Multiple Gestation
Roger B. Newman
Charles Rittenberg
Multiple gestations have become one of the most common high-risk conditions encountered by the practicing obstetrician/gynecologist. In 2003, there were 136,328 multiple gestations delivered in the United States, the highest number ever recorded. Since 1980, the number of twins delivered in the United States has risen over 80%, establishing a new height each year. Twins now represent approximately 3% of all live births. Triplets and higher-order births, formerly statistical improbabilities according to the Hellin-Zeleny hypothesis, have increased 470% over the same time period, and triplets now occur with a frequency approaching 1 in every 500 deliveries.
Although multiples account for only a small percentage of all live births, they are responsible for a disproportionate share of perinatal morbidity and mortality suffered in the United States. Multiples result in 17% of all preterm births less than 37 weeks, 23% of early preterm births less than 32 weeks, 24% of all low–birth-weight (LBW) (<2,500 g), and 26% of all very low–birth-weight (VLBW) (<1,500 g) infants. As a consequence of these high rates of both prematurity and LBW, twins are at an approximate 7-fold greater risk of dying before their first birthday compared with singletons, while triplets are at an almost 17-fold greater risk. Multiples account for 16% of all neonatal deaths in the United States.
Among survivors, there is an increased risk of long-term mental and physical handicaps. Twin pregnancies result in a child with cerebral palsy 12 times more often than do singleton births. One fifth of all triplet pregnancies and one half of all quadruplet pregnancies result in at least one child with a major long-term disability. While many cases of cerebral palsy are related to extreme prematurity, not all are the result of premature birth. Even when matched for gestational age and birth weights >2,500 g, multiples have a nearly threefold greater risk of developing cerebral palsy than do singletons.
Multiples also experience a significantly increased risk of growth restriction, which can compound the problems associated with prematurity. Growth-restricted, premature infants, regardless of plurality, experience greater morbidity and mortality than do appropriately grown infants of the same gestational age. Twins and triplets with intrauterine growth restriction (IUGR) have been shown to experience an excess of neurodevelopmental abnormalities compared with appropriately grown, gestational age–matched multiples. Multiples are at risk for numerous other complications that contribute to adverse outcomes. These complications include higher rates of congenital anomaly, twin-to-twin transfusion, monoamnionicity, cord prolapse, placental abruption, placenta previa, intrapartum asphyxia, and birth trauma.
Not unexpectedly, multiples also are associated with significantly higher health care costs. Neonatal intensive care unit (NICU) admission is required by one fourth of twins, three fourths of triplets, and virtually all quadruplets, with average NICU stays of 18 days, 30 days, and 58 days, respectively. Women who are pregnant with multiples are almost six times more likely to be hospitalized with antepartum complications—most frequently, preterm labor, preterm premature rupture of the membranes (PPROM), and preeclampsia. In addition to higher rates of antepartum admission, hospital costs for the birth admission average 40% higher than for gestational age–matched singletons due to longer lengths of stay and increased intrapartum complications with multiples.
Epidemiology and Zygosity
Monozygotic (MZ) twins are those gestations where both fetuses arise from single fertilized ova and are genetically identical. MZ twinning is considered to be a random event, independent of modifying influences such as age, race, parity, or heredity. The incidence of MZ twinning is 3 to 4 per 1,000 live births in virtually all populations. One of the few known influences on the rate of MZ twinning is the
use of assisted reproductive technology (ART) such as in vitro fertilization. The increased frequency of MZ twinning associated with infertility treatment has been attributed to a defective zona pellucida, which allows premature and partial hatching of the blastomeres.
use of assisted reproductive technology (ART) such as in vitro fertilization. The increased frequency of MZ twinning associated with infertility treatment has been attributed to a defective zona pellucida, which allows premature and partial hatching of the blastomeres.
The incidence of dizygotic (DZ) twinning, on the other hand, is extremely variable and accounts for most of the current increase in multiple births. DZ, or fraternal twins, result from multiple ovulation with fertilization by separate sperm. Multiple factors are known to affect the incidence of DZ twinning, including personal or family history. If a woman has already had one set of DZ twins, her chance of having a second set is increased twofold, and a first-degree relative with twins will increase a woman’s risk as well. The father’s side of the family contributes little or nothing to the hereditary risk.
It is estimated that approximately one third of the increase in the number of multiple births is due to delayed childbearing and the fact that DZ twinning occurs more frequently among older women, peaking in the mid thirties. The trend toward delayed childbirth has been a significant sociologic phenomenon of the past quarter century. Since 1975, the proportion of first births among women 30 years of age and older has increased from 5.3% to 22.8%, and the proportion of all births to women ≥30 years of age rose from 16.5% to 35.5%. In women younger than 20 years, the multiple birth rate is only 1.5% compared with 4.1% among women between the ages of 30 to 39 years and up to 18% for women 45 years or older. The higher frequency of multiple gestations occurring among older women also complicates prenatal genetic screening and diagnosis.
The majority of the increase in DZ twinning has been the result of ovulation induction therapy and ART. Women contemplating assisted reproduction should receive preconceptional advisories regarding the risk of multiple birth and the risks associated with those births. The 2002 report of the Society of Assisted Reproductive Technologies (SART) indicated that of all the pregnancies achieved following ART in the United States, 50.9% were singletons, 37.8% were twins, 6.9% were triplets or higher, and 4.4% were unknown. Efforts are being made to reduce the number of oocytes, zygotes, or embryos that are being transferred back in order to minimize the risk of multiple pregnancy. While fewer embryos are being transferred in ART procedures, ovulation induction continues to have a significant impact on the rate of multiple births. A similar proportion of triplets and higher-order gestations results from ART procedures (43%) and ovulation induction (38%), while spontaneous conception accounts for only a minority (19%).
Maternal race also affects the frequency of DZ twinning, which occurs approximately 7 to 10 times per 1,000 live births among whites, 10 to 40 times per 1,000 live births for persons of African descent, and only 3 times per 1,000 live births among Asians. Interestingly, white women are more than twice as likely as black women and three times as likely as Hispanic women to have a triplet or higher-order multiple, which almost certainly reflects a greater use of ART in the white population. Increased maternal parity, higher body mass index (BMI), and recent discontinuation of hormonal birth control agents are also associated with higher rates of DZ twinning.
Placentation
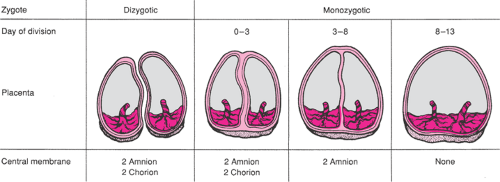
The placentation of DZ twins will always be diamniotic, dichorionic. Two complete placental units are produced, each composed of an amnion and a chorion. As a result, the membrane separating DZ twins will consist of four layers—an amnion and a chorion from each fetus. The placentas themselves may be separate or fused in DZ twins, but the dividing membrane will always consist of four layers. In MZ twins, the placentation depends on the time at which twin division occurs. If division of the zygote occurs in the first 3 days, two complete placental units will be formed and the dividing membrane will contain two amnion and two chorion layers, just as with DZ twins. The syncytiocytotrophoblast cells, which will give rise to the chorion, begin to differentiate about day 3 from the periphery of the blastocyst. If embryonic division occurs between days 3 and 8, the placentation will be a single chorion that has now already differentiated and two amnions that have not yet begun to form. As a result, the dividing membrane will be thin and wispy because it consists of only two opposed amniotic membranes without the intervening chorionic layers. This placentation is referred to as diamniotic, monochorionic. The amnion begins to differentiate by about day 8, and if embryonic division occurs between days 8 and 13, the twins will share a single amnion and chorion—a monoamniotic, monochorionic placentation. This situation, with no dividing membrane separating the fetuses, allows for potentially lethal entanglement of the umbilical cords. Different types of placental development in DZ and MZ twins are illustrated in Figure 14.1. Embryonic division, which occurs after day 13, also results in monochorionic, monoamniotic placentation but with physical attachment of the fetuses producing conjoined twins. Among MZ twins, 18% to 36% are diamniotic, dichorionic; 60% to 70% are diamniotic, monochorionic; and approximately 1% are monoamniotic, monochorionic.
Examination of the placenta(s) and a detailed description of its dividing membrane are critical for determining zygosity of the infants. The microscopic appearance of the dividing membrane consisting of either two or four layers is seen in Figure 14.2. Diamniotic, dichorionic twins are necessarily DZ if the twins are of opposite sex. If the dividing membrane contains only amniotic layers and a monochorionic placenta, the infants are MZ. If the dividing membrane has two amnion and two chorion layers (i.e., diamniotic, dichorionic) and the infants are the same sex, the twins may be either DZ or MZ. Despite these limitations, the
obstetrician can still accurately determine zygosity in the delivery room in over 50% of cases by simply observing the fetal sex and grossly inspecting the placenta. In those cases that remain uncertain, a more specific diagnosis can be made by blood or HLA antigen typing or more sophisticated DNA analyses.
obstetrician can still accurately determine zygosity in the delivery room in over 50% of cases by simply observing the fetal sex and grossly inspecting the placenta. In those cases that remain uncertain, a more specific diagnosis can be made by blood or HLA antigen typing or more sophisticated DNA analyses.
Prenatal Diagnosis
The risk of aneuploidy in multifetal gestations is primarily related to zygosity and, as a secondary factor, the mode of conception. In DZ twins, each fetus has an independent risk for aneuploidy which, like singletons, is related to maternal age. This will be a significant clinical factor, as the risk of DZ twinning increases with maternal age. Alternatively, MZ twins with rare exception will have the same karyotype, and their aneuploidy risk also will be related to maternal age.
When considering possible zygosity as well as the risk of aneuploidy, the mode of conception needs to be considered. While the percentage of naturally conceived DZ twins will vary somewhat with maternal age and ethnicity, it is generally accepted that in the United States, 33% of naturally occurring twins will be MZ and 67% will be DZ. Of those twins conceived following assisted reproduction, 93% will be DZ and only 7% MZ.
Since DZ twins carry independent risks of aneuploidy, the chance of having at least one affected live-born twin at term is twice the maternal age–associated risk. At a maternal age of 32, the age-associated risk of aneuploidy is 1 in 481 singleton births. The risk for a woman 32 years of age carrying DZ twins is 1 in 240, which is equivalent to a 35-year-old woman carrying a singleton. For a woman carrying MZ twins, her risk of having at least one affected live-born twin at term is the same as her age-associated risk (i.e., 1 in 240 at 35 years of age). Unfortunately, since her twins are MZ, this risk actually is the risk of both fetuses
being affected. Obviously, zygosity can only be determined definitely by genetic analysis of both fetuses, but certainly it can be inferred with a reasonably high degree of accuracy by noninvasive ultrasonic determination of chorionicity and fetal sex. Monochorionic (same sex) twins are always MZ, and approximately 70% of MZ twins are monochorionic. All DZ (same or opposite sex) twins will be dichorionic, while approximately 30% of MZ twins will have a dichorionic placentation.
being affected. Obviously, zygosity can only be determined definitely by genetic analysis of both fetuses, but certainly it can be inferred with a reasonably high degree of accuracy by noninvasive ultrasonic determination of chorionicity and fetal sex. Monochorionic (same sex) twins are always MZ, and approximately 70% of MZ twins are monochorionic. All DZ (same or opposite sex) twins will be dichorionic, while approximately 30% of MZ twins will have a dichorionic placentation.
Second-trimester multiple-marker screening generally has been used in twin pregnancies, although with a decreased sensitivity for aneuploidy and a higher false-positive rate compared with its use in singletons. Production of serum analytes in DZ twins can be affected by one twin differentially from the other. The median second-trimester serum analyte values for normal twins are 1.67 multiples of the median (MoM) for unconjugated estriol, 1.84 MoM for human chorionic gonadotropin (hCG), and 2.13 MoM for maternal serum alpha-fetoprotein (MSAFP) compared with normal singletons. A pseudorisk for twins can be calculated by dividing measured MoM values for the corresponding medians for unaffected twins. Using this pseudorisk approach and mathematical modeling, the estimated Down syndrome detection rates using a triple screen is 73% for MZ twins, 43% for DZ twins, and 53% for twins overall, with a 5% screen positive rate (SPR). This performance compares poorly with either the triple screen or the quad screen in singleton pregnancies, where there are detection rates of 65% and 75%, respectively, for the same 5% SPR.
A similar situation exists with first-trimester serum screening. Using free β-hCG and pregnancy-associated plasma protein A (PAPP-A) levels at 10 to 14 weeks, modeling predicted detection rates of 52% in twins discordant for Down syndrome and 55% in twins concordant for Down syndrome, with a 5% SPR compared with an estimated 60% detection rate for singletons.
An attractive alternative for women with multiples is now available with the emergence of first-trimester nuchal translucency (NT) measurement. Between 10 and 14 weeks, the NT mean (in millimeters), median (MoM), and values at the 5th, 50th, and 95th percentiles for normal twins and triplets are almost identical to normal singleton gestations. In 1996, Sebire and colleagues obtained NT measurements in 448 women with viable twins and more than 20,000 singletons between 10 and 14 weeks gestation. Among the twins, 7.3% had an NT of less than the 95th percentile, including 88% of those with Down syndrome; among the singletons, 5.2% had an NT of less than the 95th percentile, which included 79% of those with Down syndrome. In dichorionic pregnancies, the sensitivity and SPR of NT plus maternal age for Down syndrome was similar to singletons. In monochorionic pregnancies, the SPR of NT risk assessment, however, was higher than in singletons. It is possible that this difference in monochorionic pregnancies may be an early manifestation of twin-to-twin transfusion syndrome (TTTS).
In a follow-up study in 1997, Sebire and colleagues compared NT measurements obtained at 10 to 14 weeks from 116 normal monochorionic twin pregnancies and 16 that later developed severe TTTS. An NT of less than the 95th percentile had a positive predictive value of 38% for TTTS and a likelihood ratio of 4.4 (95% confidence interval [CI] 1.8 to 9.7), suggesting that the underlying hemodynamic changes associated with TTTS may manifest as increased fetal NT thickness in monochorionic twins at 10 to 14 weeksgestation.
The appeal of first-trimester NT measurement for multifetal gestations is its ability to individually assess each fetus. The addition of first-trimester serum analytes brings the same concern encountered in the second trimester where abnormalities of one twin may be normalized by the other, reducing sensitivity and increasing the false-positive rate. At present, it appears that first-trimester NT is superior to first-trimester serum screening alone for multifetal gestations. The value of combined first-trimester screening compared with NT measurement alone remains uncertain and requires further investigation.
Maternal Complications
Women who are pregnant with multiples are more likely to be hospitalized antenatally for both an increased frequency and severity of pregnancy-related complications. Some of these increased risks may be associated with maternal characteristics that predate the pregnancy, such as older maternal age, nulliparity, increased pregravid BMI, and conception by ART. However, the majority of these complications are related directly to higher plurality and the more extreme maternal adaptation required.
Cardiovascular Risks
One of the major physiologic changes occurring with a multiple pregnancy is significant expansion of the plasma volume and cardiac output above that seen in singleton pregnancies. This increased plasma volume has obvious adaptational value as the maternal host tries to meet the demands of a multiple conception. Increased cardiac demand is reasonably well tolerated in the absence of underlying cardiac disease such as undiagnosed mitral valve stenosis. However, the common use of tocolytic therapy, the common iatrogenic fluid overload, and the occasional infection all will generate significant additional cardiovascular demand. Tocolytic therapy (especially β-adrenergic agonists) has been associated with pulmonary edema, myocardial ischemia, and potentially lethal maternal tachyarrhythmias in multiples, although these complications are infrequent. An increased risk of postpartum cardiomyopathy also has been reported, especially among older gravidas with
higher-order multiples. A case-controlled study of pregnant women found that multiple pregnancy was an independent and significant risk factor (odds ratio [OR] 2.3; CI 95% 1.2 to 4.5) for admission to an intensive care unit.
higher-order multiples. A case-controlled study of pregnant women found that multiple pregnancy was an independent and significant risk factor (odds ratio [OR] 2.3; CI 95% 1.2 to 4.5) for admission to an intensive care unit.
Hematologic Abnormalities
Increased red blood cell volume expansion is unable to keep pace with plasma volume expansion in either singleton or multiple gestations. This results in a physiologic hemodilution. The average hemoglobin concentration for women pregnant with twins is 10 g/dL at 20 weeks gestation. Hemoglobin and hematocrit values decline beginning in the first trimester, reaching a nadir in the second trimester before gradually rising in the third trimester. Hemoglobin levels below 11 g/dL in either the first or third trimester accompanied by a serum ferritin less than 12 mg/dL represents iron-deficiency anemia, which complicates 21% to 36% of multiple gestations. This rate is two- to threefold higher than in singletons. Multiples generate a great demand for elemental iron, of which sufficient quantity might not be available in many diets. This need should be addressed through the consumption of heme-rich animal protein and supplementation with 60 mg per day of elemental iron and 1 mg per day of folic acid when the woman has low or absent stores of these nutrients.
Metabolic Disorders
Women who are pregnant with multiples have lower fasting and postprandial glucose levels, exaggerated insulin responses to eating, and higher levels of β-hydroxybutyrate than women pregnant with singletons. These differences suggest more rapid depletion of glycogen stores and resultant metabolism of fat between meals and during an overnight fast.
Gestational diabetes represents a disorder of relative insulin deficiency exposed as a consequence of the anti-insulin effects of several placental hormones—most notably, human placental lactogen. Multiple gestations are at increased risk for gestational diabetes due to the elevated levels of these placental hormones associated with increased placental mass. Gestational diabetes appears to be increased two- to threefold among multiples (7% among twins, 9% among triplets, and 11% among quadruplets) compared with a 3% to 4% incidence among singletons. Given the high rate of premature labor among multiples, it should be remembered that both β-adrenergic agents and corticosteroids can induce both insulin resistance and hyperglycemia.
Pregnancy-Induced Hypertension or Preeclampsia
Pregnancy-induced hypertension or preeclampsia is frequently encountered in multiple gestations. Reported frequencies increase from approximately 7% in singletons to 14% for twins, 21% for triplets, and 40% for quadruplets. A population-based study of singleton and twin births in the state of Washington found twins to have a 4-fold higher risk of preeclampsia and a 14-fold higher risk if the woman is primigravid. Pregnancy-induced hypertension or preeclampsia frequently occurs earlier, is more severe, and more often is atypical in multifetal gestations. Hypertension is not always the presenting sign, nor is proteinuria universally present, especially in higher-order multiples. Only 3 of 16 triplets and quadruplets reported in one study met traditional criteria for preeclampsia. The most common presentation among these higher-order multiples was laboratory abnormalities consistent with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome.
Placental Abruption
Antepartum maternal hemorrhage also is increased in multiple gestations. Twin pregnancies have an approximately threefold increased risk of abruption, even when controlling for maternal hypertension. Abruption occurs most frequently in the third trimester and also is a significant risk immediately after vaginal delivery of the first infant. Conformational changes in the uterine shape that occur between deliveries can predispose to a sheering off of the attached placenta.
Hydramnios
Hydramnios occurs in 2% to 5% of twin gestations, and twins account for approximately 8% to 10% of all cases of hydramnios. Hydramnios may develop as a consequence of TTTS with the cotwin experiencing both growth restriction and oligohydramnios. The development of idiopathic acute hydramnios with maternal respiratory embarrassment also has been reported in multiples.
Urinary Tract Infection
Women with multiples have a 1.4-fold increased risk of developing urinary tract infection during pregnancy. These infections usually involve only the lower urinary tract because the incidence of pyelonephritis is not significantly increased. This complication is thought to be a consequence of increased urinary stasis due to the gravid uterus.
Postpartum Hemorrhage
Overdistention of the uterus in a multifetal gestation predisposes to postpartum hemorrhage caused by uterine atony. In addition, women carrying multiples are at increased risk for retention of placental tissue, surgical or mechanical trauma to the genital tract, and pharmacologic effects of medications such as magnesium sulfate, which is frequently used to manage both preeclampsia and preterm
labor. In a British population-based study of postpartum hemorrhage, multiple pregnancy was associated with more than fourfold increased risk (relative risk [RR] 4.46; 99% CI 3.01 to 6.61). In the British study, the risk of postpartum hemorrhage among singletons was 1.2% compared with 6% for twins, 12% for triplets, and 21% for quadruplets.
labor. In a British population-based study of postpartum hemorrhage, multiple pregnancy was associated with more than fourfold increased risk (relative risk [RR] 4.46; 99% CI 3.01 to 6.61). In the British study, the risk of postpartum hemorrhage among singletons was 1.2% compared with 6% for twins, 12% for triplets, and 21% for quadruplets.
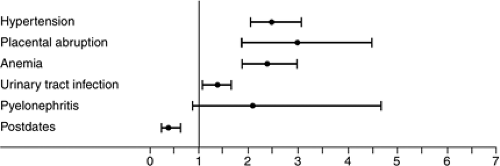
While the above scenarios represent several of the more significant maternal complications associated with multiples (Fig. 14.3), others also are encountered with increasing frequency. These include cholestatic jaundice, pruritic urticarial plaques and papules of pregnancy (PUPP), hyperemesis, and deep venous thrombosis. Women with multiple gestations also experience an increased number of somatic complaints such as shortness of breath, loss of balance, varicose veins, significant dependent edema, constipation, and hemorrhoids.
Complications Unique to Multiples
Vanishing Twin Syndrome
The loss of one or more fetuses can complicate a multiple gestation at any point during pregnancy but is most common in the first trimester. Between 20% and 50% of multiple gestations identified by ultrasound in early pregnancy are lost either as a spontaneous abortion of all fetuses or by the spontaneous loss and reabsorption of at least one of the multiples. This latter occurrence is referred to as the “vanishing twin syndrome,” and its exact frequency is difficult to ascertain for obvious reasons.
In the experience of one in vitro fertilization program, spontaneous loss of all fetuses with previously documented cardiac activity occurred in 17 of 165 twin (10.3%), 2 of 26 triplet (7.7%), and 1 of 5 quadruplet (20%) pregnancies. In addition, 33 twins spontaneously reduced to a singleton and 9 triplets spontaneously reduced to twins. Considering both the spontaneous abortion rate and the vanishing twin syndrome, the overall first-trimester pregnancy loss rate was 50 of 165 twins (30.3%), 11 of 26 triplets (42.3%), and 1 of 5 quadruplets (20%). Although these data are limited by the fact that all pregnancies were the result of in vitro fertilization, similar loss rates have been reported in spontaneously conceived multiples.
When vanishing twin syndrome does occur, there usually are no symptoms. However, in some cases, the reabsorption may be associated with a modest amount of vaginal bleeding. Some have estimated that up to 5% of all patients with first-trimester bleeding may be experiencing a vanishing twin. Maternal reassurance should be offered, as the prognosis for the surviving twin is excellent when silent reabsorption occurs in the first trimester. Following delivery, the placenta frequently will show a whitish plaque on the membranes, representing the remnant of the other gestational sac.
While vanishing twin syndrome occurs with a greater frequency than appreciated previously, it is important not to overdiagnose this event. The diagnosis of a vanishing twin should be preceded by identification of specific embryonic parts in each sac as opposed to an anembryonic cavity. Numerous sonographic findings can mimic a second anembryonic cavity, including subchorionic blood clots, chorioamniotic separations, a decidual pseudosac in the contralateral horn of a bicornuate or didelphic uterus, a cystic uterine fibroid, or even excessive transducer pressure on a thin woman. The emotional impact of a vanishing twin should not be underestimated. Parents will perceive the situation as the loss of a child, and perinatal grief counseling may be necessary in some situations.
Fetal Death in Utero (Acute Intertwin Transfusion Syndrome)
After the first trimester, single fetal demise occurs in 2% to 5% of twin gestations and in 10% to 15% of triplet gestations. The risk of a single fetal death in utero is increased three- to fourfold by monochorionicity. When death of one fetus occurs in a dichorionic gestation, the risk to the surviving cotwin is minimal, although higher rates of preterm labor or PPROM have been reported. Virtually all the adverse sequelae for the surviving cotwin occur in monochorionic gestations. Antenatal demise of a monochorionic cotwin is associated with an approximate 25% mortality rate and a similarly high rate of morbidity for the surviving fetus.
Injury to the surviving cotwin was previously thought to be a result of intravascular coagulation and embolism of tissue thromboplastins through ubiquitous placental anastomoses from the fetal demise. The passage of these tissue thromboplastins result in embolic ischemic organ injury or development of disseminated intravascular coagulopathy in the survivor. The more recent belief is that following fetal demise, there is an acute transfusion into
the dead fetus through the shared placenta. This hemorrhage into the dead fetus may cause severe fetal hypotension, hypoxic end-organ injury, and potentially lethal fetal exsanguination. This is referred to as “acute intertwin transfusion syndrome.” In prospective studies, 5% to 25% of surviving monochorionic twins have ischemic end-organ injury, most frequently neurologic. Neurologic abnormalities reported among surviving twins include necrosis and cavitation of the cerebral white matter, cerebellar necrosis, multicystic encephalomalacia, hydranencephaly, hydrocephalus, porencephaly, microcephaly, and hemorrhagic infarction. In addition to neurologic injuries, other abnormalities seen among surviving cotwins include ischemic bowel lesions, intestinal atresia, renal cortical necrosis, and cystic renal dysplasia.
the dead fetus through the shared placenta. This hemorrhage into the dead fetus may cause severe fetal hypotension, hypoxic end-organ injury, and potentially lethal fetal exsanguination. This is referred to as “acute intertwin transfusion syndrome.” In prospective studies, 5% to 25% of surviving monochorionic twins have ischemic end-organ injury, most frequently neurologic. Neurologic abnormalities reported among surviving twins include necrosis and cavitation of the cerebral white matter, cerebellar necrosis, multicystic encephalomalacia, hydranencephaly, hydrocephalus, porencephaly, microcephaly, and hemorrhagic infarction. In addition to neurologic injuries, other abnormalities seen among surviving cotwins include ischemic bowel lesions, intestinal atresia, renal cortical necrosis, and cystic renal dysplasia.
Most reported cases of demise or neurologic injury occurring in a monochorionic cotwin following death of its sibling have occurred in the third trimester. However, neurologic deficit has been reported in a surviving monochorionic twin following loss of its cotwin as early as 18 weeks gestation.
Following a fetal demise in utero, continuing pregnancy management will depend on gestational age, chorionicity, and maternal and fetal status. If the pregnancy is known to be dichorionic, then no intervention is required unless a term gestation has already been achieved or there is a specific maternal or fetal indication for delivery. A single fetal demise in a monochorionic gestation is an indication for immediate delivery if fetal maturity or near maturity can be inferred based on gestational age or documented by amniocentesis. Decisions regarding delivery at earlier gestations should be based on an assessment of the neonatal complications likely to result from delivery as opposed to the potential risk of remaining in utero. Since it is probable that hypoxic/ischemic end-organ injury occurs almost immediately after demise of the monochorionic cotwin, it is unclear if these injuries can be prevented by prompt delivery. With expectant management, increased fetal surveillance of the surviving twin should be performed, and any evidence of fetal compromise also warrants immediate delivery.
Monoamniotic Twins
Monoamniotic twins are rare, complicating fewer than 1% of MZ gestations. They carry a fetal mortality rate that approaches 40%, primarily as a consequence of cord entanglement and subsequent occlusion. Cord entanglement is present in virtually every case of monoamniotic twins. Monoamniotic twins also are at greater risk for other complications such as congenital anomaly, including conjoining and TTTS.
Some reviews have suggested that spontaneous intrauterine fetal demise due to cord entanglement is unlikely after 32 weeks gestation as intrauterine crowding limits the ability of the fetuses to make major moves in relationship to each other. However, a review of over 200 nonconjoined, monoamniotic twins demonstrated that fetal deaths occur throughout pregnancy, with a high percentage occurring after 32 weeks. In this large review, monoamniotic twins that were prenatally diagnosed and subjected to intensive antepartum surveillance enjoyed a much higher perinatal survival rate than has been reported historically. Prenatal diagnosis allows for institution of an aggressive management protocol designed to identify fetal compromise, enhance fetal lung maturity, and electively deliver once neonatal survival can be anticipated (Table 14.1).
TABLE 14.1 Management Recommendations for Monoamniotic, Monochorionic Twin Gestations | ||
|---|---|---|
|
Cesarean delivery is usually recommended due to concerns over intrapartum fetal distress related to tightening of the umbilical cord entanglement. If vaginal delivery is planned, continuous fetal monitoring is essential along with capability for immediate cesarean birth.
Discordant Twin Growth
In addition to concordant IUGR, ultrasound is useful for the detection of significantly discordant fetal growth, which is unique to multiple gestations. In terms of actual birth weight, a large review found that birth weight differed by 500 to 999 g in 18% of twins, and the difference was greater than 1,000 g in 3%. Some 15% to 30% of twins exhibit birth-weight differences of 20%. Discordance between the largest and smallest triplet is 20% in more than 40% of triplet gestations, with 7% exceeding 40% discordance.
Evidence suggests that the smaller infant may be at risk for both increased perinatal morbidity and mortality when birth-weight discordance is excessive (>20% to 25%). Another concern is that significant diversions in twin growth may predispose the smaller twin to disadvantages in long-term physical and intellectual development.
Much of the discordance in birth weight will be due to constitutional factors such as the genetic dissimilarity of DZ twins. The other major cause of growth discordance
in DZ twins is growth restriction affecting a single fetus due to local placental implantation factors (Fig. 14.4). In monochorionic gestations, discordance is more frequent, often more severe, and more likely to be associated with TTTS. Percent discordance is calculated by dividing the actual or estimated weight difference by the actual or estimated weight of the larger twin.
in DZ twins is growth restriction affecting a single fetus due to local placental implantation factors (Fig. 14.4). In monochorionic gestations, discordance is more frequent, often more severe, and more likely to be associated with TTTS. Percent discordance is calculated by dividing the actual or estimated weight difference by the actual or estimated weight of the larger twin.
It is important to appreciate that birth-weight discordance and IUGR are interrelated. When birth-weight discordance exceeds 20%, one of the fetuses will be growth restricted in more than 50% of the cases. When discordant fetuses are both appropriately grown for gestational age, differences in perinatal outcome have not been identified. Both prematurity and IUGR are much greater threats to the fetus than is the degree of discordancy.
Evaluation of discordant growth should be undertaken simultaneously with consideration of gestational age, individual fetal growth, and fetal well-being. In the absence of IUGR and in the presence of reassuring fetal testing, birth-weight discordance among preterm twins should be managed expectantly in anticipation of achieving a more advanced gestation or enhanced fetal maturity. Some caution is appropriate given that the ultrasound diagnosis of both IUGR and growth discordance is not reliable. The sensitivity of ultrasonography for substantial intertwin discordance is, at best, only 60%. Alternatively, ultrasound evidence suggesting 20% to 25% growth discordance or IUGR of either twin at ≥35 weeks gestation would be appropriate indications for delivery.
Twin-to-Twin Transfusion Syndrome (Chronic Intertwin Transfusion Syndrome)
TTTS is a serious complication affecting multiple pregnancies and is sometimes referred to as “chronic intertwin transfusion syndrome,” a complication of MZ/monochorionic twins in which intraplacental arterial venous shunts are uncompensated and preferential blood flow exists. Vascular communications are present in virtually all monochorionic placentas, and approximately one third will demonstrate at least some clinical evidence of the syndrome. Severely affected pregnancies are much less common, occurring in fewer than 5% of monochorionic gestations. Contrary to what might be expected, severe TTTS is associated with fewer, or even single, arterial venous malformations within the placenta rather than multiple vascular anastomoses. Multiple anastomoses function to restore a balance of bidirectional flow within the monochorionic placenta, while a limited number predispose to preferential flow. Severe chronic intertwin transfusion syndrome identified in the second trimester is associated with loss rates approaching 100% if untreated. Chronic intertwin transfusion syndrome accounts for 15% to 17% of all perinatal mortality in twin gestations.
In chronic intertwin transfusion, the arterial donor twin may be growth retarded, anemic, hypotensive, and oligohydramniotic. If there is little or no amniotic fluid surrounding the smaller fetus, the amniotic membrane may lie in close apposition to the smaller fetus, restricting it to the uterine wall. This is referred to as a “stuck twin.” The stuck twin can sometimes be misidentified as monoamniotic. The arterial donor twin also may experience ischemic organ damage involving the brain, kidneys, or bowel. The venous recipient twin can become hypervolemic, hyperviscous, hypertensive, and polyhydramniotic due to increased renal blood flow. Either twin may become hydropic due to volume overload in the recipient or high output failure in the donor. Polyhydramnios, which is common in the venous recipient, also contributes to a high incidence of premature labor or PPROM.
The diagnosis of chronic intertwin transfusion syndrome has become controversial. Older diagnostic criteria, focused primarily on neonatal measures (i.e., cord blood hemoglobin differences of 5 g/dL or birth-weight differences of 20%). These parameters generally have been discarded, as they did not efficiently identify those gestations thought to be affected by this disorder. Chronic intertwin transfusion syndrome now is diagnosed by using ultrasonographic criteria including:
Marked size disparity in fetuses of the same sex
Disparity in size between the two amniotic sacs
Disparity in size of the umbilical cords
A single placenta
Evidence of hydrops in either fetus
Findings of congestive heart failure in the recipient.
The monochorionic placenta, disparity in umbilical cord sizes, and severely discrepant fetal growth characteristic of TTTS are illustrated in Figure 14.5.
Doppler ultrasound may help to improve diagnostic accuracy and assess fetal well-being. The placenta in chronic
intertwin transfusion syndrome results in normal, nondiscordant systolic/diastolic (S/D) ratios. Abnormal SD ratios are more likely to reflect placental abnormalities associated with fetal growth restriction. The absence of underlying placental vascular lesions in chronic intertwin transfusion syndrome results in concordant uterine artery waveforms, helping to differentiate chronic intertwin transfusion syndrome from fetal growth restriction.
intertwin transfusion syndrome results in normal, nondiscordant systolic/diastolic (S/D) ratios. Abnormal SD ratios are more likely to reflect placental abnormalities associated with fetal growth restriction. The absence of underlying placental vascular lesions in chronic intertwin transfusion syndrome results in concordant uterine artery waveforms, helping to differentiate chronic intertwin transfusion syndrome from fetal growth restriction.
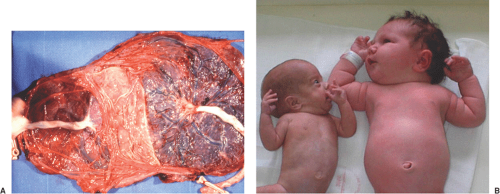 Figure 14.5 Placenta (A) and infants (B) delivery from a monochorionic, diamniotic twin gestation complicated by TTTS. Note the larger placental area associated with the recipient twin and the palor and impaired growth of the donor twin. (See Color Plate) |
Quintero and colleagues have defined TTTS as a deepest vertical pocket ≤2 cm in the donor with a deepest vertical pocket ≥8 cm in the recipient. They also developed a staging system to assess progression and prognosis (Table 14.2).
Management of chronic intertwin transfusion syndrome will be individualized depending on the Quintero stage and the gestational age at which it is encountered. The option of delivery will depend on fetal maturity and the potential morbidity that would be encountered. At earlier gestational ages, serial decompression amniocentesis and tocolytic therapy have been successful in prolonging pregnancy. With the development of fetoscopy, direct laser occlusion of the placental vascular anomaly has become an option. For those patients not delivered, fetal health should be evaluated frequently with biophysical profile scoring or fetal heart rate monitoring. Of all available management options, large volume-reduction amniocentesis is an efficacious and minimally invasive therapy that probably is the treatment of choice after attainment of viability. For the previable patient, the prognosis is extremely poor, and consideration might be given to intrauterine laser ablation of placental surface vascular anastomoses, fetoscopic cord clamping, or termination.
TABLE 14.2 Quintero Staging Criteria for Twin-to-twin Transfusion Syndrome | ||
|---|---|---|
|
Senat and the Eurofetus Consortium reported on a randomized but nonblinded prospective trial of clinical interventions for chronic intertwin transfusion syndrome prior to 26 weeks gestation. Women were randomized to either serial amnio reduction or fetoscopic laser coagulation of vascular anastamoses followed by amnioreduction to a deepest vertical pocket of 5 to 6 cm in the recipient at the conclusion of surgery. This study was halted following an interim analysis because of better outcomes in the laser group with respect to survival of at least one twin to 28 days of age (76% vs. 56%) There also was a significant reduction in the RR of both fetuses dying (RR 0.63; 95% CI 0.25 to 0.93; p = 0.009) and of both surviving at 6 months of age (p = 0.002). Additionally, surviving infants in the laser group were more likely to be free of neurologic complications at 6 to 12 months of age (52% vs. 31%, p = 0.003). While fetoscopic laser ablation may offer improved outcomes, it is available at only a limited number of centers.
The results of National Institutes of Health (NIH)-sponsored prospective randomized trial of amnioreduction versus selective fetoscopic laser photo coagulation for TTTS
were presented at the 2007 Annual Meeting of the Society for Maternal-Fetal Medicine. Forty-two patients who had failed to respond to an initial amnioreduction were enrolled with a primary outcome of 30-day postnatal survival. There was no significant difference in the primary outcome for either of the recipients (45% vs. 30%) or donors (55% vs. 55%). Additionally, there was no significant difference in 30-day survival of one or both twins between the amnioreduction and selective fetoscopic laser photocoagulation groups (75% vs. 65%) or in overall 30-day survival (60% vs. 43%). Within Quintero stages III and IV, there was a significant reduction in recipient survival for those twins treated by laser coagulation (67% vs. 12.5%; p < 0.03), suggesting that laser photocoagulation may need to be undertaken before TTTS becomes too advanced in order to be beneficial.
were presented at the 2007 Annual Meeting of the Society for Maternal-Fetal Medicine. Forty-two patients who had failed to respond to an initial amnioreduction were enrolled with a primary outcome of 30-day postnatal survival. There was no significant difference in the primary outcome for either of the recipients (45% vs. 30%) or donors (55% vs. 55%). Additionally, there was no significant difference in 30-day survival of one or both twins between the amnioreduction and selective fetoscopic laser photocoagulation groups (75% vs. 65%) or in overall 30-day survival (60% vs. 43%). Within Quintero stages III and IV, there was a significant reduction in recipient survival for those twins treated by laser coagulation (67% vs. 12.5%; p < 0.03), suggesting that laser photocoagulation may need to be undertaken before TTTS becomes too advanced in order to be beneficial.
Fetal and Newborn Complications