another syringe is attached, and the blood culture specimen (0.5 to 1 mL) is collected. A sterile needle is attached to the syringe, and the blood is inoculated into the receptacle. Of note, the catheter should be flushed with heparin or saline when the blood draw is completed.
Needleless Central Line Blood Culture
The technique for drawing blood is described in Chapter 74. Here again, the most important step is sterilizing the site. The rubber cap of the central line is swabbed well with Betadine, as are the tops of the Vacutainer tubes. The needleless cannula, which is attached to the Vacutainer needle, is then pushed into the rubber cap of the central line. The Vacutainer tube for discard is now attached, and approximately 1 mL is withdrawn and discarded. The Vacutainer blood culture tube is now attached, and 1 to 2 mL of blood is collected. When the needleless cannula is removed, the rubber cap is swabbed with Betadine, and then the line is flushed with heparin or saline.
Totally Implanted Venous Access System Blood Culture
Blood cultures are just as easily performed with implanted systems as with standard central lines. The technique for drawing blood is described in Chapter 74. Once the subcutaneous cylinder is palpated, the overlying skin is disinfected with three Betadine swabs followed by an alcohol swab. After connecting the Huber needle to tubing and a syringe, it is inserted through the diaphragm to the back of the reservoir. Again, the first 1 to 2 mL of blood is discarded, and then the blood culture specimen is collected in a separate syringe. After the blood has been collected, the system is flushed with heparin or saline.
Interpretation of Results
A great deal has been written on the interpretation of blood culture results. Certain clues are useful to distinguish between infection and contamination. The most important factor is the clinical status of the patient. Recovery of a virulent micro-organism in a well child supports contamination. Growth factors that support contamination include an organism that takes several days to grow out, one that grows in only one of several collections, one that grows in the enrichment broth only, and a culture with multiple species present. Finally, certain micro-organisms (diphtheroids, coagulase-negative staphylococci, micrococci, and bacilli) are common contaminants in immunocompetent hosts with no “hardware” (i.e., indwelling prosthetic devices).
Another area of difficulty lies with determining central line infections. It often is problematic to determine if the cultured micro-organism (usually a staphylococcus or Gram-negative rod) is a primary line pathogen. This key distinction has great import for the therapeutic regimen. If the line is the source of infection and not merely of colonization, the clinician should consider line removal.
Many authorities believe that quantitative blood cultures are important in interpreting blood culture results. Yagupsky and Nolte (25), in an extensive review of quantitative studies, note a clear trend toward low colony counts in contaminated blood cultures compared with true positive cultures. Diagnosis of coagulase-negative staphylococcal sepsis in young infants may be aided through these techniques (26). Quantitative studies also may prove useful in monitoring antibiotic
efficacy and predicting the severity of clinical disease (25,27). Several authorities believe that quantitative methods are important in diagnosing central line infections (28,29,30). Others note that this information has not been shown to be necessary for management or to correlate with outcome (31).
efficacy and predicting the severity of clinical disease (25,27). Several authorities believe that quantitative methods are important in diagnosing central line infections (28,29,30). Others note that this information has not been shown to be necessary for management or to correlate with outcome (31).
Quantitative blood cultures hold promise for resolving many of these issues of contamination, colonization, and infection. However, the most important information is gained from the history and physical examination of the patient. The clinical picture is always the crucial variable that dictates the therapeutic plan.
Complications
Complications related to the procurement of blood cultures are divided into two groups: those that can adversely affect the patient and those that can adversely affect the results. The latter mainly involve contaminated specimens. The blood culture may become contaminated from the patient’s own normal skin flora, the blood drawer’s hands, or the laboratory technician’s respiratory droplets. The American Society of Microbiologists set 3% as the maximum acceptable blood culture contamination rate (32). Complications related to phlebotomy or accessing an indwelling line are discussed in Chapters 73 and 74.
Peripheral Blood Culture
Establish rapport with the patient and family.
Consider topical analgesia and child life support to decrease the pain and anxiety associated with the procedure.
Restrain the child as for phlebotomy.
Apply a tourniquet and select a site.
Sterilize the site by scrubbing with alcohol for 60 seconds, followed by Betadine, swabbing three times concentrically starting at the center of the site.
Wait for the Betadine to dry 1 to 2 minutes and then obtain the culture while maintaining universal precautions and without repalpating the site.
Obtain at least 0.5 mL of blood and transfer to a blood culture tube or bottle without changing needles.
Central Line Blood Culture
Sterilize the cap, hub, or site depending on whether the central line is capped, needleless, or totally implanted, as per step 5 above.
Discard 0.5 mL (infant) to 1 mL (child) of blood drawn from the central line (Hickman) or discard 1 to 2 mL of blood from a needleless central line or a totally implanted device.
Obtain blood culture.
Flush the line, catheter, or system with heparin or saline.
The skin should be prepared properly so that blood culture contamination is avoided.
Most children only need to undergo one blood culture, with the exception of patients with endocarditis, central line infection, or likely anaerobic infection.
Obtaining blood cultures from an intravenous site should be avoided because the contamination rate may be increased.
A broth system for detection of blood infection is preferred to media systems because of higher sensitivity, more rapid micro-organism detection, and decreased specimen contamination.
Respiratory Specimens
Respiratory infections are one of the most common reasons pediatric patients are brought to medical attention. Despite the advances in critical care pediatrics, these infections still cause a significant number of pediatric deaths each year. It is therefore of utmost importance to know which study is indicated, how to properly collect the specimen, and how to interpret the results. An accurate diagnosis is the cornerstone of a sound therapeutic plan.
Anatomy and Physiology
It is important to understand the anatomy of the respiratory system to know where to search for a pathogen, what normal flora and cells can be expected to be found, and what structures can be damaged by the procedure. A good nasopharyngeal sample yields a specimen with ciliated, columnar epithelial cells. The majority of respiratory viruses, Chlamydia trachomatis, and Bordetella pertussis are best recovered from here. The oropharynx is the area where group A β-hemolytic streptococci (GABHS) are detected. Although the palatine tonsils regress by puberty, any inflamed areas of the posterior pharyngeal wall may harbor these causative pathogens of tonsillitis.
The trachea, because of its position inferior to the pharynx, is not an ideal location to recover potential bacterial pathogens. Procedures to recover tracheal micro-organisms are invariably contaminated by pharyngeal flora. Furthermore, it usually is unclear if the recovered tracheal micro-organisms accurately reflect the etiology of the suspected pneumonia. The bronchoalveolar lavage (BAL) and protected respiratory
brush (PRB) procedures attempt to sample as close as possible to the area of pulmonary pathology. Both techniques ideally sample secondary or tertiary bronchi. The PRB technique has the added advantage of eliminating much of the upper airway contamination.
brush (PRB) procedures attempt to sample as close as possible to the area of pulmonary pathology. Both techniques ideally sample secondary or tertiary bronchi. The PRB technique has the added advantage of eliminating much of the upper airway contamination.
Indications
The most common indication for the throat swab is the need to detect GABHS. “Strep throat” is a common cause of pharyngitis in the school-age child. Although usually seen in children 2 years of age and older, it can occur at any age. It commonly presents as sore throat with fever. Headache and abdominal pain are also common. Physical examination usually reveals pharyngeal erythema, often accompanied by tonsillar enlargement and exudate.
An important reason to culture even children with classic disease is for management of acute and postinfectious sequelae. Untreated patients may develop suppurative complications (otitis media, cervical adenitis, peritonsillar abscess) and nonsuppurative complications (rheumatic fever, glomerulonephritis). Scarlet fever, which can be recognized by its characteristic sandpaper-like erythematous rash, commonly occurs with pharyngitis and is caused by a group A streptococcal exotoxin.
Another reason for culturing patients is to appropriately manage contacts of the index patient who subsequently develop illness. Symptomatic family members should probably be cultured. Post-treatment cultures are indicated only in patients who are at high risk for rheumatic fever or who are persistently symptomatic.
Throat swabs also are commonly used to detect gonococcal pharyngitis. Gram stain and culture of an exudative pharyngitis are indicated in the appropriate clinical setting. Patients at risk include those who are sexually active and children who are suspected victims of abuse.
Another use of the swab culture is to screen the anterior nares (and other body sites) for S. aureus and its associated toxins. For example, some strains of S. aureus elaborate a toxic shock syndrome toxin (TSST-1). Demonstration of S. aureus carriage also is important for infection control purposes in newborn nurseries, hospital outbreaks, and interhospital transfers.
Many investigators have compared nasopharyngeal swab (NPS) with nasopharyngeal aspirate (NPA) for isolation of respiratory viruses. Heikkinen et al. found that the rate of detection of respiratory syncytial virus (RSV) was significantly higher with NPA than with NPS (33). This has been corroborated by several other studies. Interestingly, this same study found no difference between the two sampling methods with respect to any of the other viruses, although the numbers were small.
In the diagnosis of severe acute respiratory syndrome (SARS), Chan et al. found that pooled throat and NPS specimens provided a higher diagnostic yield than did NPA specimens (34). This is important in that the NPS procedure has a greater risk of generating infectious aerosols during its performance. Nevertheless, most experts now recommend the use of NPA over NPS as the collection method of choice for most respiratory pathogens.
Interestingly, one small study done on 32 young military recruits with pneumonia in Finland found that a sputum sample was superior to both NPS and NPA for reliable detection of Mycoplasma pneumoniae by polymerase chain reaction (PCR) assay (35). Another study compared NPS and sputum from 50 adult patients with pneumonia for the detection of another common respiratory pathogen, Chlamydia pneumoniae, and found no difference between the two samples (36).
Tracheal aspiration is probably more useful as a therapeutic rather than a diagnostic procedure. It usually is done through an endotracheal tube to remove airway secretions and debris in order to optimize gas exchange and prevent atelectasis and infection. Diagnostically, the aspirate occasionally may be helpful in the investigation of infectious respiratory disease. Bacterial tracheitis is one such example.
A useful technique in diagnosing severe or unusual lower respiratory disease is BAL. One disease in which it has proven especially useful is Pneumocystis carinii pneumonia (PCP). This disease of immunocompromised patients is readily diagnosed by staining BAL washings. This early diagnosis avoids empiric therapy and leads to early treatment and improved outcome. Other common diseases of the immunocompromised also may be diagnosed with BAL; these include infections caused by cytomegalovirus, Aspergillus fumigatus, Candida albicans, and Legionella pneumophila. Additionally, other bacterial, mycobacterial, and viral diseases can be diagnosed by BAL.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree