Obstetric and Perinatal Infections
Jill K. Davies
Ronald S. Gibbs
In this chapter, six important infections are discussed—group B streptococci (GBS), varicella-zoster virus (VZV), parvovirus B19, toxoplasmosis, cytomegalovirus (CMV), and herpes simplex virus (HSV)—focusing on their relevance during pregnancy to both the mother and the fetus. In addition, two common peripartum infections will be reviewed—intra-amniotic infection (IAI) and postpartum endometritis.
Group B Streptococci
In the 1970s, GBS became recognized as the leading cause of neonatal infection and an important cause of maternal genital tract infection. Prior to the mid 1990s, early-onset neonatal GBS disease incidence ranged between 1.5 to 2.0 cases per 1,000 live births. As prevention measures then came into practice, disease incidence declined by 70% to a rate of 0.5 cases per 1,000 live births. Most recent surveillance data indicates the rate of early onset is now 0.34 cases in 1,000 live births, and the rate of late onset disease decreased to 0.38 cases in 1000 live births.
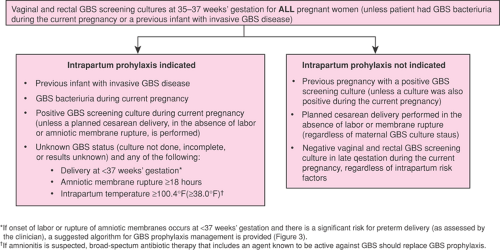
In 1996, the first national consensus guidelines were released. Based on new evidence from a large retrospective cohort study, new national prevention guidelines from the Centers for Disease Control and Prevention (CDC), American Academy of Pediatrics (AAP), and American College of Obstetricians and Gynecologists (ACOG) were released in 2002, recommending a single prevention strategy—namely, universal antenatal culture-based screening at 35 to 37 weeks gestation.
Epidemiology
It is estimated that 20% to 30% of all pregnant women are GBS carriers, but colonization can be intermittent or transient. Prenatal screening for GBS at 35 to 37 weeks gestation is recommended by the 2002 national guidelines.
In newborns, the most common GBS infections are sepsis, pneumonia, and meningitis (Fig. 19.1). Early-onset disease occurs within the first week of life; late-onset disease occurs after the first week. Outcome survival has improved recently. For term infants, survival for GBS sepsis is about 98%, but for preterm infants the survival is lower, at 90% for cases at 34 to 36 weeks gestation and 70% for cases at less than 33 weeks.
Risk factors for early-onset disease are maternal GBS colonization, prolonged rupture of membranes, preterm delivery, GBS bacteriuria during pregnancy, birth of a previous infant with invasive GBS disease, maternal chorioamnionitis as evidenced by intrapartum fever, young maternal age, African American race, Hispanic ethnicity, and low levels of antibody to type-specific capsular polysaccharide antigens.
Manifestations of maternal GBS infection include urinary tract infection, chorioamnionitis, endometritis, bacteremia, and stillbirth.
GBS are isolated in 15% of cases of amnionitis, 15% of cases of endometritis, 2% to 15% of infected abdominal wounds after cesarean delivery, and about 15% of cases of bacteriuria in obstetric patients.
Diagnosis
The 2002 CDC guidelines provide directions for collecting and processing these specimens (Table 19.1). These guidelines recommend a rectogenital specimen to obtain optimal yield of GBS.
There has been intense interest in tests for rapid identification of GBS. In one study, a polymerase chain reaction
(PCR) test reported excellent sensitivity, specificity, positive predictive value, and negative predictive value when compared with conventional anovaginal cultures. The reported laboratory turnaround time was 40 to 100 minutes for these PCR methods. The FDA approved an intrapartum rapid PCR test (IDI-Strep B, Infection Diagnostic Inc., Quebec, Canada). However, there are “real-world” limitations to this test, including turnaround time on a 24-hour, 7-day per week basis and inability to determine antibiotic susceptibility in women who cannot take penicillin or a cephalosporin. One role for PCR tests may be in testing women whose GBS status is unknown on admission in labor.
(PCR) test reported excellent sensitivity, specificity, positive predictive value, and negative predictive value when compared with conventional anovaginal cultures. The reported laboratory turnaround time was 40 to 100 minutes for these PCR methods. The FDA approved an intrapartum rapid PCR test (IDI-Strep B, Infection Diagnostic Inc., Quebec, Canada). However, there are “real-world” limitations to this test, including turnaround time on a 24-hour, 7-day per week basis and inability to determine antibiotic susceptibility in women who cannot take penicillin or a cephalosporin. One role for PCR tests may be in testing women whose GBS status is unknown on admission in labor.
 Figure 19.1 Neonatal autopsy showing congenital pneumonia due to GBS. (From Gibbs RS, Schrag S, Schuchat A. Perinatal infections due to group B streptocci. Obstet Gynecol 2004;104[5]:1064 .) |
Tests from genital specimens other than PCR (such as antigen detection) are not sufficiently sensitive for clinical use.
Therapy
Because resistance to penicillin or ampicillin has not been detected in GBS, penicillin, because of its narrow spectrum of activity, remains the agent of choice for GBS prophylaxis, with ampicillin as an alternative. Resistance to clindamycin and erythromycin among GBS isolates is widely prevalent, ranging from 7% to 30% for erythromycin and 3% to 15% for clindamycin. Resistance to also has been detected.
These resistance trends led to a revision of the first- and second-line antibiotics (Table 19.2). Because of the possibility of inducible resistance, the 2002 guidelines recommend that clindamycin or erythromycin be used only if a given patient’s GBS isolate was shown to have in vitro susceptibility to both. If there is in vitro resistance to either in a patient at high risk for penicillin anaphylaxis, then vancomycin should be used. For women at high risk of penicillin allergy colonized by clindamycin-resistant or erythromycin-resistant isolates, the 2002 guidelines recommend vancomycin. Because vancomycin use is restricted due to concerns regarding emerging vancomycin resistance, vancomycin should be reserved, in GBS prophylaxis, for highly penicillin-allergic women with isolates of unknown susceptibility and isolates with resistance to either erythromycin or clindamycin.
Because of its uniform activity, penicillin G remains the drug of choice for clinically evident maternal infection with GBS. Ampicillin is used widely and is an acceptable alternative. The usual dose of penicillin G is 5.0 million units intravenously initially, then 2.5 million units intravenously every 4 to 6 hours. Note that for prevention of GBS perinatal infections, the dosing interval is every 4 hours until delivery. For ampicillin, the usual adult dose is 2 g intravenously initially, then 1 g intravenously every 4 to 6 hours. Again, note that for GBS prophylaxis, the dosing interval is every 4 hours until delivery.
Prevention
The indications for prophylaxis under this universal prenatal screening strategy are shown in Figure 19.2.
Obstetric Procedures
Membrane sweeping (or stripping) among term patients hastens the onset of labor, with no increases in overall perinatal or peripartum infection. However, because studies using this technique did not report GBS status, there are no data to advise whether this procedure should or should not be avoided in GBS-positive women. Nevertheless, because the benefits of membrane sweeping are limited (such as a significantly greater likelihood of onset of labor within 48 hours). The authors avoid membrane sweeping in GBS-positive women. If there is an indication for delivery, there are many alternative interventions to membrane sweeping, such as vaginal prostaglandin preparations.
Vaccine Development
Immunization holds promise to prevent a larger burden of disease, protecting against both early- and late-onset
infections. Moreover, vaccination may prevent some adverse pregnancy outcomes associated with GBS, such as preterm delivery, spontaneous abortion, or stillbirth, particularly if vaccination of adolescent girls before pregnancy is a viable strategy. Additionally, immunization strategies would not contribute to emerging antimicrobial resistance among GBS. Promising GBS vaccine candidates now exist but are not available commercially.
infections. Moreover, vaccination may prevent some adverse pregnancy outcomes associated with GBS, such as preterm delivery, spontaneous abortion, or stillbirth, particularly if vaccination of adolescent girls before pregnancy is a viable strategy. Additionally, immunization strategies would not contribute to emerging antimicrobial resistance among GBS. Promising GBS vaccine candidates now exist but are not available commercially.
TABLE 19.1 Procedures for Collecting and Processing Clinical Specimens for Group B Streptococcal Culture and Performing Susceptibility Testing to Clindamycin and Erythromycin | ||
|---|---|---|
|
Varicella-zoster Virus (VZV)
VZV, a member of the herpesvirus family, is the etiologic agent that causes chicken pox, an almost universal infection of children and adolescents. Its reactivation is the cause of herpes zoster. Ninety percent of individuals will be immune prior to adulthood. Infection during pregnancy complicates 0.7 per 1,000 pregnancies. The incubation period
for varicella zoster is 10 to 21 days, and infected individuals are contagious from 1 to 2 days before the rash begins until all of the skin lesions are crusted over. The lesions are classically described as “dew drops on a rose petal.” During the prodrome and early infection, there is characteristic fever and malaise followed by development of the pruritic rash. Following varicella-zoster infection, the virus lays dormant in the dorsal horn cells of the spinal cord. Reactivation causes herpes zoster.
for varicella zoster is 10 to 21 days, and infected individuals are contagious from 1 to 2 days before the rash begins until all of the skin lesions are crusted over. The lesions are classically described as “dew drops on a rose petal.” During the prodrome and early infection, there is characteristic fever and malaise followed by development of the pruritic rash. Following varicella-zoster infection, the virus lays dormant in the dorsal horn cells of the spinal cord. Reactivation causes herpes zoster.
TABLE 19.2 Recommended Regimens for Intrapartum Antimicrobial Prophylaxis for Perinatal Group B Streptococci Disease Prevention* | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Most women are immune to varicella prior to adulthood due to a history of typical varicella infection as a child, which is considered sufficient for immunity. As most women are immune to varicella before reproductive age, most who do not specifically recall a history of chicken pox infection as a child are immune as well. Varicella vaccine (Varivax, Merck, Whitehouse Station, NJ) is now available and recommended for administration during childhood. Thus, the overwhelming majority of pregnant women are immune to varicella prior to pregnancy. Immunocompetent individuals will not get chicken pox again.
Varicella Exposure and Management
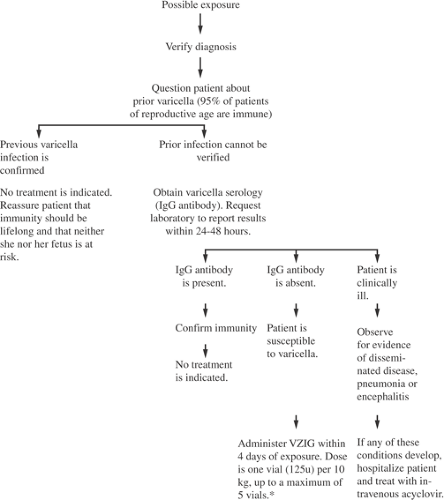
When exposure occurs in a pregnant woman, it is important to obtain her history regarding prior varicella infection. The diagnostic approach to the pregnant woman with possible varicella exposure is shown in Figure 19.3. With a prior history of typical chicken pox infection, she can be reassured. No serologic confirmation is required. In the setting of an inconclusive maternal history, a VCV immunoglobulin G (IgG) level should be checked urgently. Most laboratories can provide results of this in a day or so. If a woman’s IgG is positive shortly after exposure, this indicates prior immunity and she can be reassured. If her IgG is negative, she is susceptible to infection from the exposure. A significant exposure to varicella consists of a household contact, a face-to-face contact with an infected individual for at least 5 minutes, or an indoor contact with chicken pox or herpes zoster for at least 1 hour. If the woman meets these criteria, her varicella immunoglobulin M (IgM) antibody level should be measured. If her IgM is negative, immune globulin should be given to prevent transmission of the virus or lessen its severity. She should be given postexposure prophylaxis with varicella-zoster–specific immune globulin (VariZIG, FFF Enterprises, Temecula, CA). The adult dosage of VZIG is 625 U and is most effective when given within 96 hours of exposure. If VZIG is not available, 0.6–1.2 mL/kg of intravenous immune globulin (IVIG) can be given. In one trial, administration of VZIG to pregnant women within 96 hours of exposure was demonstrated to be highly efficacious in preventing 80% (20 of 25) of clinical infection. The 16 of 18 women who did not receive VZIG contracted infection. In addition, when VZIG was given between 3 and 10 days after exposure, the same group described attenuation of maternal infection.
Natural History and Pathophysiology
Although most pregnant women will have self-limited infection, VZV during pregnancy has been associated with increased morbidity and mortality compared with nonpregnant adult infection (http://www.fda.gov/cber/ infosheets/mphvzig092005.htm). Symptomatic therapy with antipyretics and antipruritic agents that are safe during pregnancy can be used. Because of the high infectivity of
this virus, the patient should be properly isolated from other susceptible pregnant women. When diagnosis of maternal varicella is made, patients should be treated with acyclovir 800 mg orally five times daily for 7 days to decrease severity of infection, as it has been associated with a decrease in time to lesion healing and lessens fever and other symptoms. Five percent of women in the National Institute of Child Health and Development (NICHD) review of maternal varicella developed varicella pneumonia. Women with VZV infections during pregnancy should be given strict respiratory precautions, as a significant minority of women who develop varicella pneumonia will develop respiratory failure warranting mechanical ventilation, and their respiratory status can rapidly decompensate. Respiratory symptoms most likely will develop on the second day of rash, often consisting of dry cough, hemoptysis, pleuritic chest pain, and shortness of breath. Chest radiographs most often will show a miliary or diffuse nodular pattern. Varicella pneumonia should be treated aggressively with acyclovir 10 to 15 mg/kg given intravenously three times daily for 7 days. Higher doses of acyclovir are needed to treat varicella due to its lower specificity for VZV compared with HSV.
this virus, the patient should be properly isolated from other susceptible pregnant women. When diagnosis of maternal varicella is made, patients should be treated with acyclovir 800 mg orally five times daily for 7 days to decrease severity of infection, as it has been associated with a decrease in time to lesion healing and lessens fever and other symptoms. Five percent of women in the National Institute of Child Health and Development (NICHD) review of maternal varicella developed varicella pneumonia. Women with VZV infections during pregnancy should be given strict respiratory precautions, as a significant minority of women who develop varicella pneumonia will develop respiratory failure warranting mechanical ventilation, and their respiratory status can rapidly decompensate. Respiratory symptoms most likely will develop on the second day of rash, often consisting of dry cough, hemoptysis, pleuritic chest pain, and shortness of breath. Chest radiographs most often will show a miliary or diffuse nodular pattern. Varicella pneumonia should be treated aggressively with acyclovir 10 to 15 mg/kg given intravenously three times daily for 7 days. Higher doses of acyclovir are needed to treat varicella due to its lower specificity for VZV compared with HSV.
Neonatal Varicella
Newborn infection can occur if maternal infection with rash and symptoms develop from approximately 5 days before delivery until 2 days after delivery. This is the period in which there is insufficient time for maternal IgG antibody development and passive transfer to protect the fetus. Infection in the neonate usually will occur within 5 to 10 days of life and is of variable course but can be quite severe. Passive immunization of the exposed neonate with 125 U of VZIG is recommended if maternal varicella infection occurs within 5 days of delivery to 2 days following delivery as well as to any exposed neonate born at less than 28 weeks gestation, as IgG antibody transfer at premature gestational ages is less efficient. Although VZIG will not protect all neonates from varicella, it should reduce the severity of infection. Appropriate isolation of exposed infants should occur as well.
Fetal Effects and Prenatal Diagnosis
Varicella has been shown to cause fetal effects. Although first-trimester infection is associated with a low risk for miscarriage and anomalies (0.4%), the second trimester between approximately 12 and 20 weeks is the highest risk for vertical transmission causing serious fetal abnormalities (congenital varicella syndrome). Although vertical transmission has been estimated to be as high as 10%, more recent studies suggest this risk to be less than 2%. Fetal abnormalities include skin scarring, a variety of fetal central nervous system (CNS) abnormalities,
ophthalmologic effects, limb anomalies, and gastrointestinal abnormalities. These fetal effects are thought to be as a result of immaturity of the fetal immune system as well as intrauterine herpes zoster resulting in a predilection for neuronal cells and their downstream innervated tissues. There is no clear information regarding whether maternal administration of VZIG or acyclovir will lessen or prevent these fetal effects.
ophthalmologic effects, limb anomalies, and gastrointestinal abnormalities. These fetal effects are thought to be as a result of immaturity of the fetal immune system as well as intrauterine herpes zoster resulting in a predilection for neuronal cells and their downstream innervated tissues. There is no clear information regarding whether maternal administration of VZIG or acyclovir will lessen or prevent these fetal effects.
Approximately 4 to 6 weeks following resolution of maternal varicella infection, detailed ultrasound exams to evaluate for fetal effects should be performed. A subsequent ultrasound should be performed between 8 and 12 weeks to look for delayed effects.
Herpes Zoster
Herpes zoster represents a reactivation of VZV and is commonly known as shingles. Although herpes zoster may be contagious to a nonimmune person by skin-to-skin contact, it is not spread by respiratory secretions. Fetal effects are not reportedly seen, as most herpes zoster infections do not result in viremia. Depending on dermatomal reactivation, intrauterine infection theoretically could be possible if the uterus were involved with reactivation of T10-L1 (which innervates the uterus). In most recent reviews, in utero transmission has not been reported without dissemination. These painful infections should be treated with acyclovir.
Prevention
Postpartum vaccination of varicella-nonimmune women should be offered when breast-feeding has been completed. Since it is a live, attenuated vaccine, the ACOG and AAP recommended that women avoid conception for 1 month following vaccine administration, although the manufacturer recommends a 3-month delay (http://www. merck.com/product/usa/pi_circulars/v/varivax/varivax_pi.pdf). However, since vaccine virus has not been found to be excreted into breast milk, it has been suggested that vaccine administration not be delayed in nonimmune breast-feeding women.
Parvovirus B19
Epidemiology
Erythema infectiosum and fifth disease are two common names for parvovirus B19 infection. B19 is the only parvovirus known to cause human infection. It was not until 1984 that parvovirus B19 was first described as a cause of fetal infection and nonimmune hydrops. Only 30% to 60% of adults are immune to this virus. The incidence of parvovirus B19 seroconversion during pregnancy is 1% to 2% but may be higher than 10% during epidemics.
Pathophysiology
Parvovirus is a highly contagious infection, with an attack rate of 60% to 80% of susceptible household contacts, whereas only 20% to 30% of susceptible schoolteachers or day care workers will become infected following exposure. Infection is most common in late winter or spring and is transmitted by respiratory droplets and contaminated blood. Transplacental fetal transmission occurs in one third of cases, with risk of adverse fetal outcome in approximately 10%. Parvovirus is a strong inhibitor of hematopoietic cells, including liver, myocardium, and erythroid precursor cells. The P antigen on the red blood cell is a receptor for parvovirus B19; fetal cardiac myocytes are another receptor for the P antigen. Although maternal infection can be asymptomatic, some will develop symptoms including fever, myalgias, and malaise initially followed by arthralgias and sometimes pruritis and rash—commonly a “slapped cheek”–appearing rash in children. Uncommonly, meningoencephalitis, hepatitis, or myocarditis may occur with seroconversion. Symptomatic fetal infection is seen in approximately 10% and is associated with myocarditis; nonimmune hydrops; fetal demise; spontaneous loss; and rarely, neurologic complications. When hydrops occurs, it usually does so within 2 to 6 weeks of fetal infection, although the reported range is 1 to 12 weeks, with the maximal risk for development of fetal hydrops between 17 and 24 weeks gestation. Although the mechanism behind development of hydrops is incompletely understood, severe anemia leading to high-output cardiac failure is the likely pathophysiologic mechanism.
Diagnosis
Diagnosis of maternal parvovirus B19 infection initially is made by serologic methods with enzyme immunoassay of B19 IgM and IgG. Antibodies of the IgM class will become measurable in 7 to 10 days following maternal infection, peaking at about 14 days then declining over 2 to 3 months. The IgG antibodies will rise more slowly, not peaking until about 4 months following acute infection. When IgG is positive on the initial specimen following exposure and IgM is negative, the patient demonstrates evidence of past infection. Immunity is lifelong, and she can be reassured that there is no fetal risk. If the first serum sample demonstrates negative IgM and IgG, acute seroconversion still may be occurring. Thus, repeat titers should be repeated 1 to 2 weeks after the initial titers were collected to study the evolution of the titers. To compare the sets of IgM/IgG antibodies most accurately, the laboratory should freeze the serum after running the first specimen to run together with the second specimen. Maternal B19 viremia will be present during acute seroconversion by DNA PCR, but persistent low-level viremia may persist for years following acute seroconversion. Thus, PCR testing for the virus is a less specific and a more expensive method of detection. New on the horizon include parvovirus B19 IgG avidity assays as well as IgG epitope specificity assays. These assays likely will be most useful in those patients with low-level parvovirus B19 IgM levels that may signify nonrecent seroconversion and may be associated with less, if any, fetal risk. Because fetal immunologic response is less pronounced, fetal and cord blood examinations should be confined to B19 DNA PCR.
Prenatal Diagnosis and Fetal Management
Following confirmation of acute maternal seroconversion, fetal ultrasound examinations should be serially performed for 10 to 12 weeks following seroconversion to evaluate for fetal anemia as well as hydrops and other signs of fetal viral infection such as calcifications in the liver and myocarditis. Similar to Rh isoimmunization, anemic fetuses from parvovirus B19 infection will demonstrate increased blood velocity, as measured by peak systolic velocity in the middle cerebral artery. Ultrasound evidence of anemia usually will precede the development of fetal hydrops, allowing for optimization of fetal procedures such as percutaneous umbilical blood sampling (PUBS) and intrauterine transfusion (IUT). Hydrops, defined as fluid in two or more fetal body cavities, traditionally was defined as pleural effusions, pericardial effusions, ascites, and scalp edema but also may manifest as placental edema and be accompanied by amniotic fluid abnormalities. IUT to support the fetus during the parvovirus B19 suppression of hematopoiesis can prevent hydrops formation or cause it to resolve. Resolution of hydrops may take weeks despite correction of fetal anemia with IUT. Spontaneous recovery of parvovirus-induced hydrops has been described but is uncommon. Fetal mortality is higher when hydrops is present and is lessened with IUT. Prognosis following fetal recovery from in utero parvovirus infection usually is quite good, and only rare neurodevelopmental, cardiac, and hematologic sequelae have been reported.
Toxoplasmosis
Primary maternal toxoplasmosis occurs in approximately 1 of every 900 pregnancies in the United States. This estimate
is based on a prospective study of sera from 23,000 pregnant women done in early and late gestation. That study, conducted by the National Institutes of Health, also showed that 38% of the women tested had antibodies to Toxoplasma gondii, indicating previous infection with the organism. More recent data suggest that the seroprevalence may now be somewhat lower (15% among women of childbearing age); this reduction may be due to improved hygiene, education, and meat practices. In earlier data, the presence of antibodies correlated with increasing patient age and was twice as frequent among blacks as among whites. None of the mothers tested had evidence of significant clinical disease. It has been estimated that between 400 and 4,000 babies are born with congenital T. gondii infections each year. Some data suggest that congenital infections occur in approximately 1 in 10,000 births.
is based on a prospective study of sera from 23,000 pregnant women done in early and late gestation. That study, conducted by the National Institutes of Health, also showed that 38% of the women tested had antibodies to Toxoplasma gondii, indicating previous infection with the organism. More recent data suggest that the seroprevalence may now be somewhat lower (15% among women of childbearing age); this reduction may be due to improved hygiene, education, and meat practices. In earlier data, the presence of antibodies correlated with increasing patient age and was twice as frequent among blacks as among whites. None of the mothers tested had evidence of significant clinical disease. It has been estimated that between 400 and 4,000 babies are born with congenital T. gondii infections each year. Some data suggest that congenital infections occur in approximately 1 in 10,000 births.
Microbiology: Transmission
Cats
The cat is the definitive host for T. gondii, which is a protozoan parasite (Fig. 19.4). About one half of the cats tested in the United States have antibodies to T. gondii. It is thought that cats acquire infection by eating infected wild rodents and birds. A week after infection, the cat begins to shed oocysts in its feces. Shedding of the oocysts persists for about 2 weeks only, then the cat recovers spontaneously. These animals are susceptible to reinfection and also may shed toxoplasma oocysts when infected with other organisms.
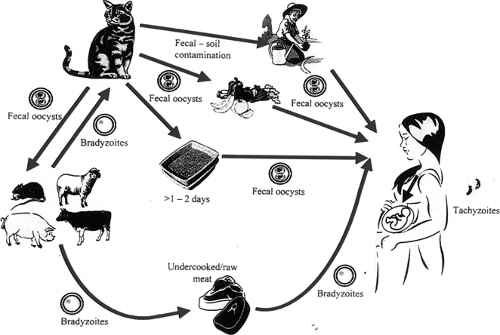 Figure 19.4 T. gondii in trophozoites or proliferative form. (From Jones JL, Lopez A, Wilson M, et al. Congenital toxoplasmosis: a review. Obstet Gynecol Surv 2001;50:297 , with permission.) |
Although the cats excrete unsporulated (i.e., noninfectious) oocysts in their feces, within days to weeks these oocysts sporulate and become extremely infectious. The fecal oocysts are an important source of infection to humans through inadvertent ingestion. Because sporulation of the organism occurs after days to weeks in cat litter, it is recommended that pregnant women not change the litter and instead it is changed daily by a nonpregant person. Care must be taken in disposing of cat litter because the oocysts can remain infectious for long periods in favorable climates.
Food
In Europe, where the use of refrigeration is more limited and foods usually are not frozen, ingestion of contaminated food is an important cause of toxoplasmosis. Although meat is the most common infected food source discussed, produce from oocyte-contaminated soil as well as unpasteurized milk and unfiltered water sources also are at risk. In the United States, most meat is frozen at some point during storage or transport. Freezing is probably one of the factors responsible for the difference in incidence of toxoplasmosis here and in Europe. Worldwide, about 1% of cattle, 20% of hogs, and 30% of sheep have toxoplasmosis.
To avoid contamination from meat, it should be cooked thoroughly at adequate temperatures. To avoid contamination of fruits and vegetables from potentially contaminated soils, thorough washing of fruits and vegetables is recommended. In addition, proper hand hygiene, prevention of cross contamination of cooking surfaces, and cleaning of food preparation areas and kitchen knives is crucial. Organ transplantation is another less common risk factor.
To avoid contamination from meat, it should be cooked thoroughly at adequate temperatures. To avoid contamination of fruits and vegetables from potentially contaminated soils, thorough washing of fruits and vegetables is recommended. In addition, proper hand hygiene, prevention of cross contamination of cooking surfaces, and cleaning of food preparation areas and kitchen knives is crucial. Organ transplantation is another less common risk factor.
Epidemiology
T. gondii has a worldwide distribution and has been reported from wherever cats are found. It is somewhat more common in tropical and coastal regions and is less common in regions that are cold, warm and arid, or at high elevation. The infection rate in the United States is significantly lower than that in France. Within the United States, seroprevalence rates are lower in the western central and mountain states and higher in eastern Atlantic and eastern central states.
Pathophysiology
Acute toxoplasmosis generally is well tolerated in immunocompetent adults but may result in vertical infection to the fetus and lead to potentially serious consequences. In the immunocompetent adult host, symptoms usually are mild or inapparent. In about 10%, fever, fatigue, malaise, headache, myalgias, and lymphadenopathy will be present. These symptoms will resolve in weeks to months without specific therapy. In immunosuppressed adult patients, signs and symptoms often will be more pronounced and can result in significant ocular and CNS abnormalities. Reactivation infection in immunosuppressed pregnant women also can cause fetal infection.
T. gondii exists in three forms: trophozoites or proliferative form (Fig. 19.4), tissue cysts, and oocytes. The organism undergoes a substantial portion of its life cycle in the cat, where after between 5 and 8 days of infection, there is peak oocyte production. As many as ten million oocysts per day can be shed in feces for periods varying between 7 and 20 days. These oocytes sporulate within 1 to 3 days and can remain infectious for several months in moist soil. At that point, they may be carried from point of deposition by other animals (e.g., flies) and deposited in food. It also has been suggested that they can become airborne from a dried-out litter box and lead to human infection. Eating undercooked or raw meat that contains tissue cysts causes approximately one half of infections in most humans. In the human, the trophozoite form of T. gondii is seen in the acute phase of the infection, and it is during this phase that host cells are invaded. Thereafter, the organism multiplies every 4 to 6 hours until the cytoplasm becomes so filled with trophozoites that the cells rupture, releasing organisms to invade other cells.
In Utero Transmission
Newborns with congenital toxoplasmosis become infected in utero by transplacental passage of the parasite when the mother has acute infection. Chronic infections (onset precedes pregnancy) do not lead to congenital infection except in the rare circumstance of an immunocompromised host (e.g., patient with systemic lupus erythematosus taking steroids, HIV infection) with reactivation. In general, the likelihood of fetal infection increases with each trimester of pregnancy, being approximately 15%, 25%, and 60% in the first, second, and third trimester, respectively. The severity of damage associated with congenital toxoplasmosis also is related to the timing of maternal infection, but in this situation, the risks decrease toward term. Severe fetal disease or fetal death occurs in about 10% of cases when infection occurs during the first trimester and is extremely rare with infection during the third trimester. Mild damage is more frequent in the second and third trimesters (about 5%). Subclinical infections increase from about 2% with first-trimester infections to 50% with third-trimester infections. The results of a case-control study of women with poor pregnancy outcomes and controls suggested that acute infection could be associated with preterm delivery and stillbirth but not with spontaneous abortion. Chronic infections were not associated with any untoward outcomes.
Diagnosis in Pregnancy
Maternal Infection
Maternal infection with T. gondii usually is asymptomatic, although 10% to 20% of infected mothers have lymphadenopathy. Posterior cervical lymphadenopathy is the most frequent finding associated with acute maternal toxoplasmosis. The infection also can result in a mononucleosislike syndrome with fatigue and lassitude and, rarely, can cause encephalitis. Acute toxoplasmosis should be considered in any pregnant woman who has lymphadenopathy, particularly involving the posterior cervical chain, or mononucleosislike symptoms. The vast majority, however, of those acutely infected with T. gondii are asymptomatic. The clinical picture can be much more severe in immunocompromised adults.
Because of the wide clinical spectrum of toxoplasmosis, clinicians are forced to rely on serologic tools for the diagnosis of toxoplasmosis in pregnancy. The diagnosis of primary infection with T. gondii during pregnancy requires either (a) the demonstration of a seroconversion to this organism, (b) a significant rise in antibody titer obtained from maternal sera taken at two different times, or (c) the detection of toxoplasma-specific IgM antibody. Adults with primary infection develop IgG and IgM antibody to toxoplasma rapidly. Toxoplasma-specific IgG antibody develops within 2 weeks after infection, peaks in 6 to 8 weeks, drops down over the subsequent several months, and then
persists for life. Toxoplasma-specific IgM develops within 10 days after infection and remains elevated for 6 months to more than 6 years.
persists for life. Toxoplasma-specific IgM develops within 10 days after infection and remains elevated for 6 months to more than 6 years.
Because IgM antibody remains elevated for many months, IgM titers may not provide useful information to document recent primary infection in pregnant women. The enzyme-linked immunosorbent assay (ELISA) test for IgM frequently shows the development of high titers of IgM antibody that can persist for many months and even years. Indirect immunofluorescence antibody (IFA) tests for toxoplasma-specific IgM usually show high titers for only about 6 months after infection; thereafter, the titer rapidly drops. The IFA test, then, frequently is more useful than ELISA in differentiating remote from recent primary infection of a pregnant woman. In any case, the presence of IgG and the absence of IgM suggest an infection that is probably at least a year old.
Because of difficulties with the reliability of some commercially available tests for IgM, it has been recommended that all positive test results be confirmed in a reference laboratory. The authors recommend the laboratory of Jack Remington and colleagues, who can be reached at 650-853-4828 or toxolab@pamf.org. Up to 40% of positive toxoplasma-specific IgM results from commercial laboratories are false positives.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree