Chapter 48
Musculoskeletal Disorders
Roanne Preston MD, FRCPC
Chapter Outline
Pregnancy commonly results in musculoskeletal complaints. Although they typically are benign and self-limited, symptoms may be disabling in some women. In addition, preexisting musculoskeletal disorders interact with pregnancy to a variable extent. These interactions range from an ameliorating effect of pregnancy on the course of the disease (e.g., rheumatoid arthritis) to the potential for a significant and possibly life-threatening deterioration in maternal condition (e.g., uncorrected severe thoracic scoliosis). The purpose of this chapter is to discuss the most common musculoskeletal disorders encountered in pregnant women and their implications for obstetric and anesthesia providers.
Lumbopelvic Pain of Pregnancy
Lumbopelvic pain is the most common musculoskeletal complaint during pregnancy; it comprises two distinct areas of discomfort: (1) the lumbar spine area (low back pain) and (2) the posterior pelvic girdle area (from the sacroiliac joints radiating down into the posterior thighs), which has been termed pelvic girdle pain.1–3 Lumbopelvic pain of pregnancy occurs at some time during gestation in more than 50% of pregnant women and impairs at least one normal activity of daily life, including sleep. Although originally it seemed to be a more significant problem in Scandinavian countries than elsewhere, it is now recognized as a universal issue, with the prevalence ranging from 25% to 70%. It is the most common reason for sick leave during pregnancy.2 Women with mainly pelvic girdle pain report more disability during pregnancy than those with lumbar pain alone.3,4 Differentiation between pelvic girdle pain and low back pain is important because the management differs and the disability of pelvic girdle pain is more likely to extend into the postpartum period for up to 1 to 2 years.2 Risk factors for lumbopelvic pain of pregnancy include a history of low back pain, young age, hypermobile joints, low socioeconomic class, multiparity, and spondylolisthesis; however, the strongest factors are prior history of lumbopelvic pain of pregnancy, previous non–pregnancy-related low back pain, and strenuous work.3–7 Unfortunately, women who suffer from lumbopelvic pain in one pregnancy have a very high risk for experiencing it during subsequent pregnancies.
The etiology includes hormonal and mechanical factors. The corpus luteum synthesizes and releases relaxin, and maternal blood concentrations of this peptide hormone increase 10-fold during gestation. Relaxin induces ligamentous softening and peripheral and pelvic joint laxity, which cause instability of the symphysis pubis and sacroiliac joints; the extent of instability and disability may be related to the maternal concentration of relaxin. There is a correlation between mean serum levels of relaxin and the occurrence of back pain during pregnancy, and women with incapacitating symptoms have the greatest serum concentrations of relaxin.8
Mechanical changes have a later onset than hormonal changes. Women with pelvic girdle pain have increased pelvic joint motion, which increases sheer forces across the joints and likely results in pain.2 In all pregnant women uterine enlargement results in a forward rotation of the sacrum and an increase in the lumbar lordotic curve, which tends to close the lumbar interlaminar space (Figure 48-1). This change exaggerates the mechanical load borne by both the facet joints and the posterior aspect of the intervertebral discs. These mechanical changes also may compromise nerve root foramina. Sciatica occurs in 1% of pregnant women, and most cases occur late in pregnancy.9 Sciatica is distinguished from pelvic girdle pain by its extension to the ankle or involvement of the foot, and it may be associated with neurologic changes.9 Disc herniation is rare in pregnancy but does occur.10 Incapacitating pain that radiates below the knee, typically accompanied by progressive neurologic deficits or bowel and bladder dysfunction, distinguishes disc herniation from the more common and benign lumbopelvic pain of pregnancy.3,10
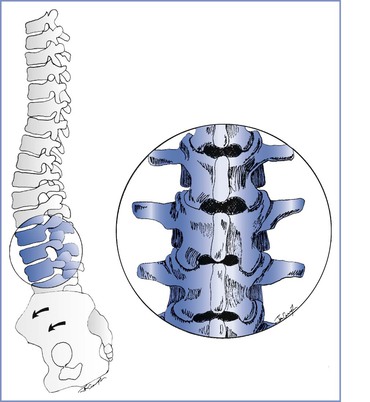
FIGURE 48-1 Musculoskeletal changes of pregnancy. Forward rotation of the pelvis and greater lumbar lordosis increase the load borne by the posterior vertebral elements and tend to close the lumbar interlaminar spaces. Inset, Lumbar vertebrae L2 to L4.
In summary, hormonal changes cause sacroiliac joint dysfunction, which is responsible for the lumbopelvic pain that occurs early in pregnancy. Mechanical changes are primarily responsible for the pain that manifests during late gestation, although symphysis pubis and sacroiliac joint instability may also continue to cause pain. Disc herniation is uncommon and is characterized by the presence of neurologic findings.
Obstetric Management
Treatment is conservative in the absence of neurologic compromise. Structured exercise programs, acetaminophen, transcutaneous electrical nerve stimulation (TENS), and acupuncture have been shown to be beneficial for women suffering from lumbopelvic pain during pregnancy.11,12 Bed rest is reserved for patients with neurologic symptoms or disability secondary to pelvic instability. Patients with severe neurologic signs or symptoms of disc herniation should be assessed by a consultant neurosurgeon who can provide recommendations for intrapartum and postpartum care. Surgical intervention may be required in women with incapacitating pain or progressive neurologic deficits.13 In a woman with severe symptoms, the obstetrician may choose to perform elective instrumental vaginal delivery to decrease maternal work and back stress during the second stage of labor. Because the disability associated with lumbopelvic pain of pregnancy, especially pelvic girdle pain, can impair the woman’s ability to function postpartum, it is important to diagnose lumbopelvic pain of pregnancy and treat it appropriately.2
Anesthetic Management
No evidence suggests that epidural or spinal anesthesia is contraindicated in patients with lumbopelvic pain. The anesthesia provider may administer neuraxial anesthesia, even to patients with sciatica. However, neurologic signs and symptoms should be first identified, delineated, and recorded. It seems prudent to administer a dilute solution of local anesthetic, with or without an opioid, to minimize motor block associated with epidural analgesia during labor to reduce any further stress on relaxed sacroiliac joints. Women with lumbopelvic pain of pregnancy may be reluctant to have neuraxial anesthesia because of concern that it may aggravate symptoms. The literature does not support this fear, and reassurance may be required.
All members of the obstetric care team must pay careful attention to the positioning of the patient with back complaints. The patient must not be placed in a position that she could not tolerate before the administration of neuraxial anesthesia. The lithotomy position puts significant stress on the lower back and should be avoided whenever possible. If it is used, care must be taken to raise and lower both legs simultaneously to prevent injury to the lumbar spine and to avoid extremes when positioning the legs. Finally, caregivers should avoid rotational movements of the spine during transfer of the patient between the bed and the operating table.2
Chronic Low Back Pain
Approximately 50% of pregnant women with a previous history of back pain or those with chronic low back pain experience a recurrence or exacerbation of their symptoms during pregnancy.6 Neuraxial anesthesia is more likely to fail in patients with chronic low back pain and in those who have had back surgery.14–16 Benzon et al.14 reported a delayed onset of epidural anesthesia in patients with back pain or sciatica; the affected roots were blocked 10 to 70 minutes later than the contralateral roots at the same level. The delay in block onset most likely results from the inability of the local anesthetic agent to diffuse into the area of the injured root. Luyendijk and van Voorthuisen17 evaluated 600 epidurograms and confirmed that contrast material failed to reach the nerve root in 33% of patients with uncomplicated disc prolapse and did not move beyond the affected disc space in 5% of cases. This may be due to epidural scarring and adhesions that may develop during healing after disc injury. During epidurography, they noted that contrast material did not diffuse past the level of an injured disc and exited through the foramina below the abnormal disc. Prolapse of an intervertebral disc may result in relative or total obstruction of the flow of local anesthetic agent within the epidural space. The unblocked area includes the affected segment but also may include all segments (either ipsilateral or bilateral) distal to the affected level.
Sharrock et al.15 reported a high rate (91%) of successful epidural anesthesia in patients with a history of limited spinal surgery. However, the success rate was lower than that achieved by the same group of anesthesiologists in a population with no history of back surgery (98.7%). They attributed the greater rate of failure to the distortion of surface anatomy and the tethering of the dura to the ligamentum flavum by scar formation, which rendered the epidural space discontinuous or obliterated it entirely. Support for this hypothesis is provided by LaRocca and MacNab’s18 description of the post-laminectomy membrane. They noted the post-laminectomy formation of organized fibrous tissue surrounding the dura and, at times, binding of the nerves to the posterior aspect of the disc and adjacent vertebral body. The fibrous response was proportional to the extent of surgical trauma and was more marked with greater operative exposures. Consequently, a local anesthetic agent injected into the epidural space may not diffuse beyond the area of scarring and an inadequate or unexpectedly high block may result.16 Post-laminectomy spinal stenosis also may lead to attenuation or obliteration of the epidural space, and the most common site of obstructive stenosis is immediately above the fusion mass.19
Obstetric Management
It is not uncommon for obstetricians to offer pregnant women who have had persistent chronic low back pain the option of cesarean delivery to decrease the potential for further back injury during labor. There are no data to either encourage or discourage this option.
Anesthetic Management
The anesthesia provider may offer epidural or spinal anesthesia to patients with previous lumbar spine pathology or surgery after an appropriate history and screening examination to identify any neurologic deficits.10 A decreased incidence of successful epidural anesthesia may be expected, especially in patients who have had extensive surgery. Nonetheless, the experienced anesthesia provider will likely administer epidural anesthesia successfully in the majority of patients. Sharrock et al.15 recommended administration of epidural anesthesia one or two interspaces above the operated segment to improve the likelihood of a successful block. Subarachnoid anesthesia is likely to be more reliable than epidural anesthesia in this patient population.
Postpartum Backache
Postpartum backache is a common complaint worldwide, occurring in at least 25% of women, with 5% to 7% of women seeking medical help.3 MacArthur et al.,20,21 citing data obtained from a postal survey of 11,701 women who had delivered 1 to 9 years previously, reported that postpartum backache, starting within 3 months of delivery and persisting for 6 weeks or longer, occurred in 23% of women. Approximately 25% of these women had experienced backache before delivery, but 14% reported new-onset backache. In many women, the pain was persistent; 70% had experienced it for more than 2 years, and 65% had pain at the time of questioning 1 to 9 years later. Back pain was more common in women who delivered vaginally with epidural analgesia than in those who did not have epidural analgesia (18.9% versus 10.5%, respectively). Women who had epidural analgesia also were more likely to have had induced labor, an abnormal fetal position, a multiple pregnancy, a prolonged first or second stage of labor, forceps delivery, episiotomy, cesarean delivery, postpartum hemorrhage, or a large infant.
MacLeod et al.22 also performed a postal survey of 2065 patients 1 year postpartum and reported a 26.2% incidence of postpartum backache in women who had received epidural analgesia, compared with a 1.7% incidence in those who had not; the latter incidence of postpartum backache (1.7%) is the lowest, by far, reported by any investigator in a postpartum population in the first year after delivery. Orlikowski et al.,23 who examined data from 992 women as a secondary analysis of a prospective randomized study on epidural analgesia versus continuous midwifery support, found no relationship between back pain at 6 months postpartum and the use of epidural analgesia. Mogren24 sent a questionnaire to 639 women who had completed an earlier postpartum survey in which they indicated that they had suffered from lumbopelvic pain during pregnancy. Mogren explored the relationship between persistent lumbopelvic pain at 6 months postpartum, mode of delivery, and the use of epidural or spinal anesthesia. She concluded that use of epidural or spinal anesthesia was not associated with persistent lumbopelvic pain.
A number of investigators have carried out prospective evaluations to eliminate the potential for reporting bias that may confound retrospective surveys. Breen et al.25 assessed 1042 women at 6 months postpartum. Although 44% of women experienced postpartum backache, there was no difference between those who had received epidural analgesia and those who had not. The most significant predictor of postpartum backache was antenatal back pain. Weight gain was greater in patients with postpartum and new-onset back pain.
Macarthur et al.26 also prospectively studied the association between epidural analgesia and early, new-onset postpartum backache in 329 women. In patients who labored without epidural analgesia, the incidence of postpartum backache was 43% at 1 day, 23% at 7 days, and 7% at 6 weeks. The incidence of symptoms in patients who had received epidural analgesia was greater on the first postpartum day (53%), but this increase was not persistent. At 1 year postpartum, 12% of the patients had back pain (9.9% in the epidural group and 13.8% in the control group). Howell et al.27 performed a randomized controlled trial comparing epidural with nonepidural analgesia during labor in 369 nulliparous women. There was no difference in the incidence or characteristics of postpartum backache at 3 and 12 months postpartum. In a follow-up study, there was no difference between the two groups in the incidence of back pain, disability, or movement restriction more than 2 years after delivery.28
The type of epidural analgesia provided has also been reviewed to determine whether alteration of the technique affects postpartum backache. Wilson et al.29 reported the incidence of postpartum backache in 1054 nulliparous women enrolled in the Comparative Obstetric Mobile Epidural Trial (COMET). The women had received either high-dose labor epidural analgesia (bupivacaine 0.25% administered with intermittent bolus injections) or low-dose mobile analgesia (either combined spinal-epidural [CSE] analgesia followed by intermittent epidural bolus injections of 0.1% bupivacaine with fentanyl or a low-dose epidural infusion of 0.1% bupivacaine with fentanyl). The incidence of backache that started within 3 months and lasted for at least 6 weeks did not differ between the three groups: 46.9% (high dose), 41.7% (CSE), and 45.8% (low-dose infusion). These findings are similar to results of previous studies of this issue.
Both transient and more persistent postpartum backaches are common, but there is little evidence that they are related to the provision of epidural analgesia during labor. Similarly, no evidence suggests that denying a parturient epidural analgesia results in a lower incidence of back problems during the postpartum period. Factors associated with more persistent postpartum backache include the presence of back pain before pregnancy, the presence of lumbopelvic pain of pregnancy, cesarean delivery, and performance of physically demanding work.3,24
Scoliosis
Scoliosis is a lateral deviation in the vertical axis of the spine. The severity of scoliosis is determined by measurement of the angle of the spinal curve, the Cobb angle, which is expressed in degrees (Figure 48-2). The incidence of minor curves is 4 per 1000 in the North American population; larger curves occur less frequently, predominantly in females. Severe scoliosis is relatively rare in pregnant women, occurring in 0.03% of pregnancies.30 Although women with moderate to severe scoliosis constitute a small population of obstetric patients, pregnancy within this population is common.31 Most cases of scoliosis are idiopathic, although some are associated with other conditions, most commonly neuromuscular disorders (Box 48-1).
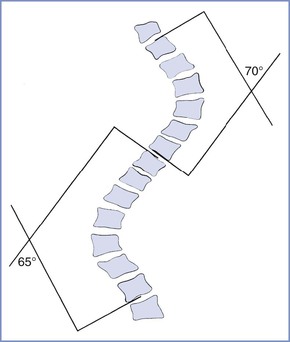
FIGURE 48-2 Schematic representation of the Cobb angle. A line is drawn parallel to the superior cortical plate of the proximal end vertebrae and another line parallel to the inferior cortical plate of the distal end vertebrae. A perpendicular line is drawn to each of these lines. The angle of intersection is the Cobb angle of the curve.
Scoliotic curves can be divided into structural and nonstructural varieties. Nonstructural curves are those seen with postural scoliosis, sciatica, and leg-length discrepancies. They do not affect the mobility of the spine and are nonprogressive. Structural curves are seen in patients with idiopathic scoliosis and with scoliosis resulting from the conditions listed in Box 48-1. Structural curves lead to reduced spinal mobility, and affected patients typically have a fixed prominence (rib hump) on the convex side of the curve. There is also a rotatory component associated with the structural scoliotic curve. The axial rotation of the vertebral body is such that the spinous processes rotate away from the convexity of the curve and back toward the midline of the patient (Figures 48-3 and 48-4).32 Deformation of the vertebral bodies results in shorter, thinner pedicles and laminae and a more narrow vertebral canal on the concave side. Vertebral deformation is unusual in patients with a Cobb angle less than 40 degrees.
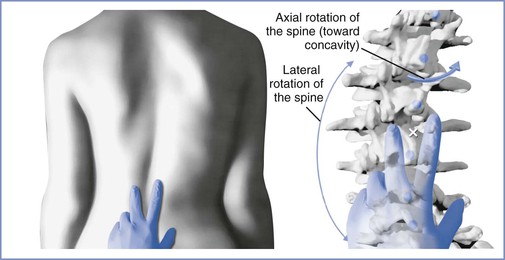
FIGURE 48-3 Spinal rotation with scoliosis. Left, View of the lumbar spine in a patient with a scoliotic curve to the left demonstrating surface landmark palpation. Right, Skeletal anatomy at the same level in the same patient. There is a reduction in the dimensions of the interlaminar space on the concave side of the curve (to the right) and an expansion on the convex side. These changes are enhanced with greater severity of the curve. As the curve increases, the spinous processes rotate into the concavity of the curve, further altering the local anatomy. Surface landmark palpation from the view at left superimposed on the skeleton reveals how the palpated midline (indicated by the white X) is to the right of the true midline. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
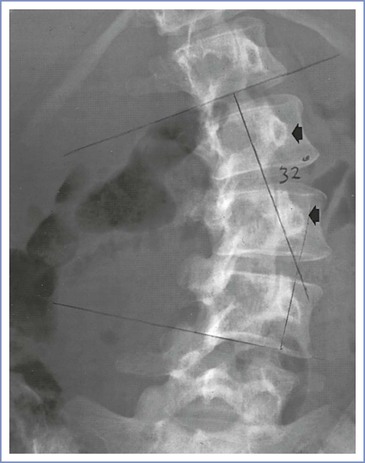
FIGURE 48-4 Radiographic study of the lumbar spine in a 26-year-old woman with idiopathic scoliosis. The spinous processes and pedicles (arrows) are rotated away from the convexity and into the concavity of the curve. (The epidural space was entered easily with direction of the needle approximately 15 degrees off the perpendicular at the skin level toward the convexity of the curve.)
Scoliosis interferes with the formation, growth, and development of the lungs; the occurrence of scoliosis before lung maturity may reduce the number of alveoli that ultimately form. The pulmonary vasculature develops in parallel with the alveoli; early-onset scoliosis and severe scoliosis may result in greater pulmonary vascular resistance and eventually lead to pulmonary hypertension. Musculoskeletal deformities also affect the mechanical function of the lungs; anatomic findings in scoliosis that are most commonly associated with respiratory compromise include the presence of a thoracic curve, thoracic lordosis, and a rib cage deformity. The most common pulmonary function abnormality is a restrictive pattern with decreases in vital capacity, total lung capacity, and lung compliance. This pattern occurs in all patients with a thoracic curve greater than 65 degrees. The functional residual capacity (FRC) is reduced, and airways may close during normal tidal breathing. If the FRC is reduced to the extent that it falls below the closing capacity, atelectasis may occur in basal alveoli. The most common blood gas abnormality is an increased alveolar-to-arterial oxygen gradient, with reduced PaO2 and a normal PaCO2. It results from both venoarterial shunting and altered regional perfusion. Venous admixture may lead to arterial hypoxemia. The natural history of severe, progressive scoliosis includes early death from cardiopulmonary failure.33
Permanent changes of the pulmonary vasculature are common in patients with a curve greater than 65 degrees. Pulmonary hypertension (a resting mean pulmonary artery pressure exceeding 25 mm Hg) occurs in many patients with severe deformity long before the onset of right-sided heart failure and is largely attributable to increases in vascular resistance resulting from chronic alveolar hypoxia, hypoxic pulmonary vasoconstriction, and anatomic changes in the vascular bed. Fixed pulmonary hypertension carries a grave prognosis in pregnancy and may prompt a recommendation to avoid or terminate pregnancy.34
Scoliosis Associated with Neuromuscular Disease
When scoliosis develops secondary to a neurologic or myopathic disorder, abnormal respiratory function results not only from the skeletal deformity but also from abnormalities in the central control of respiration and the supraspinal innervation of the respiratory muscles, as well as from the loss of muscle function caused by the underlying disorder. Respiratory function may be further compromised by (1) impairment of the defense mechanisms of the airways due to loss of control of the pharynx and the larynx, (2) an ineffective cough mechanism, and (3) infrequent or reduced large breaths. Recurrent aspiration pneumonitis may result from compromised protective airway reflexes. In general, the prognosis of scoliosis due to neuromuscular disease is worse than that of idiopathic scoliosis and is determined predominantly by progression of the primary disorder. Affected patients typically develop irreversible respiratory failure at a younger age, and pulmonary hypertension is common; pregnancy is uncommon in this population.
Interaction with Pregnancy
Pregnancy may exacerbate both the severity of the spinal curvature and cardiopulmonary abnormalities in women with uncorrected scoliosis. Progression of a curve, defined as an increase in the Cobb angle of 5 degrees or more over subsequent assessments, most likely occurs during periods of rapid growth and in patients with larger curves at the time of diagnosis. Curves that are less than 25 degrees or curves that have been stable before pregnancy typically do not progress during pregnancy.35,36 In contrast, more severe curves and those that have not stabilized may worsen. Some investigators have described a correlation between the severity of the curve and maternal morbidity and mortality. However, it is likely that the severity of functional cardiopulmonary impairment before pregnancy is a better predictor of maternal outcome than the severity of the curve.37 Patients with a severe curve (i.e., Cobb angle > 60 degrees) but good cardiopulmonary function tolerate pregnancy well, whereas in those with significant cardiopulmonary compromise, and especially in those with pulmonary hypertension, maternal mortality is high.38,39
The physiologic changes of pregnancy include decreases in both functional residual and closing capacities and increases in minute ventilation and oxygen demand. The thoracic cage normally increases in circumference during pregnancy as a result of increases in both anteroposterior and transverse diameters. If the chest cage is relatively fixed by scoliosis, the diaphragm is responsible for all increments in minute ventilation. As the enlarging uterus causes elevation of the diaphragm, diaphragmatic activity is restricted and further decreases in residual and closing capacities may occur, which may result in both greater ventilation-perfusion mismatch and decreased arterial oxygen content. The antepartum onset of new symptoms of respiratory compromise or the exacerbation of preexisting symptomatology is associated with higher maternal morbidity and a greater likelihood that assisted ventilation will be required after cesarean delivery.37
Minute ventilation typically increases by 45% during pregnancy. In normal pregnancy, the increase is primarily a result of increased tidal volume. In the scoliotic patient with restrictive lung disease, a larger tidal volume may not be possible, and the increased minute ventilation is achieved by means of a higher respiratory rate and increased work of breathing. Peak increases in pulmonary activity are reached by the middle of the third trimester, but the uterus continues to grow until term, and it may further encroach on the noncompliant thorax, causing late gestational deterioration despite stabilization of respiratory demand.
Dyspnea on exertion is uncommon in patients with scoliosis who have curves less than 70 degrees, but it becomes more common as the deformity exceeds 100 degrees. In younger patients with a curve less than 70 degrees, exercise capacity is more likely to be impaired because of the lack of regular aerobic exercise and subsequent deconditioning rather than intrinsic ventilatory impairment.40 Dyspnea is common in many pregnant women, typically begins in the first or second trimester, and is most prevalent at term. Two features help distinguish physiologic from pathologic dyspnea.41 Physiologic dyspnea tends to occur earlier in pregnancy and often plateaus or even improves as term approaches. The pathologic dyspnea of cardiopulmonary decompensation more often begins in the second half of pregnancy and is progressive, often becoming most severe as gestation advances and the physiologic loading is maximal. Second, physiologic dyspnea is rarely extreme, and patients can maintain most daily activities. Dyspnea that is extreme or has a limiting effect on normal activity may signal maternal cardiorespiratory decompensation. Dyspnea at rest is also rare in the absence of cardiopulmonary dysfunction, as is dyspnea that is acute in onset or progressive and intractable.
Minute ventilation of the unmedicated parturient increases by a further 75% to 150% in the first stage of labor and by 150% to 300% in the second stage. Oxygen consumption increases above prelabor values by 40% in the first stage and 75% in the second stage. These levels may be unattainable by the scoliotic parturient with restrictive lung disease, and respiratory failure and hypoxemia may result during labor.
Pregnant women with pulmonary hypertension have a limited ability to increase cardiac output. During normal pregnancy, cardiac output increases 40% to 50% above nonpregnant measurements; during labor and delivery, even greater increases are observed. These increases are achieved with both larger stroke volume and a higher heart rate. These demands may put an excessive burden on the cardiovascular system in parturients who had marginal cardiac reserve before pregnancy. If the right ventricle fails in the presence of pulmonary hypertension, left ventricular filling will decrease and low-output failure and sudden death may occur.34
Surgical Management
During spinal fusion and instrumentation, the spinal musculature is reflected off the vertebrae over the course of the curve and the spinous processes and interspinous ligaments are removed. The spine is subsequently extended, correcting the curve. The vertebrae are decorticated throughout the extent of the planned fusion, instrumentation is placed, and bone graft material from the ileum is placed over the decorticated vertebrae. A number of techniques for fusion have been described, but all involve both spinal instrumentation and extensive bone grafting in the axial spine (Figure 48-5).
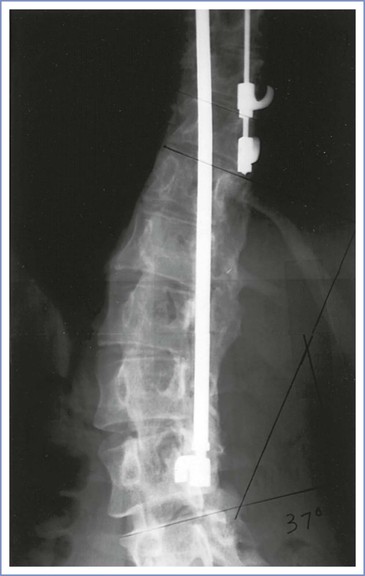
FIGURE 48-5 Harrington rod instrumentation. Radiographic study of the lumbar spine in a 31-year-old woman with thoracolumbar scoliosis corrected with spinal instrumentation. There is rotation of the vertebrae into the curve (toward the rod), and extensive bone grafting is evident adjacent to the rod. Two lumbar interspaces (L4 to L5 and L5 to S1) are not involved in the fusion.
Obstetric Management
Pregnant women with corrected scoliosis tolerate pregnancy, labor, and delivery well. In the absence of major lumbosacral deformity, there is little alteration of the pelvic cavity and malpresentation is not more common than in women without scoliosis. Uterine function is normal, and labor is not prolonged. Spontaneous vaginal delivery is anticipated, and cesarean delivery should be reserved for obstetric indications.
One uncontrolled study suggested no difference in the requirement for cesarean delivery in patients with previous Harrington rod instrumentation for correction of idiopathic scoliosis31; a second and similar study reported a higher incidence of operative delivery.42 The difference in outcomes may be influenced by the severity and etiology of the scoliosis in the populations reviewed and/or differences in the local practice patterns for managing atypical patients. Pelvic abnormalities are more common when scoliosis is associated with neuromuscular disorders and in patients with a severe, uncorrected curve.43 In addition, abdominal and pelvic muscle weakness predisposes parturients to problems with expulsion of the infant during the second stage of labor and may necessitate instrumental vaginal delivery. The need for instrumental or cesarean delivery seems to be related to the severity of skeletal deformity, the resulting maternal compromise, and cephalopelvic disproportion.
In the second stage of labor, the diaphragm has a nonrespiratory function. With expulsive efforts, maximal isometric contractions may be sustained for 20 seconds or more, and diaphragmatic fatigue has been demonstrated even in normal, laboring women. In parturients whose diaphragmatic function is compromised by neuromuscular disease or severe scoliosis, the potential for fatigue and failure is greater; expulsive forces are decreased, the second stage may be prolonged, and a trial of labor may fail, necessitating instrumental or cesarean delivery. In addition, women with severe cardiopulmonary disease (especially those with gestational decompensation) may require urgent or emergency cesarean delivery because of maternal compromise or nonreassuring fetal status.
Anesthetic Management
Pregnant women who have thoracolumbar scoliosis with a Cobb angle greater than 30 degrees or who have undergone spinal instrumentation and fusion for scoliosis should be referred to an anesthesiologist for antepartum consultation. The anesthesiologist should (1) determine the etiology of the scoliosis, as well as the severity and stability of the curve; (2) obtain a history of maternal musculoskeletal and cardiopulmonary symptoms; and (3) review prior obstetric and anesthetic experiences. For patients with scoliosis secondary to neuromuscular disorders, the anesthesiologist should also become familiar with anesthetic considerations specific to those underlying disorders.
Women with suspected or evident pulmonary compromise should undergo evaluation by a pulmonologist, and pulmonary function studies and arterial blood gas measurements should be obtained. These patients must be reevaluated periodically to ensure that they are tolerating the increasing physiologic demands of pregnancy. Echocardiography is useful to assess right-sided heart function in patients with one or more of the following: (1) a curve of 60 degrees or more, (2) hypoxemia on arterial blood gas measurement, (3) moderate or greater reductions in predicted lung volumes or flows, and/or (4) pulmonary hypertension. Radiographic studies performed before pregnancy and operative notes describing spinal surgical procedures should be reviewed before neuraxial anesthesia is given to any patient with significant scoliosis or previous spinal surgery. The anesthesiologist should also examine the spine and note the surface landmarks and interspaces that are least affected by the deformity. Modes of analgesia and anesthesia for labor and delivery can be discussed during antepartum consultation.
Invasive hemodynamic monitoring is rarely indicated during labor and delivery. Pulmonary function studies that suggest significant respiratory compromise or clinical evidence of impending respiratory failure warrant placement of an arterial catheter and serial assessment of blood gas measurements. Echocardiographic demonstration of significant right-sided heart dysfunction may warrant central venous pressure monitoring. The use of echocardiography may allow for detailed anatomic and physiologic assessment in severely ill mothers with advanced and decompensated cardiopulmonary disease.
The anesthesiologist may offer epidural analgesia for labor and delivery to patients with severe thoracolumbar scoliosis. Identification of the epidural space is more difficult in such patients, and the anesthesiologist should anticipate a greater incidence of complications. It is useful to remember the presence of the vertebral rotation during the performance of neuraxial anesthesia in a patient with a significant lumbar curve, which results in the spinous processes (which often may be structurally deformed) rotating into the concavity of the curve. Therefore, the midline of the epidural space is deviated toward the convexity of the curve relative to the spinous process palpable at the skin level (see Figures 48-3 and 48-4). The extent of lateral deviation is determined largely by the severity of the deformity.44 One method of placing an epidural or spinal needle is to direct the needle from a palpated spinous process toward the convexity of the curve, often at a significant angle. The experienced anesthesiologist can track the resistance of both the interspinous ligament and the ligamentum flavum to maintain the correct course into the epidural space. The extent of the local anatomic distortion is the limiting factor, and the selection of spaces that are least involved with the curve is advised (Figure 48-6). More recently, Huang45 suggested a modified paramedian approach based on Boon’s46 work in cadavers, in which the needle is placed lateral to the spinous process on the convex side of the curve (taking advantage of the wider interlaminar spaces on that side) and then aimed directly perpendicular to the skin. The intent is to find lamina with the needle tip and then “walk up or down” the lamina to enter the epidural space (Figure 48-7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


