Background
The obesogenic and diabetogenic effects of the environmental toxin bisphenol A during critical windows of development are well recognized. Liver and skeletal muscle play a central role in the control of glucose production, utilization, and storage.
Objectives
We hypothesized that maternal bisphenol A exposure disrupts insulin signaling in rat offspring liver and skeletal muscle. We determined the protein expression of hepatic and skeletal muscle insulin signaling molecules including insulin receptor beta, its downstream target insulin receptor substrate 1 and glucose transporters (glucose transporter 2, glucose transporter 4), and hepatic glucose-regulating enzymes phosphoenolpyruvate carboxykinase and glucokinase.
Study Design
Rat dams had ad libitum access to filtered drinking water (control) or drinking water with bisphenol A from 2 weeks prior to mating and through pregnancy and lactation. Offspring litters were standardized to 4 males and 4 females and nursed by the same dam. At weaning, bisphenol A exposure was removed from all offspring. Glucose tolerance was tested at 6 weeks and 6 months. Liver and skeletal muscle was collected from 3 week old and 10 month old offspring for protein expression (Western blot) of insulin receptor beta, insulin receptor substrate 1, glucose transporter 2, glucose transporter 4, phosphoenolpyruvate carboxykinase, and glucokinase.
Results
Male, but not female, bisphenol A offspring had impaired glucose tolerance at 6 weeks and 6 months. Both male and female adult offspring had higher glucose-stimulated insulin secretion as well as the ratio of stimulated insulin to glucose. Male bisphenol A offspring had higher liver protein abundance of the 200 kDa insulin receptor beta precursor (2-fold), and insulin receptor substrate 1 (1.5-fold), whereas glucose transporter 2 was 0.5-fold of the control at 3 weeks of age. In adult male bisphenol A offspring, the abundance of insulin receptor beta was higher (2-fold) and glucose transporter 4 was 0.8-fold of the control in skeletal muscle. In adult female bisphenol A offspring, the skeletal muscle protein abundance of glucose transporter 4 was 0.4-fold of the control.
Conclusion
Maternal bisphenol A had sex- and tissue-specific effects on insulin signaling components, which may contribute to increased risk of glucose intolerance in offspring. Glucose transporters were consistently altered at both ages as well as in both sexes and may contribute to glucose intolerance. These data suggest that maternal bisphenol A exposure should be limited during pregnancy and lactation.
The worldwide incidence of metabolic diseases, including type 2 diabetes, has increased dramatically over the past 30 years. Whereas there is little doubt that diet, exercise, and genetic factors all play a role in an individual’s susceptibility to type 2 diabetes, evidence from human and animal models suggests that predisposition to the development of metabolic disease also begins in utero. This concept of developmental programming states that early environmental exposures during pregnancy and/or lactation programs changes in gene expression that alters growth and development with consequences for the long-term health of the offspring.
Recent public health concerns have been raised regarding the potential long-term effects of exposure to the endocrine disrupter chemical bisphenol A (4,4-dihydroxyl-2,2-diphenylpropane; BPA) during the vulnerable period of perinatal development. BPA is an industrial chemical used primarily to make polycarbonate plastic and epoxy resins. It is used in the production of everyday items including carbon-free paper, sports equipment, medical devices, reusable food and drink containers, and dental sealants.
The abundant use of BPA has made this endocrine disrupter chemical ubiquitous in our environment, leading to chronic low-dose exposure. According to the 2003–2004 National Health and Nutrition Examination Survey, BPA was detected in the urine of >90% of the survey participants. Moreover, children had the highest urinary BPA concentrations, followed by adolescents and adults. In addition, BPA has been detected in maternal serum, amniotic fluid, fetal cord blood, and breast milk.
During periods of increased glucose availability, tight homeostatic regulation of blood glucose levels is primarily achieved by the actions of insulin to inhibit hepatic glucose production and to increase the uptake and storage of glucose in peripheral insulin-sensitive tissues, such as skeletal muscle. Hence, any disruption to glucose-stimulated insulin secretion or to the ability of peripheral tissues to respond to insulin action via intracellular insulin signaling pathways will likely have adverse consequences for glucoregulation.
Using National Health and Nutrition Examination Survey data (2003–2004, 2005–2006, 2007–2008), the majority of published human epidemiological studies have shown positive associations between urinary concentrations of BPA, glucose intolerance, and diabetes. Similarly, studies in animals have shown impaired glucose tolerance and altered secretion of insulin from the endocrine pancreas in adult mice and rats that were exposed to BPA perinatally.
Studies in adult mice and rats have implicated changes in pancreatic beta cell mass as well as lower expression of genes that normally optimize beta cell function, such as Pdx-1 , in the mechanism by which BPA alters glucoregulation. However, homeostatic regulation of blood glucose is determined by both pancreatic beta cell insulin secretion and its effects on peripheral, insulin-sensitive tissues.
The biological effects of insulin, including the uptake of glucose into fat and muscle cells and the suppression of glucose synthesis in the liver, are mediated by the activation of the insulin receptor and the biochemical insulin transduction pathway. Previous studies have focused on BPA-induced alterations in insulin secretion and beta cell mass or function, but whether perinatal exposure to BPA has age-, sex-, or tissue-specific effects on the protein abundance of insulin signaling components is unknown. Hence, the current study determined whether perinatal exposure to BPA alters the protein abundance of insulin signaling components in offspring liver and muscle at weaning and in adulthood.
Materials and Methods
All procedures were approved by the Animal Care Committee at the Los Angeles Biomedical Research Institute at Harbor-University of California, Los Angeles (Los Angeles, CA) and were conducted in accordance with guidelines provided by the American Accreditation Association of Laboratory Care and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Animals
Eleven virgin female Sprague Dawley rats (9 weeks old; Charles River Laboratories, Hollister, CA) were housed in a facility with constant temperature (21 ± 1°C) and a controlled 12 hour, 12 hour light/dark cycle. A rat model of maternal exposure to BPA was created using ad libitum access to BPA (Sigma-Aldrich Corp, St Louis, MO) in drinking water 2 weeks prior to and through pregnancy and lactation. The amount of BPA consumed was 239 ± 8 μg/d per body weight over the course of pregnancy and 466 ± 33 μg/d per body weight during lactation (because of increased water intake during lactation).
At birth, blood from all excess newborn pups was pooled to create sufficient volume to determine the plasma BPA level. Newborns from BPA-treated dams had a plasma BPA level (0.62 ng/mL) within the range of values measured in human umbilical cord blood, whereas BPA was undetectable in plasma from the newborns of control dams. There were no differences in body weights between control (male: 6.85 ± 0.10 g; female: 6.62 ± 0.11 g) and BPA-treated (male: 6.84 ± 0.18 g; female: 6.65 ± 0.08 g; 2-way analysis of variance [ANOVA] with sex and treatment as factors) offspring within each sex at birth or at any time point thereafter.
At 11 weeks of age, the rats were mated and continued on their respective treatments during pregnancy and lactation. After birth, at 1 day of age, litters were culled to 8 pups (4 males and 4 females) per dam to standardize nursing. All pups were nursed by their respective dams until 3 weeks of age. At weaning BPA exposure was removed and the rat pups were housed 4 per cage with the same sex. The animals were separated to 2 per cage at 125 g and into singular housing when their weight was above 250 g, such that no cage contained more than 500 g of total rat body weight.
All rats were housed in BPA-free polycarbonate cages (Ancar Corp, Bellmore, NY). The cages were filled with paper chip bedding (Sherpherd Specialty Papers, Watertown, TN) with cardboard tubes for enrichment. All weaned offspring had ad libitum access to a standard control diet (LabDiet 5001; LabDiet, St Louis, MO) and filtered drinking water with no further BPA exposure.
Glucose tolerance and plasma analyses
At 6 and 24 weeks of age, 1 male and 1 female offspring from each litter underwent a glucose tolerance test (GTT). Following an overnight fast, D-glucose (1 mg/g body weight) was injected intraperitoneally (i.p.) in conscious rats.
Blood glucose values were determined in tail bleed blood prior to (time 0) and 15, 30, 60, 120, and 180 minutes after glucose administration using a Hemocue B-glucose analyzer (HemoCue Inc, Mission Viejo, CA). Plasma insulin concentrations were determined prior to the glucose challenge (time 0; 6 and 24 weeks of age) and during the challenge at 15 and 180 minutes (24 weeks). Blood was collected into heparinized tubes at 0, 15, and 180 minutes and centrifuged immediately at 3000 × g and 4°C for 10 minutes, and the plasma was stored at –80°C. Plasma insulin concentrations were measured using a commercially available, rodent-specific enzyme-linked immunosorbent assay kit (10-1250-01; Mercodia, Uppsala, Sweden).
Tissue collection
At 3 weeks and 10 months of age, offspring were anesthetized by 5% isoflurane/2% oxygen by mask and exsanguinated via cardiac puncture. Euthanasia was confirmed by decapitation. Plasma as well as liver and gastrocnemius skeletal muscle tissue was collected, snap frozen in liquid nitrogen, and stored at –80°C until analysis.
The offspring in the current study were part of a larger study of the long-term effects of perinatal BPA exposure on offspring growth and appetite regulation (these data will be published separately). Consequently, offspring tissues were not available at the exact ages corresponding to the in vivo measurements of glucose clearance. Tissues collected at ages closest to the in vivo experiments were used to determine the protein abundances of components of the insulin signaling pathway.
Protein extraction and Western blotting
Liver and skeletal muscle protein was extracted in radioimmunoprecipitation assay buffer (1×) containing protease inhibitors (HALT cocktail; Pierce, Rockford, IL), and the total protein concentration in the supernatant was determined using a bicinchoninic acid assay (BCA kit; Pierce).
Western blotting was performed as previously described, using primary antibodies from Santa Cruz Biotechnology (Dallas, TX; 1:1000) against insulin receptor beta subunit (IR-β; sc-711), insulin receptor substrate 1 (IRS-1; sc-559), glucose transporter 2 (GLUT2; sc-9117), glucose transporter 4 (GLUT4; sc-1607), phosphoenolpyruvate carboxykinase (sc-32879), and glucokinase (sc-7908). Goat antirabbit immunoglobin G horseradish peroxidase conjugate (number 1706515; Bio-Rad Laboratories, Hercules, CA; 1:2000) and donkey antigoat immunoglobin G horseradish peroxidase conjugate (sc-2020; Santa Cruz Biotechnology; 1:2000) were used as secondary antibodies. The depicted bands had the expected molecular weights.
Statistical analysis
For all studies 5-6 offspring were studied per group (1 of the 4 male offspring and 1 of the 4 female offspring from each of 6 control and 5 BPA-treated litters), with the exception of newborn plasma for BPA measurement in which the blood was pooled from all excess pups. No differences were found in the protein abundance of insulin signaling components in skeletal muscle from the 6 week old glucose-intolerant male offspring; hence, skeletal muscle was not analyzed from 6 week old female offspring that did not show a glucose intolerant phenotype at 3 weeks of age.
Values are means ± SEM. Statistical significance (version 2.0; SigmaStat Statistical Software, San Jose, CA) was assessed using an unpaired Student t test and a 2-way, repeated-measures ANOVA, as appropriate. Statistical significance was accepted when P < .05.
Results
Fasting glucose and insulin
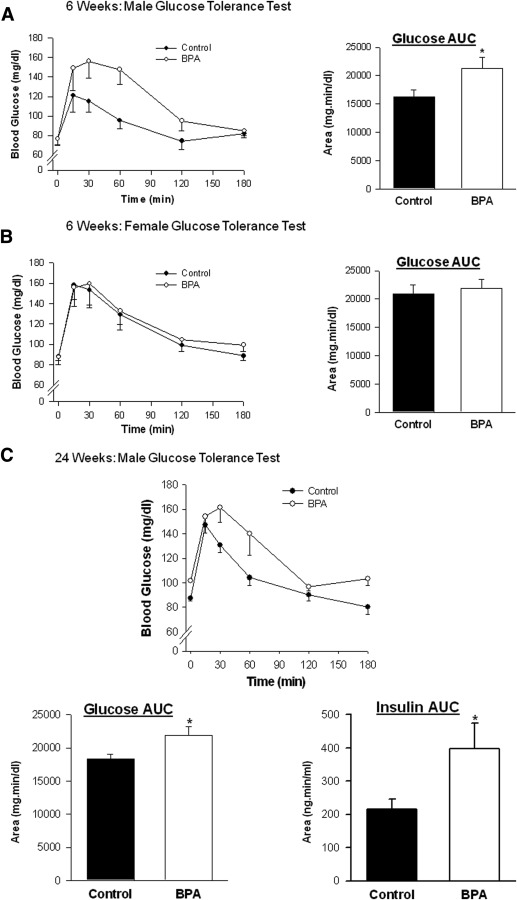
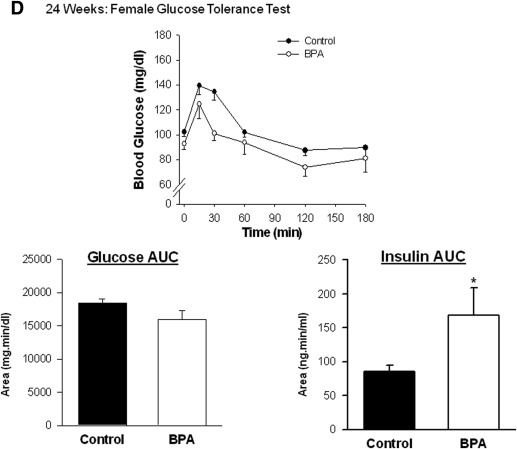
At 3 and 6 weeks of age, male and female fasting blood glucose and plasma insulin concentrations, and the ratio of insulin to glucose, were not different between control and BPA offspring. In contrast, at 24 weeks of age, male BPA offspring had higher plasma insulin concentrations, although the blood glucose concentrations and the ratio of insulin to glucose were comparable with controls.
In addition, 24 week old female BPA offspring had similar blood glucose concentrations and insulin levels, although the ratio of insulin to glucose was increased compared with controls ( Table ).
| Age | Variable | Time of data collection | Male | Female | ||
|---|---|---|---|---|---|---|
| Control | BPA | Control | BPA | |||
| 3 Weeks | ||||||
| Basal blood glucose, mg/dL | At tissue collection | 124 ± 5 | 121 ± 12 | 122 ± 5 | 101 ± 10 | |
| Basal plasma insulin, ng/mL | At tissue collection | 0.19 ± 0.01 | 0.31 ± 0.13 | 0.18 ± 0.01 | 0.18 ± 0.01 | |
| Insulin to glucose ratio | At tissue collection | 1.5 ± 0.2 | 2.3 ± 0.7 | 1.5 ± 0.2 | 1.9 ± 0.2 | |
| 6 Weeks | ||||||
| Basal plasma glucose, mg/dL | GTT 0 minutes | 80 ± 7 | 77 ± 7 | 88 ± 4 | 86 ± 6 | |
| Basal plasma insulin, ng/mL | GTT 0 minutes | 0.18 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.01 | |
| Insulin to glucose ratio | GTT 0 minutes | 2.7 ± 0.5 | 2.5 ± 0.3 | 2.3 ± 0.2 | 2.4 ± 0.2 | |
| 24 Weeks | ||||||
| Basal plasma glucose, mg/dL | GTT 0 minutes | 87 ± 2 | 102 ± 2 | 102 ± 4 | 92 ± 4 | |
| Basal plasma insulin, ng/mL | GTT 0 minutes | 0.60 ± 0.08 | 1.02 ± 0.49 a | 0.40 ± 0.03 | 0.58 ± 0.11 | |
| Insulin to glucose ratio | GTT 0 minutes | 6.8 ± 0.8 | 9.98 ± 4.67 | 4.0 ± 0.4 | 6.28 ± 1.05 a | |
| Plasma glucose, mg/dL | GTT 15 minutes | 147 ± 6 | 154 ± 8 | 139 ± 7 | 125 ± 11 | |
| Plasma insulin, ng/mL | GTT 15 minutes | 1.46 ± 0.25 | 2.98 ± 0.42 a | 0.51 ± 0.05 | 1.47 ± 0.46 a | |
| Insulin to glucose ratio | GTT 15 minutes | 9.7 ± 1.3 | 19.5 ± 2.7 a | 3.7 ± 0.5 | 11.7 ± 3.3 a | |
Glucose tolerance test
At both ages (6 and 24 weeks), administration of glucose generated an immediate increase in the blood concentration of glucose ( P < .05) that was maximal between 15 and 30 minutes after the injection in both BPA and control offspring ( Figure 1 ). In 6 week old BPA males, the area under the glucose curve was higher than in controls and remained higher in adulthood at 24 weeks of age ( Figure 1 , A and C). However, perinatal exposure to BPA did not affect the time course of glucose clearance or the area under the glucose curve in female offspring at 6 or 24 weeks of age ( Figure 1 , B and D).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


