Necrotizing Enterocolitis
Barbara Warner
Necrotizing enterocolitis (NEC) is the most common life-threatening disorder of the gastrointestinal tract among neonates, primarily occurring in infants born prematurely. It is characterized by inflammation and patchy necrosis of the bowel wall that may rapidly progress to systemic sepsis, bowel perforation, and death. Despite decades of research, the underlying etiology remains poorly understood, preventive strategies are inconsistent, and treatment options are controversial.
EPIDEMIOLOGY
The incidence of NEC is reported between 0.72 and 1.1 cases per 1000 live births.1,2 Its occurrence is inversely related to birth weight and gestational age, the most important and consistent risk factors in its development. Preterm infants comprise the overwhelming majority of cases, with near-term and term infants accounting for between 5% and 25%.3,4 For very-low-birth-weight (VLBW) infants born at less than 1500 grams, the incidence reported from large multi-center studies ranges from approximately 5% to 12% of VLBW live births.1,5,6 Reports from individual institutions, however, range widely, from as low as 0.8% to 22%.6-8 Timing of disease onset is inversely related to gestational age, with near-term and term infants typically developing disease in the first week of life, while onset is more common after the first or second week for earlier gestational ages as shown in eFigure 57.1  .9
.9
Mortality from NEC is related to gestational age, extent of bowel involvement, and surgical intervention. In 2000, the in-hospital US mortality across all weight categories was estimated to be 15.2% of all NEC cases.1 Among very-low-birth-weight infants, mortality from NEC varies from 12% to 30%,1,2 with rates as high as 50% for extremely low-birth-weight infants born at less than or equal to 1000 grams.10,11 Mortality increases with the need for surgical intervention to as high as 50% and is directly related to length of remaining intact bowel. Recent data from Blakely and colleagues11 reported 75% survival for infants with more than 80 cm of normal bowel at the time of surgical intervention, 46% for infants with 10 to 80 cm of normal bowel, and no survival in infants with less than 10 cm of normal bowel present.11 As increasing numbers of extremely premature infants survive, the numbers alive to develop NEC increase, specifically among a population also more likely to die from the disease.14,15
As increasing numbers of extremely premature infants survive, the numbers alive to develop NEC increase, specifically among a population also more likely to die from the disease.14,15
Numerous demographic and clinical risk factors for NEC have been proposed, with gestational age and birth weight the strongest and most consistent.6,8,10,16-18 Associations between gender and race are less consistent once illness severity and prematurity are controlled.1,8,17 Prenatal and postnatal factors have been variably implicated in increasing NEC risk for the preterm population. These include antenatal indomethacin exposure, low Apgar scores, presence of a patent ductus arteriosus, placement of umbilical catheters, and therapies such as use of H2-blocker therapy, indomethacin, or ibuprofen therapies. None are consistent risk factors for the preterm population. 
Occurrence of NEC in term or near-term infants commonly involves the presence of risk factors including congenital heart disease, gastroschisis, intrauterine growth retardation, birth asphyxia, sepsis, polycythemia, and exchange transfusions.3,31-34 Site of involvement is typically colonic rather than jejunal and ileal, as commonly seen in preterm infants.3
PATHOGENESIS
In 1975, it was first proposed that 3 critical factors required for the development of NEC were the presence of bacteria, feedings, and injury to the mucosa,35 with the concept of a susceptible host added later.36 Current hypotheses continue to revolve around these same concepts, although understanding of their interactions and role in NEC development has evolved (Fig. 57-1).37
 BACTERIA AND THE INTESTINAL ECOSYSTEM
BACTERIA AND THE INTESTINAL ECOSYSTEM
Evidence for the role of bacteria includes the fact that NEC is never evident in utero or in stillborns, the occurrence of clustered outbreaks, and animal studies.38,39 Many organisms have been implicated, including both gram-negative (eg, Clostridia species, Escherichia coli, Klebsiella species) and positive bacteria (eg, Staphylococcal species), as well as viruses (eg, rotavirus).40 However, no single common organism has been identified as the sole cause of NEC.41,42
Recent evidence supports the hypothesis that rather than a specific infection, NEC results from an abnormal pattern of postnatal intestinal colonization occurring in infants in the neonatal intensive care unit.43 For the premature infant, admitted to the neonatal intensive care unit and often exposed to antibiotics, bacterial colonization is delayed, with limited species diversity, often of a pathogenic type.43,44 The absence of symbiotic commensal bacteria, increasingly recognized for their contribution to continued postnatal intestinal development and health,45-47 may allow for overgrowth and invasion from colonizing intestinal flora.
 THE SUSCEPTIBLE HOST: IMMATURITY OF THE INNATE IMMUNE RESPONSE AND INFLAMMATION
THE SUSCEPTIBLE HOST: IMMATURITY OF THE INNATE IMMUNE RESPONSE AND INFLAMMATION
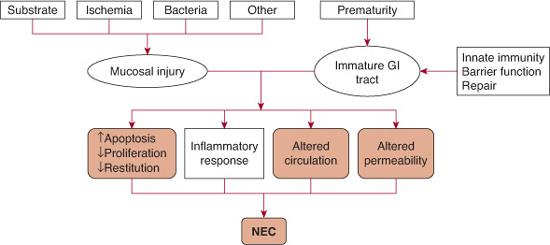
FIGURE 57-1. Pathogenesis of necrotizing enterocolitis.
The strong relationship of NEC to gestational age at birth supports the concept that a component of its pathogenesis lies in the unique characteristics of an immature gastrointestinal tract. Abnormalities in the intestinal colonization pattern are superimposed on an immature intestinal epithelium, which responds to bacterial inflammatory stimulation with excessive proinflammatory cytokine production.48,49 At least part of this response is related to the innate immune system. The response is initiated by bacterial cell wall products, referred to as pathogen associated molecular patterns (PAMPs), which bind to a family of pattern recognition receptors that include the toll-like receptors (TLRs), present on the intestinal epithelium. These receptors activate a signaling pathway that results in translocation of the nuclear transcription factor kappa B (NF-kB) to the nucleus, which initiates the inflammatory cascade, proposed to be the final pathway of intestinal injury. Toll-like receptors 2 and 4 are abundantly present on human fetal enterocytes.50 In animal models, TLR4 expression38 and NF-κB activation51 rapidly diminish after birth. In contrast, neonatal animal models of NEC demonstrate a marked increase in epithelial TLR4 and NF-kB expression and associated inflammatory mediators.38,52-54 Animals with diminished TLR4 function38,54 or that were pretreated with an NF-kB inhibitor51 experienced decreased incidence and severity of NEC.
A number of inflammatory mediators are produced once the NF-kB cascade is activated. These include tumor necrosis factor α, platelet-activating factor (PAF), the interleukins (inter-leukins 1, 6, 8, 12, and 18), nitric oxide, and oxygen-free radicals.55,56 In infants with NEC, cytokine levels are increased and correlate to disease severity.57 Among these many mediators, PAF has been shown to play a central role in increased apoptosis and mucosal permeability, activation of secondary inflammatory responses, and bowel necrosis.58
 IMMATURITY OF REPAIR MECHANISMS
IMMATURITY OF REPAIR MECHANISMS
In neonatal animals, epithelial cell turnover in response to injury is much slower than in mature counterparts.62 Epithelial repair is further diminished in NEC, with intestinal epithelium demonstrating increased apoptosis and decreased cell proliferation and migration to the site of injury, a process termed intestinal restitution. A number of factors have been implicated in altering the response to injury in the immature host,63 including the elevated expression and activation of TLR4 and NF-kB53,54,64; diminished levels of epidermal growth factor, an important modulator of epithelial cell proliferation and apoptosis18; and inflammatory mediators. including PAF,58 nitric oxide,65 and reactive oxygen species.66
 IMMATURITY OF BARRIER FUNCTION
IMMATURITY OF BARRIER FUNCTION
The intestinal barrier is a combination of epithelial, immunologic, and mucosal factors to prevent penetration of noxious substances. Many of these barrier mechanisms are not fully developed at birth including gastric acid secretion, peristaltic motility, mucus production, immunoglobulin A production, defensin antimicrobial protein production by Paneth cells, and tight junction integrity. 
 INTESTINAL CIRCULATION AND ISCHEMIA
INTESTINAL CIRCULATION AND ISCHEMIA
The role of ischemia in NEC is based on the pathologic hallmark of the disease, coagulation necrosis, which is the histologic consequence of preceding ischemia. The initial hypothesis that attributed responsibility to the diving reflex, or asphyxia and low flow, has not been borne out in epidemiologic studies or physiologic animal models.77,78 Rather, evidence now supports that perturbations in control of vascular tone result in vasoconstriction and subsequent ischemia.79 While many factors come into play, the potent vasoconstrictor endothelin 1 and vasodilator nitric oxide are factors most commonly implicated in the development of NEC. Thus, disruption of endothelial cell function by ischemia-reper-fusion, sustained low perfusion, or proinflammatory mediators alter the endothelin 1–nitric oxide balance in favor of constriction,79 perpetuating a cycle of further intestinal injury.
 SUBSTRATE
SUBSTRATE
Ninety percent of infants who develop NEC have received feedings,83 resulting in speculation that intestinal injury results from substrate within the gastrointestinal tract. Newborns’ absorption of carbohydrate and fat constituents in milk is not fully matured, and these undigested components may serve as substrate for proliferation of enteric bacteria. Their metabolic by-product, hydrogen gas, reducing substances, and organic acids, along with undigested casein and long-chain fatty acids remain intraluminal. Exposure of the intestinal epithelium to these substances is proposed to result in an “intestinal inflammation” and injury.83
In summary, it is now hypothesized that rather than one specific bacterium, the premature infants in the neonatal intensive care unit develop an inappropriate pattern of intestinal colonization, with limited diversity and paucity of synergistic bacteria. The innate immune response elicits a robust proinflammatory cascade in response to this colonization, with poor ability to repair intestinal injury. Immaturity of the mucosal barrier functions further increases susceptibility to injury from bacteria and toxic substance. The end result of mucosal injury to an immature gastrointestinal tract is NEC  .
.
 GENETICS
GENETICS
Differences in susceptibility to development of NEC maybe be explained in part by genetic variation.84 The largest retrospective cohort study of twin pregnancies (N = 1407 twin pairs) found monozygotic twins at increased risk for NEC compared to dizygotic twins (OR 4.05; 95% CI, 1.97–8.35), after adjustment for weight and age.85 In contrast, a retrospective cohort analysis of 450 twin pairs born at 32 or fewer weeks’ gestation did not find a genetic link for NEC within twins when evaluated by zygosity concordance and controlling for clinical covariants.86
A number of single gene polymorphisms have been evaluated for their relationship to development of NEC, including inflammatory mediators, vasoactive or angiogenic factors agents, pattern recognition receptors, and thrombophilic agents. Others have looked more broadly at factors associated with prematurity.95 No clear single gene association to NEC is yet evident.
CLINICAL FEATURES AND DIFFERENTIAL DIAGNOSIS
Onset of NEC may be subtle and insidious or catastrophic. Early signs can be nonspecific and common in premature infants, including temperature instability, signs of feeding intolerance such as delayed gastric emptying, and mild abdominal distention. Abdominal distention, bilious emesis or residuals, and bloody stool are more specific to NEC but can also be seen in other disease states. As the disease progresses, abdominal findings become more severe. The clinical findings of NEC are not diagnostic and can be present in other systemic and gastrointestinal pathology. The differential diagnosis includes sepsis with ileus, spontaneous intestinal perforation (SIP), inspissated meconium syndrome, Hirschsprung disease with enterocolitis, viral gastroenteritis, volvulus, feeding intolerance, milk protein allergy, and metabolic disorders.
Infants with SIP are often included in studies of NEC because clinically the presentation is pneumoperitoneum, which is difficult to distinguish from stage III NEC. Spontaneous intestinal perforation is distinct from NEC, distinguished most clearly by histopathology. The mucosal margins of the perforation appear healthy, without evidence of coagulation necrosis.97 Differences in patient populations, risk factors, and presenting signs may be helpful in distinguishing the entities and point to a difference in their underlying etiologies. Compared to infants with NEC, infants who develop SIP are smaller, more immature, and more likely to have been treated with indomethacin, postnatal steroids, or both, in the first days of life.98,99 Two subpopulations of SIP have been identified: (1) extremely low-birth-weight infants who develop SIP on day of life 7 to 10 after exposure to indomethacin and glucocorticoids and (2) larger infants who develop early SIP on day of life 0 to 3 and were less likely to have been exposed to risk factors.100 Onset of SIP is often abrupt, with pneumoperitoneum the first radiographic finding and with no evidence of pneumatosis. Infants with SIP also have a more favorable prognosis, with less mortality than infants with surgical NEC.99
 DIAGNOSTIC EVALUATION
DIAGNOSTIC EVALUATION
Laboratory findings in NEC are nonspecific and reflect the associated inflammatory process, sepsis and disseminated intravascular coagulopathy. They can include neutropenia, leukocytosis, thrombocytopenia, elevated C-reactive protein, metabolic acidosis, glucose instability, and hyperkalemia. The distinguishing feature and radiographic hallmark of NEC is pneumatosis intestinalis (eFig. 57.2 and  Fig. 412-3). Pneumatosis intestinalis represents the intramural accumulation of gaseous by-products from bacterial fermentation. Dissection of gas into the venous system results in air outlining the distribution of the portal venous collecting system, evident as linear densities over the liver on radiograph, and is termed portal venous air. The radiographic finding of pneumatosis alone is not related to disease severity; however, extensive pneumatosis or the presence of portal air suggests more severe involvement and carries a poorer prognosis.104-106 While the presence of pneumatosis intestinalis is pathopneumonic for NEC, it is important to note its absence does not rule out the diagnosis. The diagnosis of free air in the peritoneal cavity is easily missed on a flat radiograph of the abdomen. For that reason, evaluation of NEC should always include either a decubitus or cross-table lateral radiograph. Recently, use of sonography for NEC diagnosis has been proposed, offering the advantage of real-time direct images of the abdominal structures and fluid in the peritoneal cavity.107 There are, however, no studies comparing the 2 modalities, and at this time standard radiography remains the mainstay of diagnosis.
Fig. 412-3). Pneumatosis intestinalis represents the intramural accumulation of gaseous by-products from bacterial fermentation. Dissection of gas into the venous system results in air outlining the distribution of the portal venous collecting system, evident as linear densities over the liver on radiograph, and is termed portal venous air. The radiographic finding of pneumatosis alone is not related to disease severity; however, extensive pneumatosis or the presence of portal air suggests more severe involvement and carries a poorer prognosis.104-106 While the presence of pneumatosis intestinalis is pathopneumonic for NEC, it is important to note its absence does not rule out the diagnosis. The diagnosis of free air in the peritoneal cavity is easily missed on a flat radiograph of the abdomen. For that reason, evaluation of NEC should always include either a decubitus or cross-table lateral radiograph. Recently, use of sonography for NEC diagnosis has been proposed, offering the advantage of real-time direct images of the abdominal structures and fluid in the peritoneal cavity.107 There are, however, no studies comparing the 2 modalities, and at this time standard radiography remains the mainstay of diagnosis.
Table 57-1. Modified Bell Staging Criteria for Necrotizing Enterocolitis
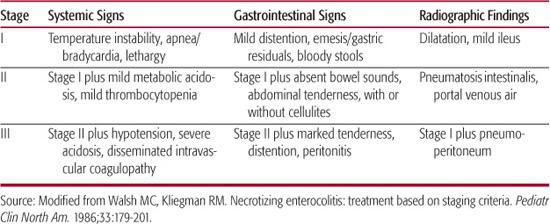
Clinical staging of NEC (Table 57-1) is based on Bell criteria, a combination of clinical, laboratory, and radiographic findings.108,109 The diagnosis is established at stage II, with the appearance of pneumatosis intestinalis or portal venous air, with stage III indicating a more advanced stage and including intestinal perforation. Bell criteria were established in the presurfactant era of neonatology, a time when survival of extremely low-birth-weight infants was rare. As increasing numbers of these infants survive, the occurrence of other acquired neonatal intestinal diseases, particularly SIP, has increased and made diagnosis and staging of NEC based on Bell criteria problematic. Gordon and colleagues99 recently proposed a guideline in the diagnosis of acquired neonatal intestinal diseases (eTable 57.1  ) that discriminates between entities according to timing of onset; clustering of cases; and clinical, radiographic, and pathologic findings.
) that discriminates between entities according to timing of onset; clustering of cases; and clinical, radiographic, and pathologic findings.
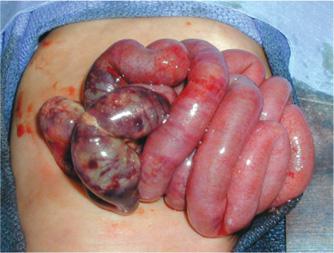
The most common site of involvement is the terminal ileum, although any part of the small or large intestines may be involved. Grossly, the bowel appears irregularly dilated, gray, or dark purple to black in areas of extensive hemorrhage and necrosis (Fig. 57-2). The dominant histopathologic finding is coagulative, or ischemic mucosal necrosis (eFig. 57.3  ).110-112
).110-112
TREATMENT
The mainstay of treatment for NEC is medical stabilization, including gastric decompression, bowel rest, cardiovascular support, and systemic antibiotics. Broad spectrum antibiotics that cover bowel flora, such as ampicillin and gentamicin, are typically used. A third agent, such as clindamycin, is commonly added to improve anaerobic bacterial coverage. Concern over coagulase staphylococcus as a pathogen and emergence of resistant organisms has led to treatment with vancomycin and third-generation cephalosporins at some centers.115,116 Choice of antibiotics should be guided by local resistance patterns and disease severity. Many patients will be hypovolemic and require fluid resuscitation as well as correction of acidemia. The acidemia is often mixed respiratory and metabolic, and endotracheal intubation may be required to treat apnea, hypercarbia, and/or hypoxia. Coagulopathy and electrolyte abnormalities may rapidly develop and require prompt attention. As systemic signs of sepsis increase, maintaining intravascular volume with fluid resuscitation and/or blood transfusion is critical. Inotropic support may be required to maintain hemodynamic stability. Close monitoring of clinical, laboratory, and radiographic status is essential. Frequent serial physical examinations, with radiographs (including left lateral decubitus or cross-table lateral to identify free air) every 6 to 8 hours, and laboratory evaluations, including an initial blood culture and serial blood counts and acid-base status, are recommended.
While many infants can be managed medically, one third to one half will develop severe necrosis or perforation and require surgical intervention.6,10,17,117,118 Perforation continues to be the only absolute indication for surgical intervention,117 with relative indications including severe clinical deterioration, abdominal wall erythema, and some advocating inclusion of extensive pneumatosis and portal venous gas.105,106,117,119,120
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


