Male Infertility
 |
Our understanding of male reproductive function and the importance of male factors in infertility has advanced significantly over the last two decades. In the past, the female partner was the primary focus of attention and male factors were regarded as a relatively uncommon cause of infertility. We now recognize that abnormalities in the male are the sole cause of infertility in approximately 20% of infertile couples and are an important contributing factor in another 20-40% of couples with reproductive failure.1
Correct diagnosis and specific treatment can help many infertile men to achieve a natural conception with their partners. In others, mild but important semen abnormalities can be overcome by treatments such as intrauterine insemination (IUI). When all else is futile or fails, modern assisted reproductive technologies (ART) still may provide the means to achieve success. In vitro fertilization (IVF) by intracytoplasmic sperm injection (ICSI), involving the injection of a single sperm directly into a mature oocyte, offers men previously considered hopelessly infertile a realistic chance to father children. Artificial insemination using donor sperm, once the only option available for many couples with male factor infertility, remains an important and highly effective treatment strategy, but now may be regarded as the treatment of last resort.
Physicians who care for infertile couples must know how to conduct a basic evaluation of male reproductive function and how to recognize men who require more extensive or sophisticated evaluation and treatment beyond the scope of their own expertise. This chapter will consider the regulation of testicular function, describe the causes of male infertility, discuss the analysis of semen and other methods for evaluation of infertile men, and review current concepts regarding the treatment of male factor infertility.
Regulation of Testicular Function
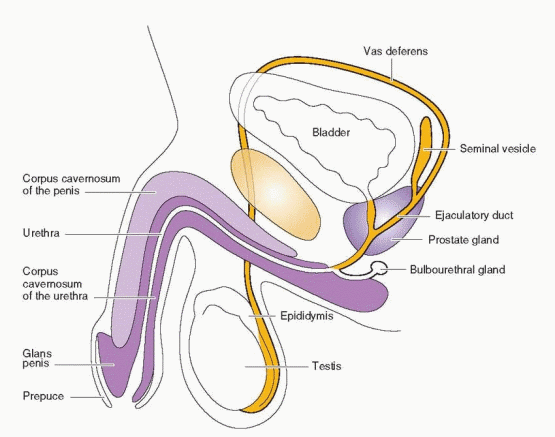
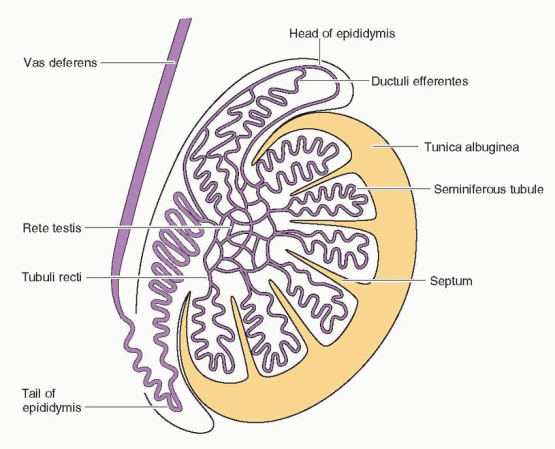
The testes have two distinct components, the seminiferous tubules (the site of spermatogenesis) and the Leydig cells (the source of testosterone). The seminiferous tubules are composed of germ cells, called spermatogonia, and Sertoli cells, which produce inhibin. Tight junctions between the Sertoli cells form a diffusion barrier known as the blood-testis barrier (similar to the blood-brain barrier), which protects the germ cells from antigens, antibodies, and environmental toxins.2 The seminiferous tubules are therefore essentially avascular, so regulatory molecules must enter by diffusion. The Leydig cells are located in the connective tissue between the seminiferous tubules.
Spermatogenesis
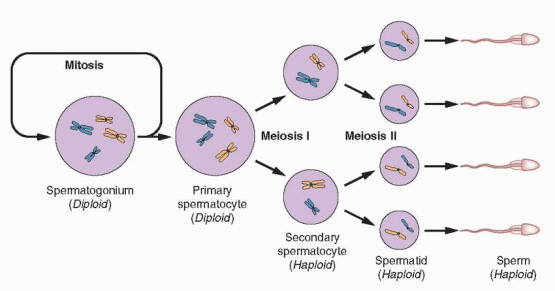
After migration of the germ cells to the genital ridge during embryogenesis, there are approximately 300 thousand spermatogonia in each gonad. Each undergoes a series of mitotic divisions and, by puberty, there are about 600 million in each testis. Continued proliferation during adult life supports the production of approximately 100-200 million sperm each day and more than 1 trillion during a normal reproductive life span.3 A spermatogonia-specific transcription factor identified in mice, Plzf, is required for maintenance of the spermatogonial stem cell pool.4,5
As spermatogenesis begins, the diploid (46 chromosomes) spermatogonia grow to become primary spermatocytes before entering meiosis. The first meiotic division yields two haploid (23 chromosomes) secondary spermatocytes, each of which gives rise to two spermatids during the second meiotic division. Thereafter, each spermatid gradually matures to become a mature spermatozoan. Approximately 3 million spermatogonia begin development each day, but about half of all potential sperm production is lost during meiosis.6
As spermatids develop into mature sperm, the nucleus moves to an eccentric position at the head of the spermatid and becomes covered by an acrosomal cap.7 The core of the sperm tail consists of nine outer fibers around two inner fibers, surrounded in the middle section by mitochondria. The tail fibers are attached to each other by arms containing the protein dynein, which is an ATPase. Hydrolysis of ATP (adenosine triphosphate) in the adjacent mitochondria provides the energy for sperm motility, which is produced by a sliding action between the fibers in the sperm tail.
The spermatogenic process is directed by genes located on the Y chromosome8 and takes approximately 70 days to complete from the spermatocyte stage.9 Another 12-21 days are required for the transport of sperm from the testis through the epididymis to the ejaculatory duct.10 During passage through the epididymis, sperm mature further to develop the capacity for sustained motility.11 The long time required for sperm development and transit implies that the results of a semen analysis reflect conditions existing many weeks earlier. Final maturation, or capacitation, of sperm may occur after ejaculation into the female genital tract. Normal spermatogenesis requires the lower temperature of the scrotum, but slight increases in scrotal temperature, such as are associated with the wearing of athletic supporters, do not appear to have any measureable adverse effect.12 Semen includes secretions contributed by the prostate, the seminal vesicles, and the distal vasa deferentia.
 |
Hormone Regulation
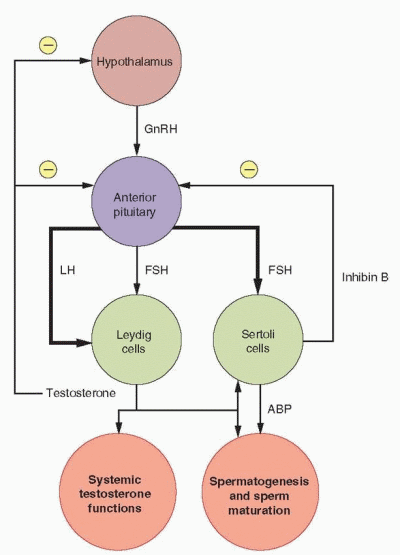
Normal testicular function requires the actions of both pituitary gonadotropins, folliclestimulating hormone (FSH) and luteinizing hormone (LH). LH stimulates the Leydig cells in the testicular interstitium to synthesize and secrete testosterone (approximately 5-10 mg per day). The actions of LH are supported indirectly by FSH, which induces the appearance of LH receptors on testicular Leydig cells13 and stimulates synthesis of androgen binding protein (ABP) in Sertoli cells.14 Testosterone is secreted both into the circulation and into the lumen of the seminiferous tubules where it is highly concentrated to the levels needed to support spermatogenesis in the germinal epithelium and sperm maturation in the epididymis; concentrations within the testes are 50-100 times higher than in blood.15,16 The actions of testosterone in support of spermatogenesis are mediated by the Sertoli cells, which line the seminiferous tubules and contain androgen receptors.16
Rising serum testosterone levels exert feedback inhibition on LH secretion, acting both at the hypothalamic level to slow the pulsatile release of hypothalamic gonadotropin-releasing hormone (GnRH),17,18 probably via a mechanism involving endogenous opiates,19 and at the pituitary level to decrease pituitary gonadotrope sensitivity to GnRH stimulation.20 Numerous studies involving infusions of testosterone, estradiol, or dihydrotestosterone (which cannot be converted to estrogen) or the administration of estrogen antagonists in normal subjects,21,22 in individuals with androgen insensitivity,23 and in men with idiopathic hypogonadotropic hypogonadism24 have established that testosterone exerts its negative feedback effects on LH secretion both directly, and indirectly via conversion to estradiol in the brain. Evidence that estradiol is involved in LH feedback control derives from the observation that LH levels are elevated in men with aromatase deficiency25 and after treatment with aromatase inhibitors.26
In contrast to its effects on LH secretion, physiologic levels of testosterone do not suppress FSH secretion. Rather, the regulation of pituitary FSH secretion is controlled by inhibin. FSH levels rise progressively after orchiectomy, the observation that led ultimately to the discovery of inhibin. Inhibin B is synthesized and secreted by Sertoli cells in response to FSH stimulation and specifically inhibits GnRH-stimulated pituitary FSH secretion.27,28 and 29 In the castrate male monkey, treatment with recombinant human inhibin can restore normal FSH levels in the absence of testosterone.30 Sertoli cell inhibin B secretion is modulated indirectly by LH via testosterone, which inhibits Sertoli cell inhibin B gene expression.31 Inhibin A is not produced in any significant amount in men. Evidence from studies in vitro suggests that other autocrine/paracrine regulatory mechanisms involving locally produced growth factors, neuropeptides, vasoactive peptides, and immune-derived cytokines also are involved, much like the complex interactions that operate in the ovarian follicle.32,33,34,35 and 36 The Sertoli cells of the testis are analogous to the granulosa cells of the ovary, and the Leydig cells are comparable to the theca cells.
The extent to which FSH and LH are needed to initiate and maintain spermatogenesis has been difficult to define because observations in various natural and experimentally-induced conditions have yielded conflicting evidence. The presence of sperm in the ejaculate of a man with an inactivating mutation in the LH β-subunit gene and in other men with isolated LH deficiency suggests that FSH alone can initiate spermatogenesis,37 although the possibility of some residual LH activity or FSH-stimulated Leydig cell testosterone production via Sertoli cell factors cannot be excluded.38 Conversely, low level sperm production in men with inactivating mutations of the FSH receptor39 and other forms of isolated FSH deficiency40,41 suggest that LH-driven testosterone production alone can initiate spermatogenesis, although the possibility of residual FSH activity in the presence of high circulating FSH concentrations must be acknowledged. Evidence that high doses of exogenous testosterone can stimulate complete spermatogenesis in immature monkeys, albeit at low levels, further suggests that FSH is not an absolute requirement,42 but descriptions of azoospermic men with mutations in the FSH β-subunit gene suggest the opposite.43,44 In men with hypogonadotropic hypogonadism of prepubertal onset, normal spermatogenesis can be stimulated by combined treatment with human chorionic gonadotopins (hCG, having potent LH-like actions) and human menopausal gonadotropin (containing FSH), but not by treatment with hCG alone.45
 |
The requirements for the maintenance of spermatogenesis are similarly controversial. The observation in monkeys that exogenous FSH can maintain testicular volume and the numbers of spermatogonia after complete suppression of gonadotropin secretion by treatment with a GnRH antagonist suggests that FSH alone can maintain spermatogenesis in primates, at least to some degree.46,47 The description of a unique individual with an activating mutation of the FSH receptor (function in the absence of FSH stimulation) and normal inhibin B
levels (a marker for FSH-stimulated Sertoli cell function)48 who had undergone hypophysectomy for removal of a benign pituitary tumor (eliminating all endogenous gonadotropin secretion) and remained fertile while receiving only physiologic exogenous testosterone replacement therapy (normally inadequate to support spermatogenesis in hypophysectomized men) serves to further illustrate the importance of FSH in maintaining spermatogenesis.49 In contrast, the restoration of fertility after treatment with only exogenous hCG in azoospermic men with isolated gonadotropin deficiency (low levels of both FSH and LH) suggests that although LH-stimulated testosterone production may be insufficient to initiate spermatogensis, it is sufficient to maintain spermatogenesis.50 In men who develop hypogonadotropic hypogonadism after puberty, during adulthood (e.g., due to a pituitary tumor), spermatogenesis stops but usually can be restored by treatment with hCG alone.45
levels (a marker for FSH-stimulated Sertoli cell function)48 who had undergone hypophysectomy for removal of a benign pituitary tumor (eliminating all endogenous gonadotropin secretion) and remained fertile while receiving only physiologic exogenous testosterone replacement therapy (normally inadequate to support spermatogenesis in hypophysectomized men) serves to further illustrate the importance of FSH in maintaining spermatogenesis.49 In contrast, the restoration of fertility after treatment with only exogenous hCG in azoospermic men with isolated gonadotropin deficiency (low levels of both FSH and LH) suggests that although LH-stimulated testosterone production may be insufficient to initiate spermatogensis, it is sufficient to maintain spermatogenesis.50 In men who develop hypogonadotropic hypogonadism after puberty, during adulthood (e.g., due to a pituitary tumor), spermatogenesis stops but usually can be restored by treatment with hCG alone.45
Regardless whether FSH or LH-stimulated testosterone alone is sufficient to initiate or to maintain spermatogenesis, both clearly are required for qualitatively and quantitatively normal sperm production. The importance of FSH has been demonstrated in a variety of experiments in nonhuman primates and men involving the selective suppression of FSH by immunization against FSH or by high dose chronic exogenous hCG treatment. FSH suppression induces both qualitative and quantitative abnormalities of semen quality that can be reversed by simultaneous treatment with exogenous FSH but not with testosterone.51,52,53,54 and 55 Moreover, in male contraceptive trials involving treatment with high doses of testosterone, alone or in combination with levonorgestrel to suppress spermatogenesis, azoospermia developed only in men whose serum FSH concentration was suppressed to undetectable levels.56,57 The importance of testosterone in spermatogenesis is evident from observations that FSH alone can induce proliferation of the seminiferous epithelium in prepubertal monkeys, but only treatment with both FSH and hCG increases testicular volume and the numbers of Sertoli cells and spermatogonia.58,59 Also, in men with idiopathic hypogonadotropic hypogonadism (due to absent GnRH stimulation), exogenous pulsatile GnRH stimulation or a combination of exogenous FSH and LH or hCG can induce spermatogenesis and achieve fertility,60,61 and 62 but treatment with FSH, alone or in combination with low doses of testosterone (insufficient to achieve the high local concentrations of testosterone required to support spermatogenesis), cannot.63
 |
 |
Aging and Male Reproductive Function
Although aging has adverse effects on male reproductive function, the impact of age is less obvious than it is in women. Semen quality and male fertility, as well as androgen production and serum testosterone levels, decrease very gradually as age increases.
Aging and Male Fertility
The relationship between age and fertility in men is more difficult to define than in women, largely due to the fundamental difference in gametogenesis between the two sexes. In women, the number of oocytes at birth inexorably declines as age advances until it is functionally exhausted at menopause, and fertility declines with the number of oocytes remaining (Chapter 27). In men, mitotic divisions in the spermatogonia throughout life replenish the supply of germ cells and spermatogenesis continues well into advanced ages, allowing men to reproduce even during senescence. Although fertility in men does appear to decline as age increases, the effects of age are much less distinct. The issue may be growing in importance because an increasing number of men are choosing to father children at older ages. In the U.S., birth rates for men between the ages of 35 and 54 increased by nearly 30% between 1980 (68.2 per 1000 men) and 2000 (88.3 per 1000 men).64,65
Semen volume, sperm motility, and the proportion of morphologically normal sperm, but not sperm concentration, appear to decrease gradually as age increases.66,67 However, semen characteristics generally do not accurately predict fertilizing capacity;68,69,70 and 71 neither do endocrine parmaters.72,73 A study in a convenience cohort of nearly 100 men ages
22-80 with no known fertility factors observed decreases in semen volume (-0.03 mL per year), total motility (-0.7% per year), progressive motility (-3.1% per year), and total (progressively) motile sperm count (-4.7% per year).67 Another study that examined the relationship between age and semen quality among over 400 male partners of women pursuing pregnancy via IVF using donor oocytes found that total motile sperm count decreased by approximately 2.5 million sperm per year.74
22-80 with no known fertility factors observed decreases in semen volume (-0.03 mL per year), total motility (-0.7% per year), progressive motility (-3.1% per year), and total (progressively) motile sperm count (-4.7% per year).67 Another study that examined the relationship between age and semen quality among over 400 male partners of women pursuing pregnancy via IVF using donor oocytes found that total motile sperm count decreased by approximately 2.5 million sperm per year.74
On balance, the available evidence indicates that pregnancy rates decrease and time to conception increases as male age increases.66,75 In studies of the effect of male partner age on pregnancy rates, female partner age and declining coital frequency with increasing age are obvious and important confounding factors.76 A study examining the effect of paternal age on pregnancy and live birth rates in couples undergoing assisted reproductive technologies found that pregnancy rates declined with age of the male partner and that each additional year of paternal age was associated with 11% increased odds of not achieving a pregnancy and 12% increased odds of not having a live birth; in first treatment cycles, each additional year of paternal age was associated with 5% increased odds of not achieving a pregnancy.77 Another study of the risk of infertility associated with paternal age, involving over 6,000 randomly selected European women ages 25 to 44, observed that the risk of infertility was increased 2- to 3-fold among women ages 35 to 39 when the male partner was 40 years or older.78 A study of IVF outcomes involving almost 2,000 women with tubal factor infertility (absent or obstructed fallopian tubes) determined that advanced paternal age (40 years and greater) increased the risk for treatment failure approximately 2-fold for women ages 35-40 years and more than 5-fold for women age 41 and older.79 Others have observed that pregnancy rates for men over 50 are 23-38% lower than for men under age 30,66 and that the probability of achieving pregnancy within a year is approximately 50% lower for men over age 35 than for those under age 25.80 Results of a British study (adjusted for partner age and coital frequency) indicate that time to conception is 5-fold longer for men over age 45 than for men under age 25, even when anal ysis is restricted to men with young partners.75 Two other studies have suggested that male fertility may start to decline before age 40.81,82 The effect of paternal age is perhaps best assessed in couples using oocyte donation, which makes male age the dependent variable (because almost all oocyte donors are ages 18-35). Unfortunately, data from such studies are conflicting, with some indicating that male age has limited or no impact on pregnancy, implantation, and live birth rates,74,83,84 and others finding that paternal age is inversely related to reproductive outcomes,85,86 including a decrease in live birth rates and an increase in miscarriage rates.
There are several possible biological mechanisms that might contribute to an age-related decline in male fertility. One involves cellular or physiologic changes in the male reproductive tract. The testes and prostate exhibit morphological changes with aging that might adversely affect both sperm production and the biochemical properties of semen.87 Autopsy studies of men who died from accidental causes have observed narrowing and sclerosis of the seminiferous tubules, decreased spermatogenic activity, and reduced numbers of germs cells and Leydig cells as age increases.88,89 Another possible mechanism is age-related changes in the hypothalamic-pituitary-testicular axis. Average FSH levels in men increase after age 30,90 suggesting that the endocrine environment may begin to change during midlife.91 Decreased semen volume may relate to reduced androgen-stimulated fluid production in the prostate and seminal vesicles because testosterone levels decrease with advancing age.92 Whatever the mechanism(s), decreasing fertility with increasing male age in healthy couples suggests that normal sperm overproduction may not fully buffer the effects of increasing age. However, because there is little or no overall measurable decline in male fertility before age 45-50, the available data suggest that male factors likely contribute relatively little to the overall age-related decline in fertility in women.
Paternal Age and Pregnancy Outcomes
Because male germ cells pass through more mitotic replications than those of females, there is greater opportunity for error. Older men also are more likely than younger men to have smoked (and for longer periods of time) and to have been exposed to gonadotoxins that may cause DNA damage.66,67 Increased paternal age has been associated with an increase in numerical and structural chromosomal abnormalities,93,94,95,96 and 97 with increased DNA fragmentation,98 and with a higher frequency of point mutations.99 There also is evidence to suggest that increasing male age may raise the risk of spontaneous abortion in young women.85,86,100
A number of studies have observed that advanced paternal age is associated with an increase in the prevalence of birth defects (e.g., neural tube defects, cardiac defects, and limb defects) and congenital diseases (e.g., Wilms tumor).101,102,103,104 and 105 In a large populationbased retrospective cohort study that included over 5 million births, the observed overall prevalence of birth defects was 1.5%; compared with infants born to fathers ages 25-29 years, the adjusted odds ratios for birth defects were 1.04 for infants with fathers aged 30-35 years, 1.08 for those aged 40-45 years and 45-50 years, and 1.15 for infants who fathers were over 50 years old.106 These data suggest that the risk for birth defects increases only slightly, if at all, with increasing paternal age.
Advanced paternal age has been associated with an increase in new autosomal dominant mutations (e.g., achondroplasia and Alpert, Waardenburg, Crouzon, Pfeiffer, and Marfan syndromes).107 At least in theory, the observation might reflect a decrease in the activity of antioxidant enzymes in the semen and sperm of older men, increasing their susceptibility to mutation.108 DNA repair mechanisms also may be impaired in older men. Although the relative risk of autosomal dominant disorders is increased markedly, the absolute risk is still very small (<1%) because autosomal dominant diseases are rare.109
Evidence indicates that advanced paternal age is associated with an increased risk for schizophrenia in offspring;110,111 and 112 overall, the incidence is increased 2- to 3-fold for children whose fathers are over age 45 years, possibly as a consequence of mutations emerging during spermatogenesis.113 Similarly, increasing paternal age has been associated with an increased risk for autism in children, which may reflect de novo mutations or errors in genetic imprinting.114,115,116,117 and 118
In older fathers, mutations resulting in X-linked disease also may be more common; examples include hemophilia A and Duchenne muscular dystrophy.119 The “grandfather effect” describes their transmission from carrier daughters to affected grandsons. Overall, advanced paternal age does not appear to be associated with any significant increase in the risk of fetal autosomal or sex chromosome aneuploidy.119,120,121,122,123 and 124 However, available data are limited and confounded by female partner age. Moreover, results from one study examining the chromosomal complement of paternal gametes suggest that the incidence of sex chromosome aneuploidy may increase with age.124 A population-based study involving more than 4 million children observed that paternal age was associated with a small but significant increase in risk of leukemia and central nervous system cancers.125
It still is not clear whether the risk for miscarriage increases with paternal age, because results of studies conducted thus far are conflicting, with some finding evidence for an association with both early and late fetal loss,100,126,127 and others not.128,129 Whereas one retrospective study of 558 pregnancies conceived using donor oocytes observed no association between paternal age and live birth rate,74 another found that risk for miscarriage increased with paternal age.83 On balance, the weight of available evidence suggests that advanced paternal age may be associated with a small increase in the risk of spontaneous abortion. Limited data suggest that advanced paternal age does not significantly increase the risk for fetal growth restriction,130,131 or stillbirth.132
Androgen Deficiency in the Aging Male
Serum total and free testosterone levels decrease in men as age increases.133 However, unlike the profound estrogen deficiency and associated symptoms that occur after menopause in women, the age-related decline in androgen levels in men is more gradual and smaller,134 and the clinical consequences of decreasing androgen levels are not yet clear.
Serum testosterone concentrations exhibit a distinct diurnal variation in young men (with highest levels in the morning), but vary relatively little in elderly men.135 In the cross-sectional European Male Aging Study, involving 3,220 men ages 40 to 79 years, serum total testosterone concentrations fell by an average of 0.4% per year and free testosterone levels by 1.3% per year.136 Longitudinal studies have observed a somewhat greater age-related decline in testosterone concentrations and found that levels decrease at a fairly constant rate.133,137,138 Because sex hormone binding globulin (SHBG) concentrations increase gradually with age, free testosterone levels decrease more than total testosterone concentrations.134 In the Massachusetts Male Aging Study, free testosterone levels fell by an average of almost 3% per year.139 SHBG levels also may rise in association with increased abdominal obesity, further contributing to the decrease in free testosterone.140
As testosterone levels fall steadily, an increasing percentage of aging men become hypogonadal, as defined by testosterone concentrations (total testosterone <300-325 ng/dL; free testosterone <5ng/dL) and/or by signs and symptoms of hypogonadism. In one population-based observational survey, the prevalence of hypogonadism ranged from 3% to 7% among men ages 30 to 69 years, and was 18% in men over age 70.141 In a longitudinal study, serum total testosterone levels in the hypogonadal range were observed in 20% of men in their 60s, in 30% of those in their 70s, and in 50% of men in their 80s.133
In some men over age 50, decreasing serum androgen concentrations may be associated with clinical symptoms and signs of androgen deficiency suggesting “andropause.” Symptoms of androgen deficiency may include decreased libido,142,143 with or without erectile dysfunction,144 reduced strength, energy, or stamina,145 irritability and perceptions of a lower quality of life,146 sleep disturbance, depressed mood, and lethargy,141 and changes in cognitive function.147,148 Symptoms may be accompanied by physical changes, including osteopenia or osteoporosis,149 decreased muscle mass,150 increased visceral adipose,151 testicular atrophy and gynecomastia. Epidemiologic studies have observed that low serum testosterone concentrations are associated with development of central obesity, increased insulin levels, the metabolic syndrome, diabetes, and increased mortality.152,153,154 and 155 Validated questionanaires now are available for use in evaluating older men.156,157 However, scores do not predict or correlate well with measured free and total testosterone levels,158 and therefore lack specificity for the diagnosis of androgen deficiency in the aging male (ADAM).159,160 and 161
Men with symptoms or signs of androgen deficiency merit evaluation by measuring the serum total testosterone level, ideally during the morning hours to minimize the influence of pulsatile and circadian rhythms in testosterone secretion. Frankly low concentrations (<200 ng/dL) should be confirmed by repeated measurements.162 Serum total testosterone includes not only free testosterone, but also testosterone bound to albumin and SHBG. “Bioavailable testosterone” is the sum of free and albumin-bound testosterone and the measurement that has correlated best with bone mineral density145 and sexual163 and cognitive function148 in epidemiologic studies. Because the serum total testosterone level occasionally may be misleading, some prefer to measure free or bioavailable testosterone, but the accuracy of free testosterone assays has been challenged164 and neither assay is widely available. A free testosterone index (FTI) calculated from measurements of total testosterone and SHBG (total testosterone/SHBG) provides an indirect measure of the amount of bioavailable testosterone. It is important to emphasize that in men with documented
androgen deficiency, a normal or low serum LH suggests a secondary hypogonadism that merits additional evaluation by measurement of serum prolactin and magnetic resonance imaging (MRI) to detect any hypothalamic or pituitary mass lesion.
androgen deficiency, a normal or low serum LH suggests a secondary hypogonadism that merits additional evaluation by measurement of serum prolactin and magnetic resonance imaging (MRI) to detect any hypothalamic or pituitary mass lesion.
Treatment
A consensus of expert opinion published in 2002 suggested that a total testosterone level under 200 ng/dL (6.9 nmol/L) is evidence of hypogonadism that warrants treatment in symptomatic men, that those with concentrations between 200 ng/dL and 400 ng/dL (6.9-13.9 nmol/L) may benefit from treatment, and that higher levels all but exclude androgen deficiency.165 A bioavailable testosterone level below the normal range for normal young adult men or an FTI less than 0.153 (nmol/nmol) also is consistent with the diagnosis of androgen deficiency.133 Evidence-based guidelines issued by the Endocrine Society in 2006 recommended that, in the absence of pituitary or testicular disease, testosterone therapy be reserved for men with clearly and consistently low serum total testosterone concentrations (<200 ng/dL) and clinically important symptoms of androgen deficiency.162
The potential risks of testosterone treatment include fluid retention, gynecomastia, increased red blood cell mass, worsening of sleep apnea, promotion of benign or subclinical malignant prostate disease, and possible added risk for cardiovascular disease.166
Accordingly, the Endocrine Society guidelines recommend against testosterone treatment in men with prostate or breast cancer, a palpable prostate nodule or induration, prostate-specific antigen (PSA) greater than 3 ng/mL without further urologic evaluation, eryrthrocytosis (hematocrit >50%), untreated obstructive sleep apnea, severe lower urinary tract symptoms (International Prostate Symptom Score > 19), or class III or IV heart failure.162
Any of the commercial formulations of testosterone may be used for treatment. Androgen therapy may involve parenteral testosterone esters (75 mg per week or 150 mg every 2 weeks), implanted pellets (225 mg every 4-6 months), scrotal (40 cm2, one patch daily) or peripheral skin patches (5 mg, one patch daily) or testosterone gel (5 g per day); treatment should be individualized. At present, there are no data to indicate that any one formulation is clearly superior. The therapeutic goal is to raise serum testosterone concentrations over pretreatment values without exceeding the normal range for young adult men. The target serum testosterone concentration should be lower than that for younger men (e.g., 300-400 ng/dL) to decrease the potential risk of testosterone-dependent disease.162 Dehydroepiandrosterone may be converted to testosterone and is commercially available as an oral dietary health supplement; standard doses (50-100 mg daily) generally do not raise serum testosterone concentrations, although higher doses may.167
In randomized, placebo-controlled studies, the effects of testosterone therapy on bone density have been inconsistent. In one, no overall increase in hip or spine bone density was observed, but treatment had greatest effect in men with the lowest pre-treatment testosterone levels.168 In another, testosterone treatment did not increase bone density, but prevented the decrease observed in men receiving placebo.169 In a third, testosterone treatment (with and without finasteride, which blocks conversion of testosterone to dihydrotestostrone) increased spine bone density by 9-10% and hip bone density by 2-3%.170 All three studies168,169,171 and a subsequent systematic review162 found that testosterone treatment increased fat free mass and decreased fat mass. However, the increase in lean mass did not result in any consistent improvement in muscle strength or physical performance.168,169,171 Testosterone treatment also was not accompanied by any demonstrable improvement in quality of life measures or sexual function, as judged by questionnaires.168,169
Androgen therapy must be monitored because the long-term health risks and benefits of treatment have not been established. A baseline physical examination (breasts, heart, lungs, prostate), serum prostate specific antigen (PSA), and complete blood count should be obtained; prostate biopsy is recommended when the digital rectal examination or serum PSA is abnormal. Within 3 months after therapy begins, men receiving androgen therapy should be evaluated for weight gain and any signs of emerging peripheral edema, gynecomastia or breast tenderness, sleep disorders, or prostate enlargement. Recommended monitoring also includes hemoglobin or hematocrit and a serum PSA. A rapid rise in PSA (>1 ng/mL) soon after treatment begins suggests the possibility of an undetected prostate cancer and is reason to discontinue treatment pending a thorough prostate evaluation.172 Serum testosterone also should be measured to ensure that treatment is achieving the target concentration, but the subjective clinical response is the most important gauge of the effectiveness of androgen therapy. Men with a good clinical response, no apparent adverse effects, and normal testosterone levels may continue treatment, but should return for similar monitoring after another 6 months, and at least annually thereafter. If osteoporosis was one of the indications for treatment, bone mineral density also should be re-evaluated approximately 1-2 years after treatment starts.
In clinical trials of testosterone treatment in elderly men, only a few cases of prostate cancer have been observed, but statistical power was insufficient to support a conclusion that testosterone treatment does not increase risk for prostate cancer. A meta-analysis including 19 trials found that testosterone treatment was associated with a higher prevalence of elevated PSA values and prostate cancer, although biopsy was more commonly performed in men receiving treatment.173 There is little evidence that short-term treatment has adverse effects on the prostate,174 but the effects of long-term treatment remain uncertain. Similarly, it is not clear whether physiologic testosterone therapy increases the risk of sleep apnea, because data are conflicting.173,175,176 and 177 However, testosterone treatment in elderly men clearly can cause erythrocytosis. In individual studies, up to one-third of treated men have developed an abnormally elevated hematocrit,170,178 and a meta-analysis concluded that testosterone treatment is associated with more than a 4-fold increased risk for erythrocytosis.173 Taken together, evidence indicates that testosterone treatment in hypogonadal men has little effect on serum concentrations of total and low-density lipoprotein cholesterol.179
Causes of Male Infertility
Male infertility may result from a variety of causes. Some, like ductal obstruction and hypogonadotropic hypogonadism, can be defined accurately and treated effectively. Others, like primary testicular failure, can be defined but are not amenable to treatment, and still others, like seminiferous tubule dysfunction cannot be corrected but can be overcome by intrauterine insemination (IUI) or ART. Although rare, male infertility also may be the first indication of a serious underlying medical condition. Unfortunately, much of male infertility is idiopathic, reflecting our still very poor understanding of the mechanisms that govern testicular function.
The list of known causes of male infertility is long and varied, but can be divided into 4 major categories: 1) hypothalamic-pituitary disorders (1-2%), which may be congenital, acquired, or result from systemic illness; 2) primary gonadal disorders (30-40%), both congenital and acquired; 3) disorders of sperm transport (10-20%); and 4) idiopathic (40-50%).
Causes of Male Infertility
Hypothalamic-Pituitary Disorders
Idiopathic isolated gonadotropin deficiency
Kallmann syndrome
Single gene mutations (e.g., involving the GnRH receptor, FSHβ, LHβ, or transcription factors involved in pituitary development)
Hypothalamic and pituitary tumors (e.g., craniopharyngioma, macroadenoma)
Infiltrative diseases (sarcoidosis, histiocytosis, transfusion siderosis, hemochromotosis)
Hyperprolactinemia
Drugs (GnRH analogs, androgens, estrogens, glucocorticoids, opiates)
Critical illness or injury
Chronic systemic illness or malnutrition
Infections (e.g., meningitis)
Obesity
Primary Gonadal Disorders
Klinefelter syndrome
Y chromosome deletions
Single gene mutations and polymorphisms (e.g., involving the androgen, estrogen, or FSH receptor)
Cryptorchidism
Varicoceles
Infections (e.g., viral orchitis, leprosy, tuberculosis)
Drugs (e.g., alkylating agents, alcohol, antiandrogens, cimetidine)
Radiation
Environmental gonadotoxins (e.g., heat, smoking, metals, organic solvents, pesticides)
Chronic illness (renal insufficiency, cirrhosis, cancer, sickle cell disease, amyloidosis, vasculitis, celiac disease)
Disorders of Sperm Transport
Epididymal obstruction or dysfunction
Congenital bilateral absence of the vas deferens (relating to CFTR mutations)
Infections causing obstruction of the vas deferens (e.g., gonorrhea, chlamydia, tuberculosis)
Vasectomy
Kartagener syndrome (primary ciliary dyskinesia)
Young syndrome
Ejaculatory dysfunction (e.g., spinal cord disease, autonomic dysfunction)
Hypothalamic-Pituitary Disorders
Any hypothalamic or pituitary disease or disorder causing a deficiency of gonadotro-pin-releasing hormone (GnRH) or gonadotropins can cause male infertility. The most common congenital cause is idiopathic isolated gonadotropin deficiency due to absent or defective GnRH secretion (resulting in sexual infantilism).180 When accompanied by one or more extragonadal abnormalities, such as anosmia, red-green color blindness, midline facial defects (e.g., cleft palate), neurosensory hearing loss, synkinesis (mirror movements), or renal anomalies, the disorder is known as Kallmann syndrome.181,182 and 183 A variety of mutations have been identified in affected men, involving genes encoding cell surface adhesion molecules or receptors, which are required for normal migration of GnRH neurons from the olfactory placode to the hypothalamus; examples include KAL1,184,185 fibroblast growth
factor 1 (also known as KAL2),186 and prokineticin-2 (PROK2) and its receptor (PROKR-2).187 Other genetic causes of hypogonadotropic hypogonadism include rare mutations affecting the GnRH receptor188 the β-subunit of FSH43 or LH,37,189,190 or transcription factors involved in pituitary development during embryogenesis, such as LHX3,191 LHX4, HESX1,192 and PROP-1.193
factor 1 (also known as KAL2),186 and prokineticin-2 (PROK2) and its receptor (PROKR-2).187 Other genetic causes of hypogonadotropic hypogonadism include rare mutations affecting the GnRH receptor188 the β-subunit of FSH43 or LH,37,189,190 or transcription factors involved in pituitary development during embryogenesis, such as LHX3,191 LHX4, HESX1,192 and PROP-1.193
Hypogonadotropic hypogonadism also can result from hypothalamic disease or treatments that inhibit GnRH secretion, abnormalities of the pituitary stalk that interfere with GnRH delivery, and pituitary disease that prevents normal gonadotropin secretion. Hypothalamic or pituitary tumors can distort the pituitary stalk or compress and suppress pituitary gonadotropes.
Infiltrative diseases of the hyopothalamus or pituitary (sarcoidosis, histiocytosis, transfusion siderosis, hemochromotosis) can inhibit GnRH or pituitary gonadotropin secretion.194,195 Hyperprolactinemia from any cause,196 and treatment with GnRH analogs (e.g., for prostate cancer) androgens (e.g., anabolic steroids),197 glucocorticoids,198 or opiates199,200 and 201 can suppress gonadotropin secretion. Critical illness202 or injury (e.g., head trauma)203 and chronic systemic illness (e.g., diabetes mellitus) or malnutrition also have been associated with hypogonadotropic hypogonadism. Infections (e.g., meningitis) are another rare but recognized cause of hypopituitarism.204
Obesity in men is associated with hypogonadotropic hypogonadism, involving several mechanisms.205 Serum free testosterone concentrations are inversely related to body weight and body mass index, independent of changes in SHBG levels,206,207 and 208 and estrogen concentrations are elevated due to increased aromatase activity in adipose.209 Obstructive sleep apnea is a separate but related additional factor, resulting in hypoxia.
Primary Gonadal Disorders
Primary gonadal failure (hypergonadotropic hypogonadism) is a major cause of azoospermia and oligospermia and can result from a variety of congenital or acquired disorders, including Klinefelter syndrome, Y chromosome deletions, single gene mutations, cryptorchidism, varicoceles, and other less common causes.
Klinefelter Syndrome
Klinefelter syndrome is one of the most common causes of primary testicular failure, affecting approximately 1 in 1,000 males,210,211 and is characterized by sex chromosome aneuploidy. Although an extra X chromosome (47,XXY) is the most common form, some men with Klinefelter syndrome have a greater or lesser number of X chromosomes (e.g., 48,XXXY, 46,XY/47,XXY);212 46,XX males, resulting from translocation of the testis-determining gene (SRY) to an X chromosome, also have Klinefelter syndrome. The phenotype varies with the number of extra X chromosomes, and possibly also with the number of trinuceotide CAG repeats on the androgen receptor gene (a polymorphism); as the length of the repeat sequence increases, receptor activity decreases. A longer CAG repeat sequence has been associated with taller stature, lower bone mineral density, gynecomastia, and decreased penile length.213,214
Men with Klinefelter syndrome generally have small, firm testes, resulting from damage to both seminiferous tubules and Leydig cells. Serum concentrations of FSH and LH are elevated and testosterone levels are decreased to varying extent. Affected men
have severely reduced sperm counts and are under-virilized.212,215 Cryptorchidism is more common in men with Klinefelter syndrome and causes more severe testicular damage.216
have severely reduced sperm counts and are under-virilized.212,215 Cryptorchidism is more common in men with Klinefelter syndrome and causes more severe testicular damage.216
The length of the arms and legs is increased in men with Klinefelter syndrome, due both to testosterone deficiency and to an independent abnormality of the long bones. Men with Klinefelter syndrome also exhibit a number of psychosocial abnormalities,217 which have been described as a marked lack of insight, poor judgment, and an impaired ability to learn from adverse experience.218 They also may have difficulty with complex speech and a decreased attention span.219 Later in life, they have an increased risk for developing pulmonary diseases, breast cancer,220 mediastinal germ cell tumors,221 varicose veins and leg ulcers,222 systemic lupus erythematosus,223 and diabetes mellitus.224
Other chromosomal abnormalities associated with primary gonadal failure include the 46,XY/45,X karyotype, causing a syndrome characterized by short stature and other features of Turner syndrome.225 Because the testes may be streaks, dysgenetic, or normal, the phenotype varies from female to normal male. In those with a streak and a dysgenetic testis (mixed gonadal dysgenesis), the risk of gonadoblastoma is increased (approximately 20%), and gonadectomy is therefore indicated.
Y Chromosome Deletions
Microdeletions of the long arm of the Y chromosome are now recognized as a relatively common cause of severe oligospermia and azoospermia, affecting up to 20% of men with infertility.226 Most map to the Yq11 region (named azoospermia factor, or AZF), which contains three regions, AZFa, AZFb, and AZFc. Deletions of the AZFa or AZFb regions typically result in azoospermia. Mutations in the AZFc region cause infertility of varying severity, ranging from oligospermia to azoospermia and are the largest known recurrent deletions in humans.227,228 The DDx3Y and USP9Y genes, both located in the AZFa region, have been implicated as having an important role in spermatogenesis; azoospermia is consistently observed when both are deleted.229,230 Y chromosome deletions also have been identified in men with cryptorchidism, varicocele, and obstructions of the vas deferens.231,232
Because all Y chromosome abnormalities will be transmitted to sons of affected men conceived via intracytoplasmic sperm injection (ICSI), genetic testing and counseling should be offered to affected men before their sperm are used for that purpose. Given the importance and potential consequences of Y chromosome deletions, there is a need to standardize the tests for their detection.233
Single Gene Mutations and Polymorphisms
Normal male sexual differentiation and spermatogenesis require both normal androgen production and normal androgen receptors (Chapter 9). The androgen receptor plays an important role in the differentiation of spermatids and their release from the seminiferous epithelium. Consequently, it is not surprising that defects in androgen synthesis or androgen sensitivity are associated with infertility.234,235
As discussed above, the number of trinucleotide CAG repeats in exon 1 of the androgen receptor gene is inversely correlated with its transcriptional activity.213,214 In one study in normal fertile men, those with short repeat sequences had the highest sperm concentrations.236 However, studies in men with idiopathic infertility have yielded inconsistent results, with some finding an association between longer CAG repeat segments and male
infertility,237,238 and 239 and others not.240 A meta-analysis including 33 studies concluded that men with idiopathic infertility had significantly longer CAG repeat lengths than fertile men, suggesting that even subtle abnormalities in androgen action may adversely affect male fertility.241
infertility,237,238 and 239 and others not.240 A meta-analysis including 33 studies concluded that men with idiopathic infertility had significantly longer CAG repeat lengths than fertile men, suggesting that even subtle abnormalities in androgen action may adversely affect male fertility.241
Evidence suggests that disorders of estrogen synthesis or action also may be associated with infertility in men. Impaired spermatogenesis has been observed in mice and in men lacking a functional estrogen receptor (alpha),242,243 and in mice with an inactivating mutation in the aromatase enzyme.244 Polymorphisms involving variations in the number of TA tandem repeats in the promoter region of the estrogen receptor gene also have been related to sperm production, with higher numbers of TA repeats being associated with lower sperm counts.245 Inactivating mutations in the FSH receptor gene are a rare cause of male infertility.39,246
Several other autosomal and X-linked genes have been identified as important regulators of spermatogenesis. Men with myotonic dystrophy (an autosomal disorder associated with impaired motor function, cataracts, premature balding, mild mental retardation, and hypo-gonadism) also can exhibit abnormal spermatogenesis.247 Mutations in the SYCP3 gene (involved in regulation of the synapse between homologous chromosomes during meiosis) have been implicated as a potential cause of male infertility.245 Others include polymorphisms of DAZL (an autosomal homolog of the DAZ, deleted in azoospermia, gene),248,249,250,251 and 252 PRM1 and PRM2 (protamines involved in chromatin compaction), TNP1 and TMP2 (transition nuclear proteins), and USP26 (de-ubiquitinating enzyme family).245
Cryptorchidism
Cryptorchidism results from a failure of testicular descent during fetal development, which is an androgen-dependent process. Consequently, it is common in men with abnormalities of testosterone production, such as Kallmann syndrome, androgen resistance, and defects in testosterone synthesis. Cryptorchidism can be unilateral or bilateral and, in either case, is associated with impaired spermatogenesis and an increased risk for developing testicular tumors. Even in the absence of cryptorchidism, the incidence of testicular cancer is increased in infertile men.253,254
In men with cryptorchidism, serum FSH levels often are elevated, but LH concentrations generally are normal, reflecting normal Leydig cell function. The severity of the semen abnormality relates to the duration of time the testes have been outside of the scrotum. Because the testes are more easily retractable early in life, very young boys may appear transiently to have cryptorchidism but, in most, the testes descend and remain in the scrotum by age 1.255 Men having low serum inhibin B levels, increased FSH concentrations, and decreased sperm density after repair of cryptorchidism are at increased risk for infertility.256
Varicoceles
Varicoceles result from dilation of the panpiniform plexux of the spermatic veins in the scrotum. They are more prevalent in infertile men (up to 30%) than in fertile men (10-15%) and are 10 times more commonly found on the left than on the right, probably because the left spermatic vein is longer and joins the left renal vein at a right angle.257 Although increased testicular temperature, delayed removal of local toxins, hypoxia, and stasis are viewed as the mechanisms likely responsible for the association between varicoceles and infertility, no causal relationship has been established.258,259 and 260
Other Causes of Primary Gonadal Failure
Certain infections are associated with male infertility. Mumps orchitis is widely recognized as a cause of male infertility. Although rare in prepubertal males, it occurs in up to 25% of adult men with mumps, some of whom become infertile. The mechanism may involve damage to the germinal epithelium, ischemia, or immune dysfunction.261,262 Gonorrhea and chlamydial infections also can cause orchitis. Other infections associated with male infertility include tuberculosis, which may cause epididymal obstruction, leprosy,263 and human immunodeficiency virus (HIV).264,265
Drugs that can adversely affect spermatogenesis or Leydig cell function include alkylating agents (e.g., cyclophosphamide, chlorambucil), anti-androgens (e.g., flutamide, cyproterone, spironolactone), ketoconazole, cimetidine, and anabolic steroids.266 Doses of radiation as low as 0.015 Gy (15 rads) can suppress spermatogenesis and doses above 6 Gy generally cause permanent azoospermia and infertility.267
Environmental exposures that may act as gonadotoxins include heat, smoking, metals, organic solvents, and pesticides. A modest increase in scotal temperature can adversely affect spermatogenesis and a febrile illness can result in dramatic, if also transient, decreases in sperm density and motility. Hyperthermia also may explain the infertility associated with spinal cord injuries, and chronic sauna or spa exposure.268 In theory, environmental sources of heat, including tight-fitting underclothing, hot baths and spas, and occupations that require long hours of sitting (long-distance driving) might decrease fertility, but none has ever been substantiated in clinical studies.12 Smoking or heavy use of marijuana, alcohol, or cocaine can decrease semen quality and testosterone levels.269,270 and 271
Disorders of Sperm Transport
Even when sperm production is normal, epididymal obstruction or dysfunction can result in infertility. The cause of infertility is clear in men with obstruction, but relatively little is known about epididymal function. Isolated asthenospermia is presumed to result from epididymal dysfunction, and intrauterine exposure to diethylstilbestrol may be one cause.275
Congenital or acquired abnormalities of the vas deferens can cause obstruction and infertility. Approximately 1-2% of infertile men have congenital bilateral absence of the vas deferens (CBAVD), almost always related to mutations in the cystic fibrosis trans-membrane conductance regulator (CFTR) gene.276 Most affected men do not exhibit any respiratory and pancreatic disease. Infections (gonorrhea, Chlamydia, tuberculosis) and vasectomy are other causes of vasal obstruction. Primary ciliary dyskinesia (Kartagener syndrome) is a genetic disease that adversely affects cilia structure and function and generally presents as recurrent sinus and pulmonary infections, bronchiectasis, situs inversus, and male infertility due to oligo-asthenospermia.277,278 Young syndrome is another genetic disease, in which inspissated secretions in the vas and epididymis result in obstructive azoospermia.279,280
Ejaculatory dysfunction resulting from spinal cord disease or injury, sympathetomy, or autonomic disease is another cause of infertility relating to disorders of sperm transport.
The Male Infertility Evaluation
The evaluation of the infertile male should be directed towards achieving all of the following goals281:
To identify and to correct specific causes of infertility, when possible.
To identify individuals whose infertility cannot be corrected but may be overcome by IUI or use of various forms of ART.
To identify individuals having a genetic abnormality that may affect the health of any offspring that may be conceived through the use of ART.
To identify individuals whose infertility can neither be corrected nor overcome with ART, for whom adoption or the use of donor sperm are options worthy of consideration.
To identify any important underlying medical condition that may require specific medical attention.
Evaluation of the male partner should begin at the same time as in the female partner, generally when pregnancy fails to occur after 1 year of reasonably regular unprotected intercourse. Earlier evaluation is indicated for men with any obvious infertility risk factor, those whose partner is age 35 or older, (where it is important to identify all potential infertility factors as quickly and efficiently as possible), and men who have reason to question their fertility.
In the male partner, the most relevant parts of the medical history and physical examination include the following:281
History
Duration of infertility and previous fertility.
Coital frequency and any sexual dysfunction.
Results of any previous evaluation or treatment for infertility.
Childhood illnesses and developmental history.
Previous surgery, its indications and outcome, and systemic medical illnesses.
Past episodes of or exposures to sexually-transmitted infections.
Exposures to environmental toxins, including heat.
Current medications and allergies.
Occupations and use of tobacco, alcohol, and other drugs.
Physical Examination
Examination of the penis, to include the location of the urethral meatus.
Palpation of the testes and measurement of their size.
The presence and consistency of both the vasa and epididymides.
Presence of any varicocele
Secondary sexual sex characteristics, including body habitus, hair distribution, and breast development.
Digital rectal examination.
A history of cryptorchidism or mumps orchitis suggests the possibility of testicular atrophy.261,282 The timing and extent of secondary sexual development may alert one to the possibility of an endocrinopathy. Ductal obstruction can result from sexually-transmitted infections. Diabetes mellitus (bladder neck dysfunction resulting in retrograde ejaculation) and cystic fibrosis (highly associated with congenital absence of the vas deferens) are medical illnesses that may hinder fertility in men. Inguinal hernia repair, renal transplant, and scrotal surgery are associated with risks for unrecognized injury to the vas deferens.283 Retroperitoneal surgery may disrupt neural pathways and cause ejaculatory dysfunction;
treatment with alpha-blockers, phentolamine, methyldopa, guanethidine, or reserpine may have similar effects.
treatment with alpha-blockers, phentolamine, methyldopa, guanethidine, or reserpine may have similar effects.
When the infertility evaluation is directed by the gynecologist or primary clinician, physical examination of the male may be deferred pending the results of the first semen analysis when there is no history of any male genital abnormality, trauma, surgery, or sexual dysfunction. However, an abnormal reproductive history or semen analysis is indication for additional formal evaluation that may be conducted by the gynecologist having the necessary training and experience, but is most often performed by the urologist or other specialist in male reproduction.
Semen Analysis
If a male infertility factor exists, it almost always will be revealed by an abnormal semen analysis, although other male factors (sexual dysfunction) may be involved even when semen quality is normal. The initial evaluation for male factor infertility should include at least one properly performed semen analysis. If abnormal, another semen analysis should be obtained after at least 4 weeks.281 Semen parameters can vary widely over time, even among fertile men,284,285,286 and 287 and also exhibit seasonal variations.288,289 and 290 Considering that the overall objective is to gain a sense of the usual semen quality, over time, more than one analysis is helpful, because a single semen sample yields only a point estimate that may or may not be representative. However, with relatively few exceptions, a normal initial semen analysis generally excludes an important male factor when there is no complaint or suspicion of sexual dysfunction. Conversely, abnormal semen parameters suggest the need for additional endocrine, urologic, or genetic evaluation.
Standard but detailed instructions for semen collection should be provided, to include a defined abstinence period of 2-3 days. Shorter intervals of abstinence decrease the semen volume and sperm density but generally have little or no impact on sperm motility or morphology.291 Longer abstinence intervals increase semen volume and sperm density, but also increase the proportion of dead, immotile or morphologically abnormal sperm.292 Ideally, the semen specimen should be collected by masturbation directly into a clean container. If necessary, semen may be collected via intercourse using a specially manufactured silastic condom that does not contain spermicidal agents like those in condoms intended for contraceptive purposes. Collection after withdrawal during intercourse risks loss of the initial portion of the specimen, which generally contains the highest concentration of sperm. If possible, the semen specimen should be collected in a private room within or near the laboratory. When necessary, the specimen can be collected at home but should be kept at room or body temperature during transport. Regardless of the method of collection, the semen sample should be examined within an hour after collection.
Normal Reference Values
The normal reference values in wide use are based on comparisons of the values observed in the male partners of fertile and infertile couples without specific exclusion of female infertility factors,293,294 and 295 and therefore do not necessarily represent the average ranges observed in fertile men. Unfortunately, there is considerable overlap between the semen parameters observed in fertile and infertile men.296 The normal reference ranges certainly do not represent the absolute minimum values needed for conception; many men with values outside the normal ranges are fertile and many with normal values are nonetheless infertile.296,297,298 and 299 Values outside of normal ranges suggest a male infertility factor that may
require additional clinical or laboratory evaluation, but each parameter must be considered in the context of the whole. A mildly low sperm density may have little significance when semen volume, sperm motility, and the proportion of abnormal sperm are normal. Conversely, a normal sperm density offers little reassurance when semen volume is frankly low or the proportion of motile or normal sperm is grossly abnormal. Overall, the odds of male infertility increase with the number of major semen parameters (concentration, motility, morphology) in the subfertile range; the probability is two to three times higher when one is abnormal, five to seven times higher when two are abnormal, and 16 times greater when all three are abnormal.296
require additional clinical or laboratory evaluation, but each parameter must be considered in the context of the whole. A mildly low sperm density may have little significance when semen volume, sperm motility, and the proportion of abnormal sperm are normal. Conversely, a normal sperm density offers little reassurance when semen volume is frankly low or the proportion of motile or normal sperm is grossly abnormal. Overall, the odds of male infertility increase with the number of major semen parameters (concentration, motility, morphology) in the subfertile range; the probability is two to three times higher when one is abnormal, five to seven times higher when two are abnormal, and 16 times greater when all three are abnormal.296
Although detailed procedures for semen analysis have been established by the World Health Organization (WHO), the methods and accuracy of semen analyses as they are performed in physician offices, hospitals, and specialty andrology laboratories may vary. Ideally, to ensure accurate and reliable results, semen analyses should be performed in a laboratory having an established quality control program that conforms to the standards outlined in the Clinical Laboratory Improvement Amendments (CLIA; www.hcfa.gov/medicaid/clia/cliahome.htm).300,301 The traditional WHO normal reference values are as follows302,303 and 304:
Semen Analysis: Normal Reference Values
Volume | 1.5-5.0 mL |
pH | >7.2 |
Viscosity | <3 (scale 0-4) |
Sperm concentration | >20 million/mL |
Total sperm number | >40 million/ejaculate |
Percent motility | >50% |
Forward progression | >2 (scale 0-4) |
Normal morphology | >50% normal302 |
>30% normal303 | |
>14% normal304 | |
Round cells | <5 million/mL |
Sperm agglutination | <2 (Scale 0-3) |
Over time, the methods and normal reference values for determining sperm concentration and motility have changed little, but those for sperm morphology have changed rather substantially. Using the most recent and rigorous standard, even fertile men have relatively few normal sperm. The rationale for the change in the morphology standard and its clinical relevance are discussed below (see Sperm Morphology).
In 2010, the WHO published revised lower reference limits for semen analyses, which represent the fifth centile in a population of over 1,900 men from eight countries on three continents whose partners conceived within 12 months:305
Semen Analysis: Lower Reference Limits (95% CI) in Fertile Men
Volume | 1.5 (1.4-1.7) mL |
Sperm concentration | 15 (12-16) million/mL |
Total sperm number | 39 (33-46) million/ejaculate |
Total motility | 40 (38-42) % |
Progressive motility | 32 (31-34) % |
Normal morphology | 4 (3-4) % |
Vitality | 58 (55-63) % |
These data provide reliable, clinically relevant reference values for use in the evaluation of infertile men and in assessing their prognosis for achieving pregnancies with their partners.
Ejaculate Volume and pH
A low or absent ejaculate volume suggests the possibility of failed emission, incomplete collection, a short abstinence interval, congenital bilateral absence of the vas deferens (CBAVD), ejaculatory duct obstruction, hypogonadism, or retrograde ejaculation. Other semen parameters can help to differentiate the cause.
The majority of semen volume comes from the seminal vesicles which share a common embryology with the vasa deferentia. Seminal vesicle secretions are alkaline and contain fructose. Because the seminal vesicles are hypoplastic or absent in most men with CBAVD, they generally produce a low-volume acidic (pH less than 7.2) ejaculate that contains little or no fructose and reflects the greater contribution of acidic prostatic secretions.306,307 and 308 Men with ejaculatory duct obstruction produce an ejaculate having similar characteristics because the ejaculatory ducts are formed by the union of the vasa with the ducts exiting the seminal vesicles, proximal to the prostate; semen fructose concentrations decrease with increasing severity of ejaculatory duct obstruction.309,310 and 311 When both ejaculatory ducts are completely obstructed, the semen is acidic (containing only prostatic secretions) and contains neither fructose nor sperm. Hypogonadal men with either primary or secondary testicular failure also may exhibit low ejaculate volumes because the secretions of the seminal vesicles and prostate are stimulated by androgens; volume is therefore decreased when androgen levels are low.
A post-ejaculatory urinalysis can detect retrograde ejaculation and should be considered whenever the ejaculate volume is less than 1 mL, except when hypogonadism, CBAVD, collection problems, or a short abstinence interval offers an obvious explanation. When indicated, the post-ejaculatory urinalysis involves centrifugation for 10 minutes at no less than 300 g, followed by microscopic examination of the pellet (400X). In men with no or low semen volume and azoospermia (no sperm in the ejaculate), the observation of any sperm on post-ejaculatory urinalysis suggests retrograde ejaculation. More substantial numbers of sperm must be observed in men with low volume oligospermia before making the diagnosis of retrograde ejaculation because sperm found in the urine may simply have been washed from the urethra during urination.306
Sperm Concentration and Total Sperm Count
Azoospermia describes the absence of sperm on standard microscopic examination. The prevalence of azoospermia is approximately 1% in all men312 but up to 10-15% in infertile men.313 To establish the diagnosis, the semen specimen should be centrifuged at high speed (3,000 g for 15 minutes) and the pellet examined at high magnification (400X)304; the absence of sperm should be documented on at least two separate occasions. Azoospermia is generally classified as obstructive (normal sperm production) or non-obstructive (decreased or absent spermatogenesis).
Obstructive azoospermia may result from a blockage anywhere in the ductal system, from the efferent ductules to the ejaculatory ducts, as the consequence of severe infection, iatrogenic injury during scrotal or inguinal surgery, or congenital anomalies (CBAVD); approximately 40% of azoospermic men have an obstruction.306 Non-obstructive azoospermia is caused by intrinsic testicular disease (primary testicular failure) or endocrinopathies and other conditions that suppress spermatogenesis (secondary testicular failure). Men with non-obstructive azoospermia may have low level sperm production that is insufficient to drive epididymal transport and to permit sperm to enter the ejaculate.314 Careful examination of a centrifuged semen sample will identify sperm in the ejaculates of up to one-third
of men with a preliminary diagnosis of non-obstructive azoospermia.315 The observation has practical significance because men in whom even a modest number of sperm can be recovered from the ejaculate may not require surgical sperm retrieval for IVF (testicular sperm extraction; TESE).
of men with a preliminary diagnosis of non-obstructive azoospermia.315 The observation has practical significance because men in whom even a modest number of sperm can be recovered from the ejaculate may not require surgical sperm retrieval for IVF (testicular sperm extraction; TESE).
Oligospermia is defined traditionally by a sperm density less than 20 million/mL and is considered severe when the sperm concentration is below 5 million/mL. The probability of conception increases with increasing sperm concentrations up to approximately 40-50 million/mL, but does not rise further with higher sperm densities.296,297 The results of a large U.S. study comparing semen parameters in fertile and infertile men with normal partners indicate that the likelihood of male infertility is increased approximately 5-fold (5.3, 95% confidence interval 3.3-8.3) when sperm density is less than 13.5 million/mL.296 In an earlier European study of similar design, the density representing the tenth percentile for fertile men was 14 million/mL.316 These values are consistent with the lower reference limit for fertile men recommended recently by the WHO (15 million/mL).305 Oligospermia may be associated with a varicocele, hypogonadism, or specific microdeletions in the Y chromosome. Endocrine and genetic evaluation is indicated for men with severe oligospermia (discussed below).
Total sperm count is simply the product of multiplying the semen volume and sperm concentration. The total sperm count may be normal in oligospermic men when volume is high, and also normal when volume is low but density is high. The two parameters fluctuate and must be considered together in making judgments regarding semen quality. Numerous studies have suggested that the average sperm count in men has been decreasing steadily over the past few decades,317,318 raising concerns that environmental toxins and chemicals having estrogen-like activity (xenoestrogens) might be responsible. However, numerous others have observed no evidence of any significant change.319,320,321,322,323 and 324 Most importantly, the prevalence of infertility has not increased significantly over the same intervals, indicating that any decrease in semen quality that may have occurred has had no global clinical impact.
Sperm Motility, Forward Progression, Total Motile Count, and Vitality
Sperm motility is estimated as a percentage of the total sperm population exhibiting any motion. Forward progression generally is graded on an arbitrary scale (grade 0-4) and most often reported as the percentages exhibiting rapid (grade 3-4), slow (grade 2), and non-progressive motility (grade 0-1). Total progressive motility generally represents an estimate of the percentage of sperm exhibiting purposeful forward motion (grades 2-4). The probability of conception rises with increasing motility up to approximately 60%.296 According to one large U.S. study, the likelihood of male infertility is increased approximately 5-fold (OR 5.6, 95% CI 3.5-8.3) when progressive motility is less than 32%.296 In another, the threshold separating fertile and infertile men was 45% and the tenth percentile motility for fertile men was 28%.316 Again, these values compare well with the lower reference value for progressive motility now recommended by the WHO (32%).305
The total motile sperm count is calculated from the total sperm count and the percentage of progressively motile sperm and represents an estimate of the total number of active sperm in the ejaculate. Allowing for the inevitable procedural losses associated with processing a semen sample for IUI (up to approximately 50%), the total motile sperm count can be used to estimate the likely processed total motile sperm count, which correlates with the probability of pregnancy achieved with IUI in the treatment of male factor infertility (see Treatment, below).325,326,327,328 and 329
In general, poor sperm motility (asthenospermia) suggests testicular or epididymal dysfunction. Asthenospermia has been associated with sperm autoantibodies (predisposing to aggregation), genital tract infections (leukocytes in the semen), partial obstruction of the ejaculatory ducts or at the site of a vasectomy reversal (reanastomosis), varicoceles, and prolonged abstinence intervals.
Large numbers of viable nonmotile sperm suggest the rare possibility of primary ciliary dyskinesia (Kartagener syndrome), in which sperm tails have a structural abnormality and cannot flagellate. The cilia of the respiratory tract usually also are involved; affected individuals are infertile and predisposed to chronic respiratory tract infections. Diagnosis is made by examination of sperm using electron microscopy.
When no motile sperm are observed, a sperm vitality test can differentiate viable nonmotile sperm from dead sperm. One method involves mixing fresh semen with a supravital dye (eosin Y or trypan blue); sperm with intact membrane function do not take up the stain. Another method, the hypo-osmotic sperm swelling test, involves incubation of sperm in a hypo-osmotic solution; the tails of sperm with normal membrane function swell and coil as fluid is transported across the membrane. In men with few or no motile sperm, the hypo-osmotic swelling test can be used to identify living nonmotile sperm for ICSI.330
Sperm Morphology
Sperm morphology reflects the quality of spermatogenesis. Morphological abnormalities (teratospermia) are categorized by location, involving the head, neck (midpiece), or tail. Cytoplasmic droplets in the midpiece that occupy more than approximately one half of the area of a normal sperm head represent another specific defect. Sperm classified as normal must be normal in all respects. Teratospermia has been associated with varicocele and with both primary and secondary testicular failure. It may be observed in association with abnormalities in sperm concentration and motility or occur as an isolated abnormality.
The most recent WHO reference values (since 1999) for the evaluation of sperm morphology are very similar to those known as the Kruger (Tygerberg) or “strict” criteria,331,332 which arose from efforts to identify predictors of fertilization in IVF cycles. When sperm morphology was judged according to a strict normal standard, fertilization efficiency in vitro correlated with the percentage of morphologically normal sperm.331,333,334 Conventional fertilization rates were highest when the percentage of normal sperm was 14% or higher, very poor (7-8%) when less than 4% of sperm had normal morphology, and intermediate when values fell between the two threshold values.331 After several studies confirmed the predictive value of strict sperm morphology in IVF,335,336,337,338,339,340,341 and 342 severe teratospermia (0-4% normal sperm by strict criteria) became widely accepted as an indication for ICSI in IVF cycles. However, others have observed no differences in the fertilization, pregnancy, and live birth rates achieved with ICSI and conventional fertilization and argue that isolated teratospermia is not a valid indication for performing ICSI.343,344,345 and 346 Controversy continues, but strict sperm morphology remains the best available predictor of sperm function (the capacity to fertilize a mature oocyte).
It was logical to anticipate that if strict sperm morphology could predict fertilization efficiency under optimized conditions in vitro, it also might have value for predicting the likelihood of successful fertilization in vivo and help to discriminate fertile and infertile men. A number of studies have examined semen parameters in couples with no known infertility factors who were attempting pregnancy,297,347 or compared the semen parameters of fertile and infertile men;296,316,340,348 two have included only men whose partners had no apparent infertility factors.296,316 Whereas sperm concentration and progressive motility had value
for distinguishing fertile from infertile men, strict sperm morphology (as determined by an individual having extensive training and experience) was the one most discriminating value.296,316 In the larger of the two studies, the likelihood of male infertility was increased approximately 4-fold (OR = 3.8, 95% CI = 3.0-5.0) when strict sperm morphology was less than 9% normal.296 The 9% threshold value had a sensitivity of 43% and a specificity of 81% for identifying infertile men; lowering the threshold value to 5% normal forms decreased sensitivity to only 19%, but increased specificity to 94%.296 In a smaller study of similar design, the threshold value that identified infertile men was 10% and the value corresponding to the tenth centile among fertile men was 5% normal forms.316
for distinguishing fertile from infertile men, strict sperm morphology (as determined by an individual having extensive training and experience) was the one most discriminating value.296,316 In the larger of the two studies, the likelihood of male infertility was increased approximately 4-fold (OR = 3.8, 95% CI = 3.0-5.0) when strict sperm morphology was less than 9% normal.296 The 9% threshold value had a sensitivity of 43% and a specificity of 81% for identifying infertile men; lowering the threshold value to 5% normal forms decreased sensitivity to only 19%, but increased specificity to 94%.296 In a smaller study of similar design, the threshold value that identified infertile men was 10% and the value corresponding to the tenth centile among fertile men was 5% normal forms.316
Strict sperm morphology is perhaps most relevant for couples with mild oligospermia or asthenospermia or with unexplained infertility (normal ovulatory function, female reproductive anatomy, and semen parameters). In such couples, IUI (with or without ovarian stimulation) and IVF are the treatment options that offer the greatest likelihood for success (Chapter 27). Most,349,350,351 and 352 but not all,353,354 studies have observed that cycle fecundability in IUI cycles correlates with the proportion of morphologically normal sperm and is generally poor when strict morphology is less than 5% normal. Although no threshold value excludes the possibility of pregnancy with expectant management or IUI, the relationship between strict sperm morphology and cycle fecundability with IUI merits careful consideration and discussion when planning treatment for couples with male factor and unexplained infertility. Other important considerations include age of the female partner, the duration of infertility, and the comparative costs, logistics, risks, and prognosis associated with alternative treatment strategies, including IVF with and without ICSI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


