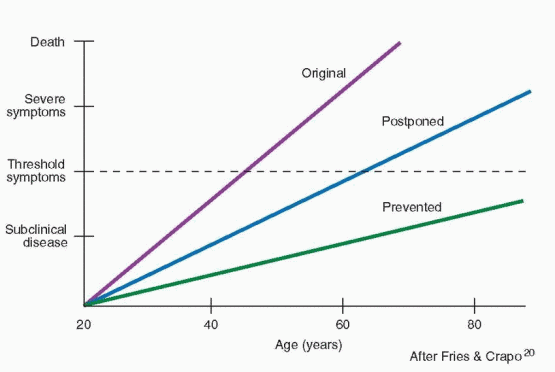
The menopause should serve to remind patients and clinicians that this is a time for education. Certainly preventive health care education is important throughout life, but at the time of the menopause, a review of the major health issues can be especially rewarding. Besides the general issues of good health, attention is appropriately focused on cardiovascular disease and osteoporosis.
Vasomotor Symptoms
The vasomotor flush is viewed as the hallmark of the female climacteric, experienced to some degree by most postmenopausal women. The term “hot flush” or “hot flash” is descriptive of a sudden onset of reddening of the skin over the head, neck, and chest, accompanied by an increase in heart rate and a feeling of intense body heat. The flush is sometimes concluded by profuse perspiration. The duration varies from a few seconds to several minutes and, rarely, for an hour. The frequency may be rare to recurrent every few minutes. Flushes are more frequent and severe at night (when a woman is often awakened from sleep) or during times of stress. In a cool environment, hot flushes are fewer, less intense, and shorter in duration compared with a warm environment.
203 Most importantly, hot flushing can affect a woman’s quality of life and interfere with work or recreational activities.
In the longitudinal follow-up of a large number of women, fully 10% of the women experienced hot flushes before menopause, while in other studies as many as 15-25% of premenopausal women reported hot flushes.
9,89,204,205 The frequency has been reported to be even higher in premenopausal women diagnosed with premenstrual syndrome.
206 In the Massachusetts Women’s Health Study, the incidence of hot flushes increased from 10% during the premenopausal period to about 50% just after cessation of menses.
89 By approximately 4 years after menopause, the rate of hot flushes declined to 20%. In a community-based
Australian survey, 6% of premenopausal women, 26% of perimenopausal women, and 59% of postmenopausal women complained of hot flushing.
207 A large American cross-sectional survey reported that 57% of perimenopausal women and 49% of early postmenopausal women experienced significant hot flushing.
200 Another national survey in the U.S. reported hot flushing in 79% of perimenopausal women and 65% of postmenopausal women.
208In cross-sectional surveys, up to 40% of premenopausal women and 85% of menopausal women report some vasomotor complaints.
205 A longitudinal study in Gothenburg, Sweden, recorded a maximal prevalence of 60% at age 52-54, with a decline to 30% at age 60 and 9% at age 72.
209 In the SWAN study, 57% of perimenopausal women experienced hot flushing, and about 50% after menopause up to age 55.
210 There is no difference in the prevalence of vasomotor complaints in U.S. surveys of black and white women.
211,212 Overweight women report more hot flushing, perhaps reflecting the effect of body fat causing a higher core body temperature.
200,202,213 Exact estimates on prevalence are hampered by inconsistencies and differences in methodologies, cultures, and definitions.
214 The prevalence in different societies is influenced by personal and social attitudes, individual psychological and physical health, familiarity with the portrayal of menopausal issues in the literature and media, ethnic variation, different diets, and dissimilar living conditions; however, accounting for cultural differences, the overall prevalence and experience are similar throughout the world.
215,216Although the flush can occur in the premenopause, it is a major feature of postmenopause, peaking in the first year after the last menses, lasting in 50% of women for 4 to 5 years, but in some (as many as 25%) for longer than 5 years, and up to 15 years in 10%.217 In an excellent Australian longitudinal cohort study, the average duration of vasomotor symptoms was 5.2 years (with a range of 2 to 10 years) in nonusers of hormone thrapy, and slightly longer, 5.5 years, in hormone users.218
The physiology of the hot flush is still not understood. Studies suggest that women with hot flushes have a more narrow zone of temperature regulation, and therefore, smaller changes in core body temperature produce compensatory responses, such as shivering or flushing.
219 MRI scanning of the brain during hot flushing indicates widely distributed cortical activation rather than a precise location.
220 Hot flushes are definitely brought about by a decline in estrogen; however, not all hot flushes are due to estrogen deficiency. Flushes and sweating can be secondary to diseases, including pheochromocytoma, carcinoid, leukemias, pancreatic tumors, and thyroid abnormalities.
221 Unfortunately, the hot flush is a relatively common psychosomatic symptom, and women often are unnecessarily treated with estrogen.
When the clinical situation is not clear and obvious, estrogen deficiency as the cause of hot flushes should be documented by elevated levels of FSH.The correlation between the onset of flushes and estrogen reduction is clinically supported by the effectiveness of estrogen therapy and the absence of flushes in hypoestrogen states, such as gonadal dysgenesis. Only after estrogen is administered and withdrawn do hypogonadal women experience the hot flush. Although the clinical impression that premenopausal surgical castrates suffer more severe vasomotor reactions is widely held, this was not borne out in the only objective study ever performed.
222Although the hot flush is the most common problem of the postmenopause, it presents no inherent health hazard. The flush is accompanied by a discrete and reliable pattern of physiologic changes.
219,223 The flush coincides with a surge of LH (not FSH) and is preceded by a subjective prodromal awareness that a flush is beginning. This aura is followed by measurable increased heat over the entire body surface. A flush is triggered by a small elevation in core body temperature. The body surface experiences an increase in temperature, accompanied by changes in skin conductance, and then the flush is followed by a fall in core temperature—all of which can be objectively measured. In short, the flush is not a
release of accumulated body heat but is a sudden inappropriate excitation of heat release mechanisms. Its relationship to the LH surge and temperature change within the brain is not understood. The observation that flushes occur after hypophysectomy indicates that the mechanism is not dependent on or due directly to LH release. In other words, the same brain event that causes flushes also stimulates gonadotropin-releasing hormone (GnRH) secretion and elevates LH. This is probably secondary to hypothalamic changes in neurotransmitters that increase neuronal and autonomic activity.
224Premenopausal women experiencing hot flushes should be screened for thyroid disease and other illnesses. A comprehensive review of all possible causes is available.
225 Clinicians should be sensitive to the possibility of an underlying emotional problem. Looking beyond the presenting symptoms into the patient’s life is an important service to the patient and her family that eventually will be appreciated. This is far more difficult than simply prescribing estrogen, but confronting problems is the only way of reaching some resolution. Prescribing estrogen inappropriately (in the presence of normal levels of gonadotropins) only temporarily postpones, by a placebo response, dealing with the underlying issues.
A striking and consistent finding in most studies dealing with menopause and hormonal therapy is a marked placebo response (at least 51% in the first weeks of treatment)
226 in a variety of symptoms, including flushing. In an English randomized, placebo-controlled study of women being treated with estrogen implants and requesting repeat implants, there was no difference in outcome in terms of psychological and physical symptoms comparing the women who received an active implant to those receiving a placebo.
227A significant clinical problem encountered in our referral practice is the following scenario: a woman will occasionally undergo an apparently beneficial response to estrogen, only to have the response wear off in several months. This leads to a sequence of periodic visits to the clinician and ever-increasing doses of estrogen. When a patient reaches a point of requiring large doses of estrogen, a careful inquiry must be undertaken to search for a basic psychoneurotic or psychosocial problem. To help persuade a patient that her symptoms are not due to low levels of estrogen, we find it very helpful and convincing to measure the patient’s blood level of estradiol and share the result with her.
Atrophic Changes
With extremely low estrogen production in the late postmenopausal age, or many years after castration, atrophy of vaginal mucosal surfaces takes place, accompanied by vaginitis, pruritus, dyspareunia, and stenosis. Genitourinary atrophy leads to a variety of symptoms that affect the ease and quality of living. Urethritis with dysuria, urgency incontinence, and urinary frequency are further results of mucosal thinning, in this instance, of the urethra and bladder. Recurrent urinary tract infections are effectively prevented by postmenopausal intravaginal estrogen treatment.
228 Vaginal relaxation with cystocele, rectocele, and uterine prolapse, and vulvar dystrophies are not a consequence of estrogen deprivation.
Deprived of estrogen, the vagina loses collagen, adipose tissue, and the ability to retain water. As the vaginal walls shrink, the rugae flatten and disappear. The surface epithelium loses its outer fibrous layer and thins to a few layers of cells, markedly reducing the ratio of superficial to basal cells. As a result, the vaginal surface is left friable, prone to bleeding with minimal trauma. While these changes are occurring, the blood vessels in the vaginal walls narrow, and secretions from sebaceous glands diminish. Over time the vagina itself contracts and loses flexibility, while the labia minora become paler and smaller. In addition, pH becomes more alkaline, making the vaginal environment less hospitable to lactobacilli and more susceptible to infection by urogenital and fecal pathogens. Infecting organisms can ascend into the urinary system to cause urethritis, urinary tract infections, and cystitis.
Dyspareunia, sometimes with postcoital bleeding, is the inevitable consequence of a severely atrophied vagina and scanty lubrication. Even for women who are not sexually active, atrophic vaginitis can cause itching, irritation, and burning. These symptoms often go unmentioned, and it is important to inspect for signs of vaginal atrophy even in the absence of complaints.
Measuring pH is a simple way to determine estrogen’s influence or absence. A pH greater than 4.5 is almost always observed with estrogen deficiency.229,230Dyspareunia seldom brings older women to our offices. A basic reluctance to discuss sexual behavior still permeates our society, especially among older patients and physicians. Gentle questioning may lead to estrogen treatment of atrophy and enhancement of sexual enjoyment. Objective measurements have demonstrated that vaginal factors that influence the enjoyment of sexual intercourse can be maintained by appropriate doses of estrogen.
231 Both patient and clinician should be aware that a significant response can be expected by 1 month, but it takes a long time to fully restore the genitourinary tract (6-12 months), and clinicians and patients should not be discouraged by an apparent lack of immediate response. Raloxifene and tamoxifen have little impact on the vaginal epithelium, and vaginal dryness is worse with aromatase inhibitors. Sexual activity by itself supports the circulatory response of the vaginal tissues and enhances the therapeutic effects of estrogen. Therefore, sexually active older women have less atrophy of the vagina even without estrogen.
Although it is argued that genuine stress incontinence is not affected by treatment with estrogen, others contend that estrogen treatment improves or cures stress incontinence in over 50% of patients due to a direct effect on the urethral mucosa.
232,233 and
234 A meta-analysis concluded that improvement was reported only in nonrandomized studies.
235 Two randomized trials dedicated to this clinical problem failed to demonstrate a beneficial effect of estrogen treatment.
236,237 Most cases of urinary incontinence in elderly women are a mixed problem with a significant component of urge incontinence that is believed to be improved by estrogen therapy. However, the Heart and Estrogen-progestin Replacement Study (HERS) randomized trial indicated a worsening of incontinence with hormone therapy for both urge and stress incontinence, and the Nurses’ Health Study reported a small increase of incontinence in hormone users.
238,239 There is no convincing support for a beneficial impact of estrogen treatment on incontinence. In the SWAN study, only 15% of incontinent women reported a worsening of urinary incontinence during the perimenopausal transition, largely because of weight gain.
240 The majority of incontinent women experienced either no change or an improvement. The SWAN study strongly documents that urinary incontinence is not a major symptom of menopause and the perimenopausal transition.
240,241 Incontinence at midlife is not a consequence of hormonal changes, but largely the effect of excess body weight or diabetes mellitus.A decline in skin collagen content, elasticity, and skin thickness that occurs with aging can be considerably avoided by postmenopausal estrogen therapy.
242,243,244,245 and
246 The effect of estrogen on collagen is evident in both bone and skin; bone mass and collagen decline in parallel after menopause, and estrogen treatment reduces collagen turnover and improves collagen quality.
247,248 One study demonstrated not only an increase in facial skin thickness, but an improvement in wrinkles with topical estrogen.
249 A randomized trial demonstrated improvements in skin elasticity, skin hydration, and skin thickness comparing hormone treatment with placebo.
250 More impressively, data from the U.S. First National Health and Nutrition Examination Survey indicated that estrogen use was associated with a lower prevalence of skin wrinkling and dry skin.
251 Smoking is a major risk factor for facial skin wrinkling, and hormone therapy cannot diminish this impact of smoking.
252 In a 1-year clinical trial, hormone therapy did not improve skin wrinkling already present.
253One of the features of aging in men and women is a steady reduction in muscular strength. Many factors affect this decline, including height, weight, and level of physical activity. Women currently using estrogen have been reported to demonstrate a lesser decline in muscular strength, although at least one study could detect no impact of estrogen.
254,255,256,257,258 and
259 This is an important issue because of the potential protective consequences against fractures, as well as a benefit due to the ability to maintain vigorous physical exercise.
Psychophysiologic Effects
The view that menopause has a deleterious effect on mental health is not supported in the psychiatric literature, or in surveys of the general population.
204,205,260,261 The concept of a specific menopause-induced psychiatric disorder (involutional melancholia) has been abandoned. Indeed, depression is less common, not more common, among middle-aged women, and the menopause cannot be linked to psychological distress.
3,4,5,6,7,8 and
9,262 The longitudinal study of premenopausal women indicates that hysterectomy with or without oophorectomy is not associated with a negative psychological impact among middle-aged women.
263 Longitudinal data from the Massachusetts Women’s Health Study document that menopause is not associated with an increased risk of depression.
264 Although women are more likely to experience depression than men, this sex difference begins in early adolescence, not at menopause.
265The U.S. National Health Examination Follow-up Study includes both longitudinal and cross-sectional assessments of a nationally representative sample of women. This study has found no evidence linking either natural or surgical menopause to psychologic distress.
266 Indeed, the only longitudinal change was a slight decline in the prevalence of depression as women aged through the menopausal transition. Results in this study were the same in estrogen users and nonusers.
A negative view of mental health at the time of the menopause is not justified; many of the problems reported at the menopause are due to life events.11,12,267,268 Thus, there are problems encountered in the perimenopausal transition and the early postmenopause that are seen frequently, but their causal relation with estrogen is unlikely. These problems
include fatigue, nervousness, headaches, insomnia, depression, irritability, and palpitations. Indeed, at this stage of life both men and women express a multitude of complaints that do not reveal a gender difference that could be explained by a hormonal cause.
269,270 Nevertheless, midlife women report complaints more often than men,
270 perhaps reflecting the generally negative perceptions and connotations our cultures and societies have attributed to the menopause.
Two longitudinal cohort studies assessed the new onset of depressive symptoms and disorders during the perimenopausal transition. The Penn Ovarian Aging Study followed over 8 years 436 women with no history of depression and correlated hormonal changes with the onset of depressed mood.
271 Fifty percent of the women developed an increase in measures of depression and 26% met the criteria for a clinical diagnosis of depressive disorder. Using the women as their own controls, the depression group was 2.5 times more likely to develop clinical depression comparing status during the perimenopausal transition to the premenopausal state. These symptoms during the perimenopausal transition were associated with greater variability (but no average differences) in estradiol levels, suggesting that fluctuations of estradiol can be an important destabilizing factor.
The Harvard Study of Moods and Cycles is a prospective cohort of women with and without histories of depression.
272 In the women who entered the perimenopausal transition, the risk of new depression was almost doubled compared with premenopausal women, from 9.5% to 16.6%, and this risk was linked to the presence of vasomotor symptoms. Importantly, a statistically significant increase in risk of new depressive symptoms was present only in women with a history of adverse life events (the events are not defined or specified in the report). Also of note, 83% of the women experienced no mood changes.
The SWAN study reported similar results. A first episode of depression in perimenopausal women was linked to poor physical health, anxiety disorders, stressful life events, and hot flushing.
273This area of concern has been very difficult to study. Inconsistent results can reflect variations in study designs, selection of subjects, methods used to measure mood, and the definition of menopausal status.
However, the best reports provide reliable evidence of a vulnerable population of women. Depressive mood changes are influenced by other factors, including body weight, smoking, premenstrual syndrome (PMS, defined in
Chapter 14), employment, and marital status. Premenopausal PMS is a strong predictor of depressive symptoms arising in the menopausal transition.
The most important questions are whether truly normal women experience an increase in depression during the menopausal transition, and are there subtle or even clinically apparent psychological problems that identify a susceptible subgroup? The cohort studies support the argument that there is a vulnerable group of perimenopausal women who are responsible for the increase of new depression observed during the perimenopausal transition. The data are consistent with the idea that fluctuations in hormone levels are related to mood symptoms, but it is impossible to know if this is a true cause and effect relationship.
In summary, most women (about 85%) experience the perimenopausal transition without mood difficulties. Some women are at greater risk of new onset depressive symptoms, and this is probably enhanced by hormonal variations and vasomotor symptoms. These vulnerable women are likely derived from a group of premenopausal women with underlying psychological problems (although “problem” may be too strong of a word). It is also possible that perimenopausal hormone changes create a state that makes an individual less able to deal with adverse events in life.
Attempts to study the effects of estrogen on these problems have been hampered by the subjectivity of the complaints (high placebo responses) and the “domino effect” of what
a reduction of hot flushes does to the frequency of the symptoms. Using a double-blind crossover prospective study format, Campbell and Whitehead concluded many years ago that many symptomatic “improvements” ascribed to estrogen therapy result from relief of hot flushes—a “domino” effect.
274 Studies that have controlled for menopausal symptoms conclude that mood is very affected by vasomotor symptoms and sleep disturbances, besides reflecting life problems.
139,275A study of 2,001 Australian women aged 45-55 focused on the utilization of the health care system by women in the perimenopausal period of life.
14 Users of the health care system in this age group were frequent previous users of health care, less healthy, and had more psychosomatic symptoms and vasomotor reactions. These women were more likely to have had a significant previous adverse health history, including a past history of premenstrual complaints. This study emphasized that perimenopausal women who seek health care help are different from those who do not seek help, and they often embrace hormone therapy in the hope it will solve their problems. Similar findings have been reported in a cohort of British women.
276 It is this population that is seen most often, producing biased opinions among clinicians regarding the menopause. We must be careful not to generalize to the entire female population the behavior experienced by this relatively small group of women. Most importantly, perimenopausal women who present to clinicians often end up being treated with estrogen inappropriately and unnecessarily. Nevertheless, it is well established that a woman’s quality of life is disrupted by vasomotor symptoms, and estrogen therapy provides impressive improvement.
277,278 and
279 Patients are grateful to be the recipients of this “domino” effect.
The Women’s Health Initiative (discussed in
Chapter 18) concluded that estrogen-progestin therapy had no beneficial impact on health-related quality of life.
280 However, only 12.7% of the participants had moderate to severe vasomotor symptoms at entry to the study, and the severity can be questioned because the participants were willing to take placebo medication. The overall baseline quality of life in this study was relatively high, and the study participants were older (the average number of years distant from menopause was 12+). This randomized, clinical trial did not study the appropriate population of women in order to assess the effect of hormone therapy on measures of quality of life.
The Women’s International Study of Long Duration Oestrogen after the Menopause (WISDOM) trial was a randomized, controlled trial in the U.K., Australia, and New Zealand, of 3,721 women aged 50-69 treated with either combined 0.625 mg conjugated estrogens-2.5/5.0 mg medroxyprogesterone or placebo.
281 The original plan was to randomize 22,300 women to the study that would last 10 years. The study was canceled in October 2002 in reaction to the initial reports from the WHI. Unfortunately, the premature cancellation precludes the possibility of any long-term data from WISDOM. In 2,130 women who completed one year, there were statistically significant improvements in the treated women in the categories of vasomotor, sexual, and sleep symptoms. Treated women reported a reduction in aching joints and muscles, night sweats, insomnia, and vaginal dryness. The treated group reported more breast tenderness, but the percentages were notably low (16% in the treated group and 7% in the placebo group).
The WISDOM trial investigators argued that the small effects on quality of life reported by the WHI and HERS can be attributed to the insensitive measurement tools used in those clinical trials. The WISDOM trial used a survey tool specifically designed to assess postmenopausal physical and emotional wellbeing, plus a validated, generic questionnaire, the European quality of life instrument. Only the specific questionnaire detected significant changes; the European generic tool did not. This emphasizes the importance of using the appropriate study tool to investigate this area of postmenopausal health. Similar results with vasomotor symptoms, sleep, and joint complaints were actually reported by the WHI, but with a smaller difference between treated and placebo groups. The WHI survey had only one question devoted to sexuality.
The results of the WISDOM trial are not surprising; they reflect what all clinicians have observed in their own practices. The most important point to be made is this: the WISDOM, WHI, and HERS trials were all similar in that they enrolled postmenopausal women heavily tilted towards the oldest age group without symptoms. It is a simple and logical conclusion that hormone therapy in a younger, symptomatic group of postmenopausal women would produce greater quality of life benefits than that quantified in the clinical trials. All three clinical trials, therefore, underestimated the beneficial impact because of age and symptom status of their participants. However, in the WISDOM trial, even older postmenopausal women who were symptomatic benefited from hormone therapy. Age should not be the sole guiding factor in decision-making.
Emotional stability during the perimenopausal period can be disrupted by poor sleep patterns. Hot flushing does have an adverse impact on the quality of sleep.
282,283 and
284 Estrogen therapy improves the quality of sleep, decreasing the time to onset of sleep and increasing the rapid eye movement (REM) sleep time.
277,285,286 In the SWAN study, one-third of the women reported sleep problems, even without hot flushes or night sweats, and the prevalence of vasomotor symptoms was associated with an increased risk of sleep disturbances; hormone therapy improved sleep quality.
287,288 Perhaps flushing may be insufficient to awaken a woman but sufficient to affect the quality of sleep, thereby diminishing the ability to handle the next day’s problems and stresses. An improvement in sleeping with estrogen treatment can even be documented in postmenopausal women who are reportedly asymptomatic.
286Thus, the overall “quality of life” reported by women can be improved by better sleep and alleviation of hot flushing. However, it is still uncertain whether estrogen treatment has an additional direct pharmacologic antidepressant effect or whether the mood response is totally an indirect benefit of relief from physical symptoms and, consequently, improved sleep. Utilizing various assessment tools for measuring depression, improvements with estrogen treatment were recorded in oophorectomized women.
289,290 In the large prospective cohort study of the Rancho Bernardo retirement community, no benefit could be detected in measures of depression in current users of postmenopausal estrogen compared with untreated women.
291 Indeed, treated women had higher depressive symptom scores, presumably reflecting treatment selection bias; symptomatic and depressed women seek hormone therapy. Others report that estrogen therapy has a more powerful impact on women’s well-being beyond the relief of symptoms such as hot flushes.
277,292,293 In elderly depressed women, improvements in response to fluoxetine were enhanced by the addition of estrogen therapy.
294 In a 12-week, randomized, placebo-controlled trial of 55 perimenopausal women with clinically significant major depression, estradiol treatment with the 100 mg transdermal method significantly improved mood.
295 A similar American short-term study of 34 perimenopausal women with both major and minor depressions treated with 50 mg estradiol transdermally demonstrated improvements independently of an effect on vasomotor symptoms.
296 These small clinical trials argue that estrogen treatment is beneficial for the treatment of clinical depression. This conclusion is supported by the successful treatment of postpartum depression with estradiol treatment.
297,298The most common cause of perimenopausal mood problems is already-existing depression,10,299 but there does exist a small population of women whose moods are sensitive to hormonal changes. In the American SWAN study, the prevalence of mood changes increased from the premenopause to the early perimenopause, from about 10% to about 16.5%.
299 There are three possible explanations: (1) the decline in estrogen at menopause affects neurotransmitters that regulate mood; (2) mood is adversely affected by vasomotor symptoms (the domino theory); (3) mood is affected by the vicissitudes of life that are commonly prevalent around menopause. Some would argue that these mood swings are in response to the hormonal fluctuations that occur during the perimenopausal years. These fluctuations do indeed occur,
64 but whether they cause any symptoms remains to be determined. It seems logical that individuals with mood problems can reflect all of these mechanisms.
Cognition and Alzheimer’s Disease
Depending on the method of assessment, evidence for beneficial effects of estrogen on cognition can be found in the literature, especially in verbal memory.
300,301 However, the effects in healthy women are not impressive, and perhaps of little clinical value. A short-term study failed to document an objective improvement in memory, although a slight improvement in mood was recorded.
302 Another short-term (3 months) randomized, double-blind study could detect no improvement in cognitive performance compared with placebo treatment.
303 The Melbourne Women’s Midlife Health Project could not document an effect on verbal memory during the menopausal transition.
304 A longitudinal study in Chicago could not detect a cognitive decline through the menopause, as assessed by working memory and perceptual speed.
305 On the other hand, estrogen treatment of women immediately after bilateral oophorectomy was associated with improvement in certain, but not all, specific tests of memory, and healthy postmenopausal women taking estrogen scored higher on tests of immediate and delayed recall.
306,307 and
308 In a case-control study of women aged 55-93 years, estrogen users had better recall of proper names, but no improvement in word recall.
309 Women in the Baltimore Longitudinal Study of Aging who were using estrogen performed better in tests of visual learning and memory.
310,311 In a New York City cohort of women, the use of estrogen was associated with better performance in tests of cognition, and better performance in verbal memory, but the cohort in the Study of Osteoporotic Fractures demonstrated no effect of estrogen use on the age-related decline in cognition.
312,313 In Connecticut, a randomized, placebo-controlled trial demonstrated better reading ability and verbal memory in the estrogen-treated group of postmenopausal women.
314 Perhaps a lack of agreement is due to the variability in test vehicles and the specific aspects of memory function studied. Furthermore, there is impressive individual variability, and when differences have been observed they have not been large, and perhaps of little clinical importance. In addition, any beneficial effects may be attenuated by progestational agents.
301Another possibility for the variable effects of estrogen treatment on cognition is the variability among women in endogenous estrogen levels. Using sensitive assays for free, non-protein-bound estradiol and bioavailable (loosely bound) estradiol, cognitive decline occurred at a greater rate in women with low estradiol levels.
315 Studies of cognition may have to differentiate between low- and high-risk women according to endogenous, biologically active estradiol levels. Similarly, a beneficial effect on cognitive decline has been observed only in women negative for the gene associated with Alzheimer’s disease,
APOE-e4, which encodes the e4 allele of the glycoprotein known as apolipoprotein E, which has as one of its functions, the shuttling of lipids during neuronal repair.
316Up to three times as many women as men develop Alzheimer’s disease. Estrogen is capable of protecting central nervous system function by means of multiple mechanisms. For example, estrogen protects against neuronal cytotoxicity induced by oxidation; estrogen reduces the serum concentration of amyloid P component (the glycoprotein found in Alzheimer’s neurofibrillary tangles); and estrogen increases synapses and neuronal growth, especially dendritic spine density.
317,318 and
319 Estrogen protects against the cerebrovascular toxicity exerted by amyloid peptides, and promotes synaptic formation and neuronal growth and survival.
320,321 and
322 Progestational agents do not exert similar actions.
Case-control and cohort findings indicated that Alzheimer’s disease and related dementia occurred less frequently (perhaps as much as 60% less) in estrogen users, and the effect was greater with increasing dose and duration of use.
323,324 and
325 In the Baltimore Longitudinal Study of Aging (a prospective cohort), the risk of Alzheimer’s disease was 54% reduced; in a cohort in New York City, the risk was reduced 60%; and in the Italian Longitudinal Study of Aging, the risk was 72% reduced in estrogen users.
326,327 and
328 The findings are not uniformly positive; a case-control study with accurate information on clinical diagnoses and estrogen use from the U.K. General Practice Research Database could detect no impact of estrogen
treatment on the risk of developing Alzheimer’s disease, but the number of estrogen users was very small.
329The short-term administration of unopposed estrogen to patients with Alzheimer’s disease (secondary prevention) has been reported to improve cognitive performance, but mostly to have no effect.
330,331,332,333 and
334 The administration of combinations of estrogen and progestin has also failed to demonstrate a beneficial impact in Alzheimer’s disease.
335 The presence of estrogen therapy has been reported to enhance the beneficial response to tacrine in women with Alzheimer’s disease,
336 but overall, the evidence is consistent with a failure of estrogen to influence already-existing Alzheimer’s disease or other forms of dementia.
337The data do support, however, a primary preventive effect. Most revealing is a prospective cohort study of women living in Cache County, Utah.
338 Hormone therapy provided about a 41% reduced risk of developing Alzheimer’s with any use and an 83% reduction with 10 or more years of use. This cohort also demonstrated improved cognition in estrogen users.
339 Most importantly, if women had initiated hormone therapy within a period of time that encompassed 10 years before the development of clinical symptoms, there was no effect. The Utah study strongly suggested that hormone therapy must be used for a significant duration of time very early in the postmenopausal period in order to have an impact on the risk of Alzheimer’s disease. As neurons become changed by the pathology of dementia, they lose their ability to respond favorably to estrogen.
The importance of timing is supported by findings from the large Women’s Health Initiative (WHI) clinical trial. The oldest women in the WHI being treated with either estrogen alone or combined estrogen-progestin (treatment began at age 65 or older) had impaired cognition and an increased risk of dementia.
340,341 and
342 In a subset of these women, MRI scans demonstrated greater brain atrophy in the women receiving hormone therapy.
343 The mechanism for this adverse effect of hormonal therapy in old women may be a neurotoxic action because the WHI study with MRI scanning could not detect an increase in ischemic brain lesions.
344 Smaller MRI studies of younger women treated with hormone therapy found beneficial trophic changes in brain morphology, associated with improved cognition.
345,346 and
347The theme that emerges is that maintenance of health in target organs by estrogen requires normal tissue, a principle of timing that will also be discussed in regards to the heart in Chapter 18.
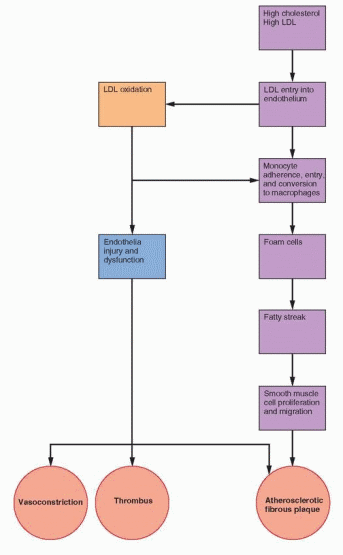
Following the failure of secondary prevention trials to demonstrate a beneficial impact of hormone therapy on coronary disease in older women, it is increasingly argued that healthy cardiovascular endothelium is needed to respond to estrogen; that by the time, the endothelium is involved with excessive atherosclerosis, it is too late for estrogen to exert a beneficial effect. A similar argument is made for brain tissue, focusing on biochemical and signaling pathways that are progressively compromised with the neuronal involvement with disease.
348 The requirement for normal tissue, at least in the heart and in the brain, would explain the beneficial effects in studies of primary prevention and the lack of effect in secondary prevention trials.