Neonatal Seizures
INTRODUCTION
A seizure may be the first sign of neurological dysfunction and serious systemic disease. The potential etiologies are diverse and range from very serious brain injury to benign, age-limited genetic conditions. Because the underlying cause of the seizures may be an acute, potentially harmful, and treatable metabolic derangement (eg, hypoglycemia) or infection, early recognition, evaluation, and treatment are important.
EPIDEMIOLOGY
Seizures occur more often during the first week of life than at any other time.1 The reported incidence ranges from 1 to 5 per 1000 newborns in developed countries.2–4 The incidence is much higher in developing countries.5 Premature and low birth weight infants also have a higher incidence of seizures than term and normal weight newborns.4,6,7
Etiology of Seizures in the Neonate
The most common etiology of seizures in the neonate is a hypoxic-ischemic insult as a result of conditions such as perinatal asphyxia, cardiac disease, and respiratory distress. Other common causes include infections, cerebral dysgenesis, and stroke. Less-common etiologies are the benign epilepsy syndromes that present in the neonatal period. A large variety of rare genetic and metabolic conditions are associated with severe seizures and a poor long-term prognosis, although some are treatable. The most severe seizure disorders in neonates are caused by early infantile epileptic encephalopathy (EIEE; Otahara syndrome) and early myoclonic epilepsy (EME).8,9
Hypoxic-Ischemic Encephalopathy
Hypoxic-ischemic encephalopathy is the most common etiology of neonatal seizures. Approximately two-thirds of all neonatal seizures are a result of this cause.10 Both animal studies and human case studies showed that the frequency and severity of neonatal seizures in the setting of hypoxic-ischemic encephalopathy usually parallel that of the encephalopathy.11,12
Seizures caused by hypoxic encephalopathy usually manifest in the first 2 days of life.13 They are often persistent and difficult to control acutely. When they do come under control from a clinical perspective, they frequently persist on electroencephalogram (EEG). Seizures caused by an acute hypoxic insult are often transient, so seizure medication may only be needed in the short term. However, there is an independent long-term risk of epilepsy later in life. Approximately one-third of these infants will develop epilepsy.14
Infections
Meningitis
The organisms most commonly responsible for neonatal seizures during the first few days of life are β-hemolytic group B streptococci (GBS), Escherichia coli, and other gram-negative organisms reflecting maternal flora. Later-onset meningitis may be the result of community exposure as well.15 Seizures are among the common presenting signs of meningitis in the neonate, along with irritability, lethargy, poor feeding, poor tone, and apnea.16 Furthermore, seizures in the setting of neonatal meningitis are a concerning prognostic sign indicating a higher risk of an abnormal neurological outcome.17
Encephalitis
Central nervous system infection with herpes simplex virus (HSV) commonly presents with lethargy, poor temperature regulation, and seizures. The neonatal form is most often caused by type 2 HSV. Seizures are a poor prognostic sign. But, treatment with a full course of acyclovir does correlate with a better outcome. Still, over 50% of children with neonatal herpes will develop epilepsy.18–21
Congenital cytomegalovirus (CMV) infection is a relatively common infection, affecting as many as 1% of newborns worldwide. Seizures may be caused by either the active infection or, in most cases, brain injury and malformation caused by intrauterine infection.22
Intracranial Hemorrhage and Stroke
Hemorrhage
Subarachnoid, intraventricular, and intraparenchymal hemorrhages may cause seizures in term and preterm infants. Subdural and subarachnoid hemorrhages may cause seizures in infants even when a vaginal delivery does not appear to have been traumatic.23 Focal clonic seizures, presumably caused by irritation of primary motor cortex, are a common manifestation. Typically, these seizures are easier to control than those caused by more diffuse insults, such as hypoxia-ischemia. Exclusion of systemic metabolic and infectious disease is important to have confidence that the hemorrhage is the etiology and not merely incidental. Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain will show subarachnoid blood over the convexities of the hemispheres and along the tentorium. The seizures are usually self-limited, and the prognosis is favorable.24
In the premature infant, intraventricular hemorrhages occur as a result of bleeding in the subependymal germinal matrix. Large hemorrhages can be associated with parenchymal injury and may present with seizures. Intraventricular hemorrhage accounts for a large proportion of seizures presenting in premature infants.25
Parenchymal hemorrhage, including hemorrhage with subdural and subarachnoid extension, may result from venous sinus thrombosis. Perinatal risk factors such as complicated delivery and preeclampsia are frequently present and are more common than prothrombotic factors.26 Seizures occur in about half of cases.27 Symptoms are commonly present in the first 2 days after birth. Babies with intraparenchymal hemorrhage caused by sinovenous thrombosis are at significant risk for an abnormal neurological outcome.28 Acute treatment with anticoagulation is controversial because of the perceived risk of additional hemorrhage and the high rate of spontaneous recanalization.
Stroke
Arterial occlusion may occur acutely because of complicated delivery, but stroke, particularly involving the middle cerebral artery distribution, may occur in utero. Thromboembolism caused by congenital heart disease may also lead to ischemic stroke. An acute stroke in a neonate will usually be associated with encephalopathy. But, a baby with a remote intrauterine stroke may look entirely normal at birth and have good bilateral motor function. Seizures, particularly focal clonic seizures, are a frequent first symptom of an ischemic stroke in the neonate. Infants frequently develop spastic hemiparesis as a long-term sequela of stroke, but fewer than half of such infants will develop epilepsy.29
Cerebral Malformations
Malformations of cortical development are a common cause of epilepsy in children. The majority present before 16 years of age. A sizable proportion present at less than a month of age.30 The type of lesions range from diffuse malformations with dismal prognoses, such as lissencephaly, to subtle focal cortical dysplasia that may not be apparent with initial neuroimaging.31
Tuberous sclerosis (TS) is a particular genetic disorder associated with multifocal cortical malformations (“tubers”). The seizures commonly associated with TS may present in the neonate in conjunction with cutaneous hypopigmented macules, cardiac rhabdomyomas, and renal angiomyolipomas.32,33 TS may present in the neonatal period with a variety of seizure types, ranging from infantile spasms to more subtle partial seizures.34 When TS presents with seizures in the neonatal period, the prognosis for normal neurological development is poor. Epilepsy and mental retardation are common.33
Transient Metabolic Disorders
Hypoglycemia
When hypoglycemia is a cause of seizures in the neonate, the condition must be recognized quickly so appropriate therapy can be instituted and brain injury averted. Hypoglycemic seizures will be seen most commonly in infants born to mothers with gestational diabetes. Other conditions associated with hypoglycemia include hypoxic-ischemic encephalopathy, sepsis, intrauterine growth retardation, genetic disorders of glycogen metabolism, and hyperinsulinism.
Although most newborns with hypocalcemia are asymptomatic, seizures can be a sign of low calcium. Focal clonic seizures are most common. There are both early and late causes of hypocalcemia. Early causes include prematurity, birth asphyxia, and intrauterine growth retardation. Later presentation after 2 or 3 days of age may be because of genetic disorders such as DiGeorge syndrome (22q11 deletion), maternal hyperparathyroidism, or deficient calcium intake. As with hypoglycemia, early recognition and correction of the underlying electrolyte abnormality will be more efficacious than simply administering antiseizure medication. Hypomagnesemia most often occurs in conjunction with hypocalcemia, although isolated cases are reported.
Hyponatremia
Hyponatremia is described less often than hypoglycemia and hypocalcemia as a cause of neonatal seizures. There are case reports of maternal water intoxication leading to neonatal seizures. Other reported causes include congenital adrenal hypoplasia, congenital hypothyroidism, and oxytocin administration.35
Persistent Metabolic Disorders
Inborn Errors of Metabolism
There are numerous inborn errors of metabolism associated with seizures during the newborn period. Most of these disorders present with seizures and progressive encephalopathy after a few days of age, once the baby has begun feeding and is dependent on his or her own metabolism instead of the mother’s. Among the more common of these rare disorders are urea cycle defects, organic acidurias, and aminoacidopathies. Some of these are amenable to treatment with cofactor administration (eg, biotinidase deficiency) or avoidance of “toxic” nutrients via feeding with a specially formulated diet (eg, maple syrup urine disease). Measurement of ammonia, urine organic acids, and serum amino acids will usually reveal diagnostic abnormalities.
Nonketotic hyperglycinemia (glycine encephalopathy) is caused by a defect in cleavage of the excitatory amino acid glycine. This disorder often causes seizures characterized by frequent myoclonic seizures. Nearly all patients with nonketotic hyperglycinemia have severe neurologic sequelae, although a rare, transient form does exist and carries a more favorable prognosis. Measurement of glycine concentration in the cerebrospinal fluid (CSF) will suggest the diagnosis.
Pyridoxine dependency is caused by an inborn abnormality in the synthesis of γ-aminobutyric acid (GABA). This is a rare autosomal recessive disorder. Neonates without obvious seizure risk factors present with frequent, medically refractory seizures or status epilepticus in the newborn period or early infancy. Seizures may be present from the time of birth, an exception to the general rule that seizures caused by inborn errors of metabolism present after feeding has begun. EEGs are typically dramatically abnormal with burst suppression or hypsarrhythmia. Treatment with B6 (pyridoxine) may lead to a rapid resolution of the seizures and the EEG abnormalities, but the absence of an immediate EEG improvement does not preclude the diagnosis. Serum and CSF analysis show elevated α-aminoadipic semialdehyde and pipecolic acid.36,37
Folinic acid-responsive seizures are the result of another rare, cofactor-responsive inborn error of metabolism. As with pyridoxine dependency, the seizures are distressingly refractory to routine seizure medications. The seizures respond promptly to administration of folinic acid. CSF analysis shows elevated α-aminoadipic semialdehyde and pipecolic acid as in pyridoxine dependency, and the 2 disorders may be allelic.38,39
Familial or Idiopathic Epilepsies
There are 2 benign idiopathic epilepsy syndromes routinely considered in the neonate. One is benign familial neonatal seizures (BFNSs), and the other is benign idiopathic (nonfamilial) neonatal seizures (BINSs). Two other neonatal epilepsy syndromes are considered “catastrophic” seizure disorders: EME and EIEE.
The BFNS was the first idiopathic epilepsy proven to have a genetic etiology. The syndrome was recognized as an autosomal dominant condition with high penetrance (85%). It was first linked to chromosome 20 and soon afterward was attributed to mutations in genes encoding for voltage-gated potassium channels (KCNQ2 and KCNQ3). It is estimated to occur in 14 per 100,000 births. The seizures typically present in the first week of life as focal (or multifocal) clonic seizures or as tonic seizures. The babies respond well to treatment with traditional antiseizure medications. The interictal EEG is usually normal or only mildly abnormal. The seizures typically remit spontaneously at less than a year of life.40,41
The BINSs are distinguished from BFNSs by the absence of a family history. The etiology is therefore less clear. There still may be a genetic etiology with seizures arising because of de novo mutations. Most children affected by BINSs have seizure onset between 4 and 6 days of life (fifth-day fits). Most of the time, the seizures present as focal clonic seizures. Remarkably, the seizures typically remit within a few days, and there may not be a need to treat with seizure medication beyond a few days. BINSs are diagnosed by exclusion of other seizure-producing conditions and by the characteristic benign clinical course.
Early myoclonic epilepsy is characterized by erratic, fragmentary myoclonic jerks that begin in the first month of life. The myoclonus is eventually replaced by partial seizures, massive erratic myoclonus, and tonic seizures. The EEG reveals a burst-suppression pattern. There are a variety of possible etiologies, particularly inborn errors of metabolism (eg, nonketotic hyperglycinemia, pyridoxine dependency, urea cycle defects) and certain congenital structural abnormalities, such as Aicardi syndrome. The prognosis is, unfortunately, poor. Affected infants are developmentally delayed and have a variety of other symptoms, such as abnormal muscle tone and microcephaly.42
Early infantile epileptic encephalopathy presents with frequent tonic spasms in the neonatal period or early infancy. The EEG is characterized by an interictal burst-suppression pattern and diffuse attenuation during the spasms. The underlying cause is most frequently a structural abnormality, such as lissencephaly, porencephaly, hemimegalencephaly, and Aicardi syndrome. Congenital metabolic disorders are less frequently found than with EME. Infants with EIEE are severely developmentally delayed and have medically refractory seizures.
SEIZURE CLASSIFICATION
There are numerous neonatal seizure classification schemes that have been used over the years. The most recent classification proposed by the Commission on Classification and Terminology of the International League Against Epilepsy does not classify neonatal seizures separately from seizures in older individuals.43 Nevertheless, there are common differences in the way seizures appear in neonates compared with older children and adults.
The most widely used scheme divides neonatal seizures into the following descriptive types (Table 15-1)44:
Table 15-1 Seizure Types in Neonates
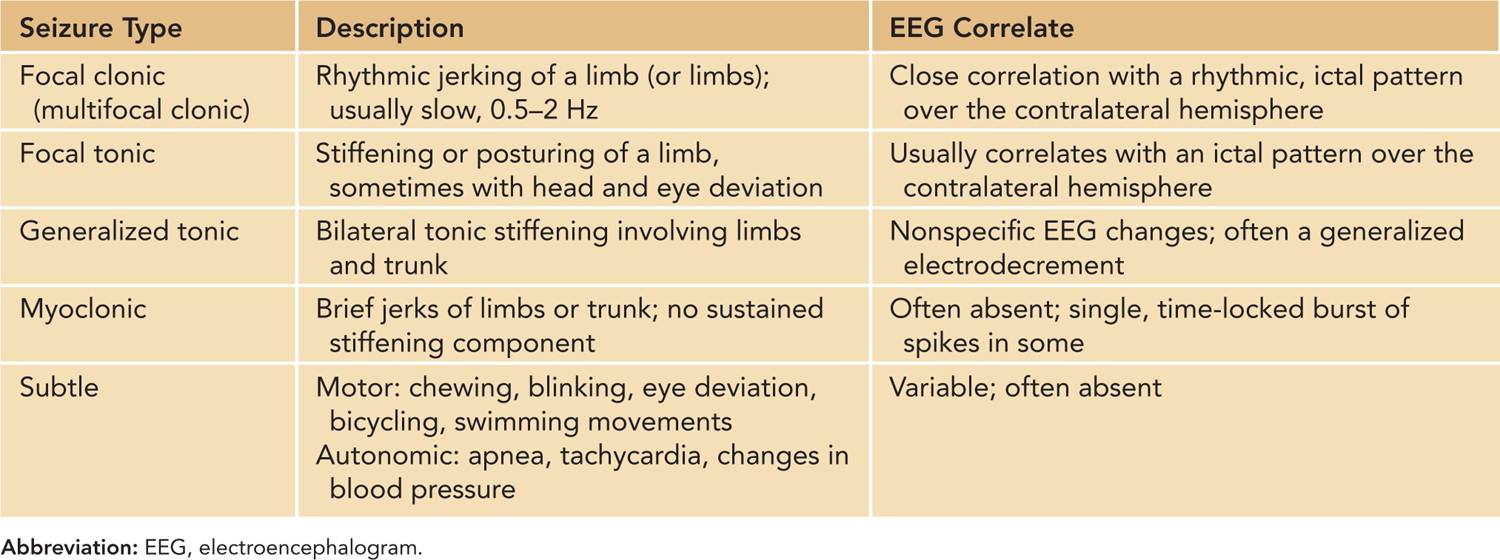
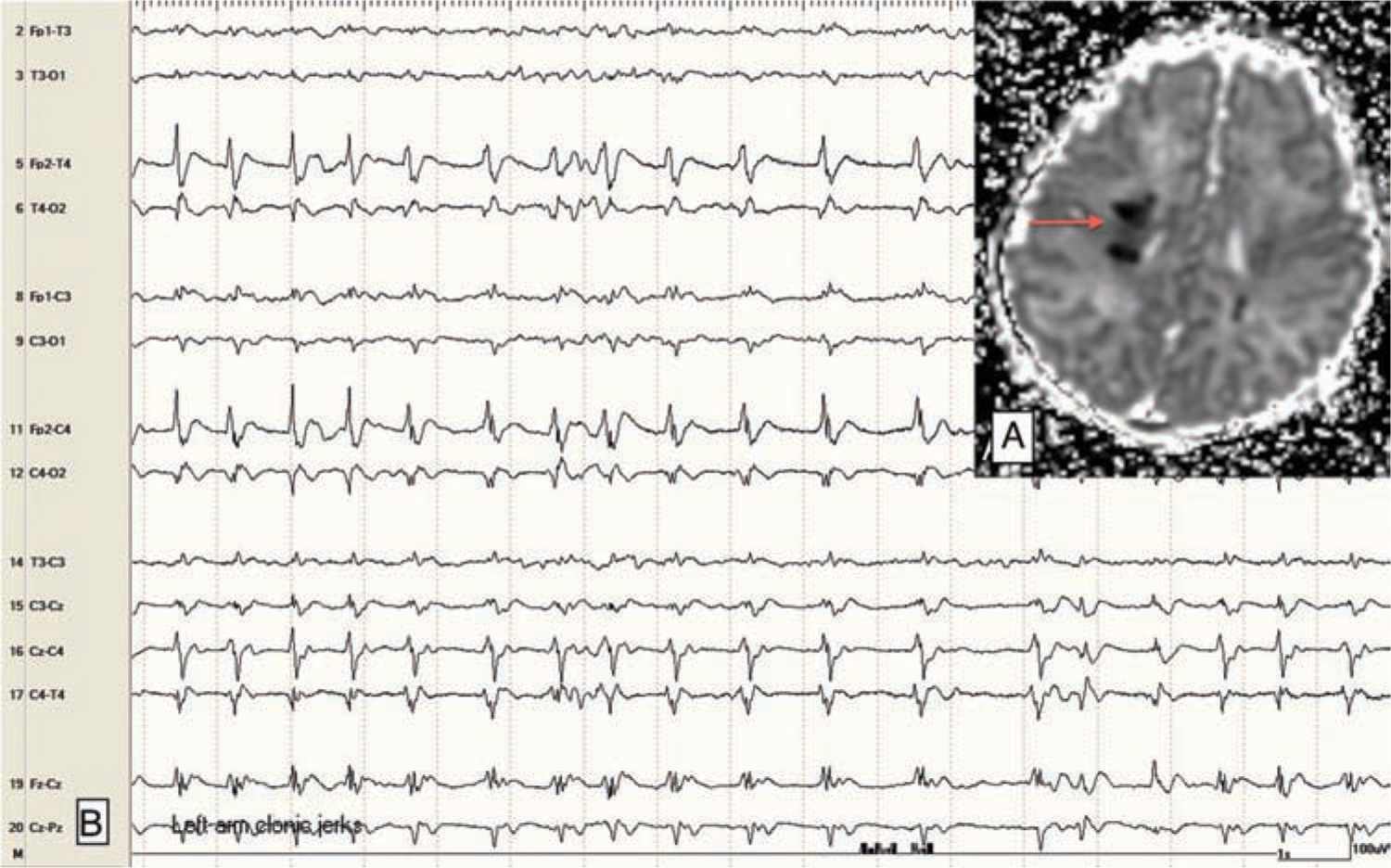
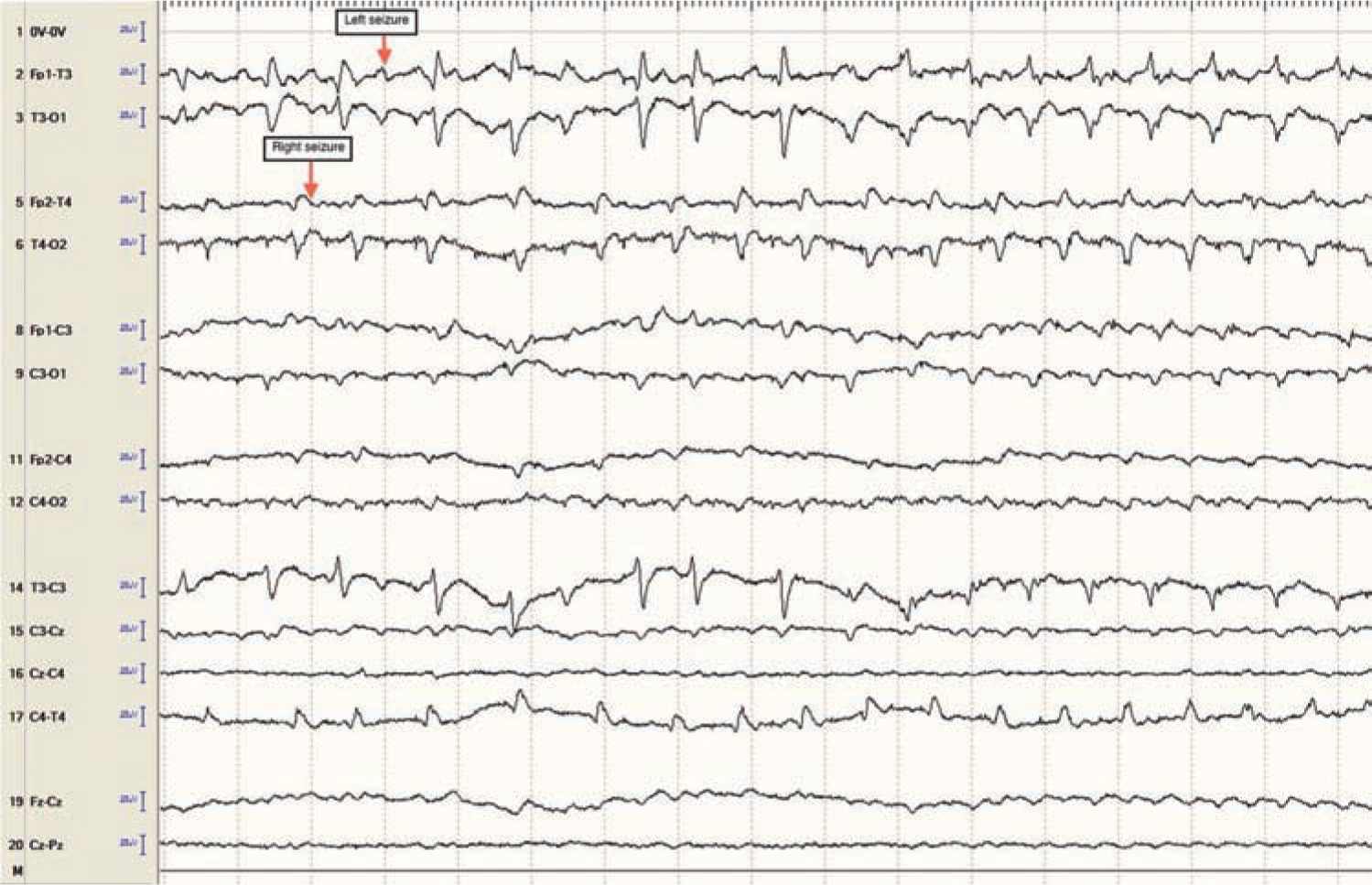
Focal clonic seizures: These seizures consist of rhythmic jerking of the hand, arm, leg, face, or trunk. The rhythmic clonic movements of focal clonic seizures in the neonate are usually slow (0.5–2 Hz) compared to the clonic movements in an older child or adult. Focal clonic seizures most commonly have a distinct ictal EEG pattern that correlates with the rhythmic clonic movements (Figure 15-1). Focal clonic seizures may be multifocal and sometimes have a generalized appearance, although EEG will typically show that there are bilateral, independent seizures occurring (Figure 15-2).
FIGURE 15-1 A, Magnetic resonance imaging (apparent diffusion coefficient map) showing focal abnormality (stroke; arrow) in the right posterior frontal lobe in a 2-day-old term baby with venous infarction caused by polycythemia. B, Electroencephalogram with rhythmic ictal discharge over the right central-temporal region; correlates with focal left arm clonic jerking.
FIGURE 15-2 Independent bilateral ictal electroencephalographic rhythms (arrows) in a 1-day-old term baby with severe neonatal encephalopathy after decreased fetal movement, Apgar scores of 0 at 1 and 5 minutes, and 14 minutes of resuscitation. No ictal movements noted.
Focal tonic seizures: These consist of sustained posturing of one or more limbs, sometimes with more complex features, such as head and eye deviation. The seizures are transient, usually lasting seconds. They can be differentiated from nonepileptic movements by their repeated, stereotyped appearance and their persistence after repositioning or restraining the affected limb. Focal tonic seizures most often have a corresponding ictal EEG pattern.
Generalized tonic seizures: These seizures consist of bilateral tonic stiffening of the limbs and trunk. These seizures last for seconds and may evolve slowly or rapidly (spasms). They are often triggered by stimulation or a state change. These seizures, along with the “subtle seizures” described further in this list, may be thought of as a reflex phenomenon secondary to a depressed cortex that is not inhibiting the brainstem and other subcortical structures. The EEG will usually not show a characteristic evolving, rhythmic ictal pattern. There may be nonspecific paroxysmal changes on the EEG (eg, an electrodecrement) that reflects a sudden state change, but this pattern is not generally regarded as “epileptic” in the same way as an evolving rhythmic discharge is.
Myoclonic seizures: Myoclonic seizures are sudden jerks of a limb, the face, or the trunk. They may be focal or generalized. The jerks are sudden and do not have sustained stiffening. This distinguishes them from brief focal or generalized tonic seizures. Sometimes, they are localized to the same body region; at other times, they may be multifocal. Myoclonic seizures may be generated by cortical or subcortical structures. When they have a cortical origin, there is often a clear ictal EEG correlate simultaneous with the clinical myoclonus. When the myoclonus is caused by an insult to deep gray nuclei (eg, acute hypoxic-ischemic insults), there will usually be no consistent, simultaneous EEG correlate.
Subtle seizures: These behaviors present the greatest diagnostic and treatment challenge among the various seizure types. Subtle seizures may be complex motor phenomena, such as rhythmic chewing and sucking movements, rhythmic tongue thrusting, blinking, eye deviation, or movements resembling swimming or bicycling. Autonomic changes such as tachycardia, apnea, and sudden changes in blood pressure may be manifestations of seizures as well, although in a nonparalyzed patient, apnea seldom occurs in isolation from subtle or overt motor seizure phenomena.44,45
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree