Leiomyomata Uteri and Myomectomy
Carla P. Roberts
Jennifer F. Kawwass
DEFINITIONS
Intravenous leiomyomatosis—Smooth muscle tumor that consists of polypoid intravascular projections into the veins of the parametrium and broad ligaments.
Leiomyomatosis peritonealis disseminata—A benign reparative process in which fibroblasts replace soft peritoneal decidua on subperitoneal surfaces of the uterus and other pelvic and abdominal viscera resulting in nodules with a pseudoleiomyomatous pattern.
Menorrhagia—Prolonged (>7 days) or heavy (>80 mL) menstrual bleeding occurring at regular intervals.
Metrorrhagia—Uterine bleeding occurring at irregular intervals, sometimes of prolonged duration.
Menometrorrhagia—Heavy, prolonged bleeding occurring at irregular intervals.
Submucosal—Present within the uterine myometrium just below the basal layer of the endometrial lining.
Subserosal—Present within the uterine myometrium just below the serosal or peritoneal covering of the uterus.
Leiomyomata are the most common tumors of the uterus and the female pelvis. This chapter discusses the pathologic and clinical features of uterine leiomyomata, the choice of treatment, and the indications and techniques for myomectomy.
Hysterectomy is sometimes required for the management of leiomyomata. It is also performed for many other indications, but leiomyomatous uteri are the most common indication for hysterectomy. Refer to Chapter 32 for a complete discussion of hysterectomy.
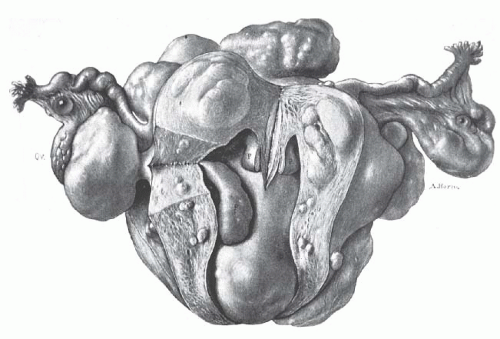
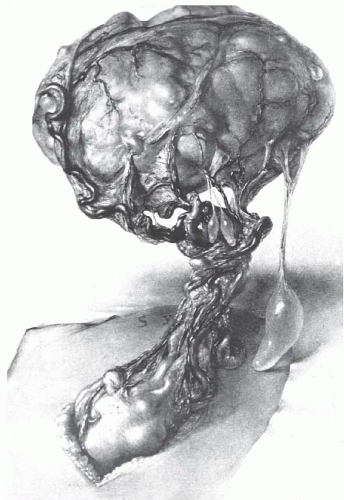
Advances in gynecologic surgery in the early 1900s finally brought this common, sometimes fatal, disease of women under reasonable control. Before the 20th century, no effective treatment was available. Uterine leiomyomata often grew to enormous size and caused great suffering from bleeding, pain, and emaciation (Fig. 31.1). Death from this benign disease was not uncommon. Progress in gynecologic surgery and anesthesia finally allowed the safe removal of these tumors by skilled gynecologic surgeons.
No one played a more important role in this endeavor than Drs. Kelly and Cullen. Working together at Johns Hopkins Hospital, they gradually developed surgical techniques that were successful in preventing and controlling intraoperative hemorrhage. Several illustrations from their magnificent treatise, Myomata of the Uterus, published in 1907, are included in this chapter. In the preface, Cullen wrote:
It was my good fortune to come to Baltimore in 1891, shortly after the hospital opened. At that time many cases of myoma were considered inoperable, and even when hysterectomy was undertaken it was only in the cases in which a stout rubber ligature could be temporarily tied around the cervix and when, as happened in some cases, this ligature slipped, alarming hemorrhage followed. Then came the systematic controlling of each of the cardinal vessels; later the bisection, and finally the transverse severance of the cervix as a preliminary feature of the operation in exceptionally difficult cases, until at present a myomatous uterus that cannot be removed is almost unheard of. I have watched the gradual simplification of the surgical procedures with the greatest interest. Many American surgeons have had much to do with the wonderful advance in this direction, but I know of no other man, either here or abroad, who has done as much toward this advancement as Howard A. Kelly.
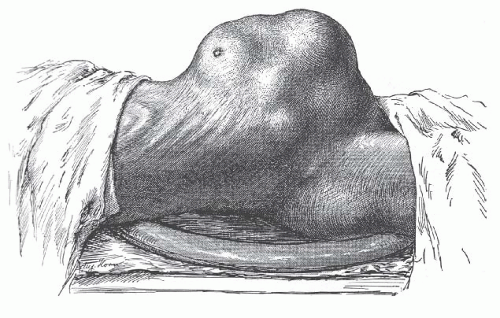 FIGURE 31.1 The patient is thin and emaciated, the outline of the ribs being prominent. Such advanced and neglected cases of multiple uterine leiomyomata are rarely seen today. |
The mortality rate for 1,373 operations performed for uterine leiomyomata at Johns Hopkins Hospital between 1889 and 1906 was 5.75%; it was less than 1% for 238 operations performed between 1906 and 1909. In 55 patients, no operation was attempted because of patients’ refusal or weakened condition. Among these patients, 21 deaths occurred in the hospital. Death from uterine leiomyomata rarely occurs today. The near elimination of mortality secondary to uterine fibroids represents a major milestone in the health care of women.
During the past century, hysterectomy and myomectomy by the traditional and classic techniques have been the main treatment for women with uterine leiomyomata and significant symptoms; they continue to be so today. Each year in the United States, more than 200,000 hysterectomies are performed with uterine leiomyomata as the primary indication. However, this traditional management is currently evolving toward more conservative, less invasive techniques for several reasons:
Concern regarding the increasing costs of health care has focused on the need to use effective but less expensive methods of management of uterine leiomyomata.
Advances in surgical technology now allow certain patients to be treated with new, minimally invasive techniques, including robotic hysterectomy, laparoscopic hysterectomy, laparoscopic-assisted vaginal hysterectomy, robotic myomectomy, laparoscopic myomectomy, laparoscopic myoma coagulation (myolysis), and hysteroscopic resection of submucous myomata. Under proper circumstances, these procedures can be safe, effective, and less costly.
Interest in nonsurgical management also appears to be increasing with more data available regarding minimally invasive procedures, including uterine artery embolization (UAE). This procedure has emerged from an investigational realm to common clinical practice. As more long-term data become available, outcomes and prognosis are becoming more clearly delineated.
A medical approach to the management of patients with leiomyomata is now available. Gonadotropin-releasing hormone (GnRH) analogs, administered for 3 to 6 months, cause most uterine leiomyomata to shrink. However, the myomata regain their original size several months after the GnRH analog is discontinued. This medical regimen has
been useful as an adjunct to surgical management. Women who become symptomatic with leiomyomata just before menopause can be treated temporarily with GnRH analogs and can possibly avoid surgical therapy.
Uterine leiomyoma are a major public health and women’s health care problem. Society has a legitimate reason for interest and concern and has questioned the advisability of hysterectomy for the management of most cases of uterine leiomyomata. Many women insist on the preservation of uterine function for future childbearing and sometimes even when future childbearing is not desired or not likely to occur. A greater emphasis has developed on expectant management, medical management, minimally invasive surgical procedures, and conservational management of uterine leiomyomata in the future.
In the future, the traditional and classic techniques of hysterectomy and myomectomy will be required less often for patients with symptomatic leiomyomata. At present, however, these operations are still appropriate in many situations.
ETIOLOGY, PATHOLOGY, AND GROWTH CHARACTERISTICS OF UTERINE LEIOMYOMATA
A leiomyoma is a benign tumor composed mainly of smooth muscle cells but containing varying amounts of fibrous connective tissue. The tumor is well circumscribed but not encapsulated. Various terms are used to refer to the tumor, such as fibromyoma, myofibroma, leiomyofibroma, fibroleiomyoma, myoma, fibroma, and fibroid. The latter designation is the one most commonly used, but it is the least accurate and acceptable. The term leiomyoma is a reasonably accurate one that emphasizes the origin of this tumor from smooth muscle cells and the predominance of the smooth muscle component. The tissue culture work of Miller and Ludovici suggested an origin from smooth muscle cells, and the studies of Townsend and associates affirm a unicellular origin for leiomyomata.
Leiomyomata are the most common tumors of the uterus and female pelvis. It is impossible to determine their true incidence accurately, although the frequently quoted incidence of 50% found at postmortem examinations seems reasonable. Leiomyomata are responsible for about one third of all hospital admissions to gynecology services. It is well recognized that the incidence is much higher in African American than in Caucasian women. In a careful study of leiomyomata among women in Augusta, Georgia, Torpin and associates found the incidence among African American women to be three and one third times that among Caucasian women. There is no known explanation for this racial difference. Leiomyomata also are larger and occur at a younger age in African Americans. In fact, many African American women develop leiomyomata before 30 years of age. However, development prior to age 20 is extremely rare, regardless of race. Patients with uterine leiomyomata often have a positive family history of uterine leiomyomata. This suggests the presence of a gene, which has yet to be discovered, encoding for their development.
About 40% to 50% of leiomyomas show karyotypically detectable chromosomal abnormalities that are both nonrandom and tumor specific. Identified chromosomal abnormalities include t(12;14) (q15;q23-24), del(7) (q22q32), rearrangements involving 6p21, 10q, trisomy 12, and deletions of 3q. Interestingly, a recent study of 217 myomas found a positive correlation between the presence of a cytogenetic abnormality and the anatomic location of the myoma. In this study by Brosens and colleagues, submucous myomas were consistently shown to have fewer cytogenetic abnormalities when compared with intramural and subserous lesions (12% vs. 35% and 29%, respectively). An increased prevalence in certain races, twin studies indicating higher correlation with hysterectomy in monozygotic twins, and increased incidence in first-degree relatives all seem to support an inherited predisposition. The true genetic contribution to the development of uterine leiomyoma remains to be defined.
Most of the data concerning the incidence of uterine leiomyomata are based on gross examination of the uterus, routine pathology reports, or the clinical diagnosis of uterine leiomyomata. Cramer and Patel subjected 100 uteri to gross serial sectioning at 2-mm intervals. They found 649 leiomyomata, roughly threefold the number identified by routine pathologic examination. Admittedly, some were only a few millimeters in diameter, but all were grossly visible. In 48 uteri with no mention of leiomyomata in the routine report, 27 were found to have small tumors. The incidence of leiomyomata was the same in premenopausal and postmenopausal uteri, although the average number of leiomyomata and the average size of the largest leiomyoma were greater in the premenopausal women. This work has important implications for future epidemiologic studies. It also suggests that it is almost never possible to surgically remove all leiomyomata when a myomectomy is performed.
The growth of leiomyomata is dependent on estrogen production. The tumors thrive during the years of greatest ovarian activity. Continuous estrogen secretion, especially when uninterrupted by pregnancy and lactation, is thought to be the most important underlying risk factor in the development of myomata. After menopause, with regression of ovarian estrogen secretion, growth of leiomyomata usually ceases. Actual regression in the tumor size may occur. There are rare instances, however, of postmenopausal growth of benign leiomyomata, suggesting the possibility of postmenopausal estrogen production either in the ovary or elsewhere. Postmenopausal ovarian cortical stromal hyperplasia may be associated with an increase in estrogen secretion by the ovary. The postmenopausal ovarian stroma in a variety of presumably inactive ovarian tumors, including mucinous cysts and Brenner tumors, can also produce estrogen. When a central pelvic tumor presumed to be uterine leiomyomata enlarges after menopause, one should think of the possibility of malignant change in the leiomyoma itself or in the adjacent myometrium, or of the growth of a new pelvic tumor of extrauterine origin.
Older nulliparous women have an increased risk of developing leiomyomata. However, in multiparous women, the relative risk decreases with each pregnancy. A woman who has had five term pregnancies has only one fifth the risk of a nulliparous woman of developing myomata. The risk is reduced in women who smoke and is increased in obese women; this is possibly related to the conversion of androgens to estrogen by fat aromatase.
The observation that leiomyomata may show significant enlargement during pregnancy provides further clinical evidence of the relation of estrogen and progesterone to the growth of these tumors. However, a better blood supply during pregnancy might also encourage their growth. In a prospective ultrasonographic study of 29 pregnant patients with uterine leiomyomata, Aharoni and associates found no evidence of enlargement of the myomata in 78%. Lev-Toaff and colleagues also confirmed that some but not all leiomyomata enlarge during pregnancy in response to estrogen and progesterone.
In the initial two decades following the introduction of oral contraceptives containing high-dose estrogen, there was a striking increase in the occurrence of large leiomyomata among young women of all racial backgrounds who took these pills. Although the growth of uterine leiomyomata is not invariably stimulated, oral contraceptives containing highdose estrogen should not be prescribed for women with these tumors. Oral contraceptives with low-dose estrogen are less likely to stimulate growth. According to Parazzini and associates, there is no significant relation between the occurrence or growth of leiomyomata and the newer oral contraceptives that contain much smaller amounts of estrogens and progestins, and some believe that the risk of developing myomata is reduced with these low-dose pills.
Scientific investigators have been intrigued by the observation that leiomyomata develop during the reproductive years, sometimes grow during pregnancy, and regress after menopause. Nelson, Lipschutz, and others have produced multiple leiomyomata artificially on the serosal surface of the uterus and other peritoneal surfaces in guinea pigs given prolonged estrogen injections. Spellacy and coworkers found that levels of plasma estradiol were the same in patients with and without leiomyomata. However, Wilson and associates found a significantly higher concentration of estrogen receptors in leiomyomata than in myometrium. Farber and colleagues reported that these tumors bind about 20% more estradiol per milligram of cytoplasmic protein than does the normal myometrium of the same organ. This observation was not uniformly true for all leiomyomata, suggesting that different cellular components with a leiomyoma may be associated with different biologic activity. Otubu and coworkers found the concentration of estradiol to be significantly higher in leiomyomata than in normal myometrium, especially in the proliferative phase of the menstrual cycle. Soules and McCarty reported that leiomyomata had more estrogen receptors than did normal uterine tissues in the first phase (days 1 through 9) and in the second phase (days 10 through 18) of the menstrual cycle. Gabb and Stone found that the ability to convert estradiol to estrone was similar in leiomyomata and myometrium. However, Pollow and associates found the conversion of estradiol into estrone to be significantly lower in leiomyomata than in myometrium. This difference in conversion rate could result in a relative accumulation of estrogen in a leiomyoma, causing a hyperestrogenic state within the tumor and surrounding tissues. The enzyme 17β-hydroxy dehydrogenase accelerates the conversion of estradiol to estrone. Leiomyomata have a low concentration of 17β-hydroxy dehydrogenase, which results in a relative accumulation of estradiol in leiomyomatous tissue. These findings may explain the myometrial hypertrophy that is invariably present with leiomyomata.
Other abnormalities in endocrine function have also been suggested. Ylikorkala and colleagues found that pituitary function may be abnormal in women with leiomyomata. Patients with leiomyomata had a low follicle-stimulating hormone level and a diminished follicle-stimulating hormone response to pituitary GnRH. There was an excessive prolactin response to thyrotropin-releasing hormone. Spellacy and colleagues found that the peak levels of human growth hormone reached during a hypoglycemic test were twice as high in patients with leiomyomata as in the control group. Reddy and Rose suggested the possibility that 5α-reduced androgens may play a role in the pathophysiology of uterine leiomyomata, because a significant increase in 5α-reductase has been found in leiomyoma tissue as compared with the myometrium and endometrium. Influenced by the experimental investigations of Lipschutz and associates, Goodman in 1946 treated patients with uterine leiomyomata with progesterone and noted a decrease in tumor size in all patients. However, Segaloff and colleagues reported no effect in their study. Goldzieher and coworkers produced histologic evidence of extensive degenerative changes in leiomyomata by administering high-dose progestin therapy (medrogestone in high doses for 21 days). Filiceri and associates have reported the regression of a uterine leiomyoma after longterm administration of a long-acting luteinizing hormone-releasing hormone agonist given to suppress ovarian estrogen secretion. Coutinho successfully used a potent 19-norsteroid antiestrogen-antiprogesterone to treat excessive uterine bleeding in 16 patients with uterine leiomyomata. A reduction in the size of the tumors was noted.
Although the exact etiology of uterine leiomyomata is not known, the puzzle may be solved bit by bit by the research of Kornyei and colleagues, Wilson and coworkers, Tamaya and associates, Buchi and Keller, Sadan and colleagues, and others who continue to investigate estrogen and progesterone as possible growth factors. Although some data are conflicting, evidence suggests that both estrogen and progesterone are involved in the growth of uterine leiomyomata. The possibility that progesterone may play a role in the growth of leiomyomata is suggested by the work of Kawaguchi and coworkers, who found a higher mitotic count in leiomyomata obtained in the proliferative phase of the menstrual cycle.
Anderson and associates have shown that medroxyprogesterone acetate, a progestin, causes a decrease in connexin-43 messenger ribonucleic acid levels in primary cultures of human myometrium and leiomyoma. Connexin-43 is a gap junction protein whose formation is stimulated by 17β-estradiol.
According to the research data of Brandon and colleagues, progesterone receptor messenger ribonucleic acid is overexpressed in uterine leiomyomata, compared with normal adjacent myometrium, suggesting that amplified progesteronemediated signaling is instrumental in the abnormal growth of these tumors. It is possible that the increased amount of progesterone receptor is caused by an alteration of estrogen or estrogen receptors in leiomyomata. The work of Kastner and coworkers and Nardulli and associates has demonstrated that progesterone receptor expression is regulated by estrogen.
Research in recent years has also focused on polypeptide growth factors in the stimulation of growth of leiomyomata. Polypeptide growth factors that have been investigated include epidermal growth factor, transforming growth factor alpha and beta, insulin-like growth factor (IGF), platelet-derived growth factor, vascular endothelial growth factor, and basic fibroblast growth factor. Other growth factors may also be involved. Polypeptide growth factor research has been performed by Goustin and colleagues, by Hoffmann and coworkers, by Lumsden and associates, and by others. A brief review of this research has been written by Vollenhoven and associates, who have been involved in the study of IGFs in uterine leiomyomata.
Results from a study by Strawn and colleagues demonstrate that IGF-I stimulates leiomyoma growth in a dose-related manner over that of normal myometrial tissue in monolayer culture. This stimulatory effect, in the absence of sex steroid hormones or other growth factors, provides additional support that IGF-I may play an important direct role in the pathogenesis of these tumors, possibly by modulating the response of these tumors to various levels of sex steroids. Dawood and Kahn-Dawood were unable to find any significant elevation in peripheral levels of serum IGF-I in nonpregnant premenopausal women with uterine leiomyomata of 14 weeks gestational size. The authors state, “Nevertheless, the finding does not detract from the potential paracrine or autocrine role that
IGF-I produced by leiomyoma cells may have either on the growth of its own or adjacent myomas or on the vascular supply and blood flow of the uterus and myomas.”
IGF-I produced by leiomyoma cells may have either on the growth of its own or adjacent myomas or on the vascular supply and blood flow of the uterus and myomas.”
Rein and coworkers have proposed a hypothesis to explain the pathogenesis of myomata. This hypothesis suggests a critical role for progesterone in the growth of myomata. They state:
The initiation and growth of myomas likely involves a multistep cascade of separate tumor initiators and promoters. The initial neoplastic transformation of the normal myocyte involves somatic mutations. Although the initiators of the somatic mutations remain unclear, the mitogenic effect of progesterone may enhance the propagation of somatic mutations. Myoma proliferation is the result of clonal expansion and likely involves the complex interactions of estrogen, progesterone, and local growth factors. Estrogen and progesterone appear equally important as promoters of myoma growth.
To treat patients with uterine leiomyomata properly, the gynecologic surgeon must be familiar with their pathology, growth characteristics, and clinical features. Leiomyomata may be single, but most are multiple. They develop most commonly in the uterine corpus and much less often in the cervix. They may develop in the round ligaments, but this is rare. Because they arise in the myometrium, they are all interstitial or intramural in the beginning. As they enlarge, they can remain intramural, but growth often extends in an internal or external direction. Thus, the tumor can eventually become subserous or submucous in location. A subserous tumor can become pedunculated and occasionally parasitic, receiving its blood supply from another source, usually the omentum. A submucous tumor can also become pedunculated and may gradually dilate the endocervical canal and protrude through the cervical os. Indeed, a submucous myoma may descend through the vagina. Rarely, chronic uterine inversion results if the prolapsing submucous leiomyoma is attached to the top of the endometrial cavity and pulls the uterine fundus downward through the cervix.
In general, subserous leiomyomata contain more fibrous tissue than submucous leiomyomata. However, submucous leiomyomata contain more smooth muscle tissue than subserous leiomyomata. Sarcomatous change is more common in submucous tumors.
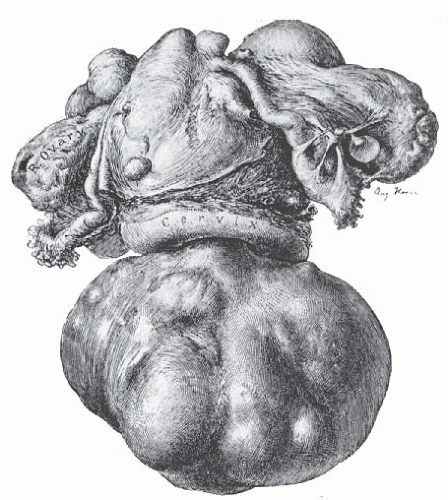
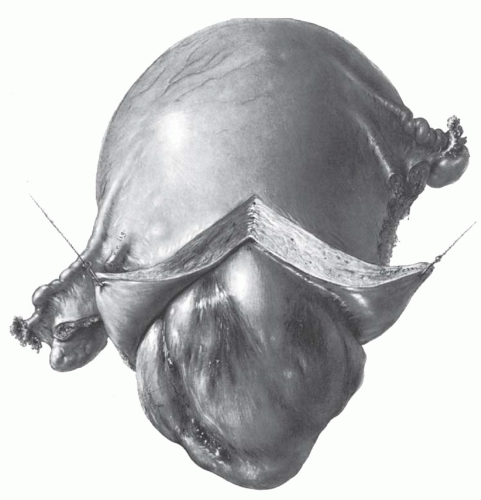
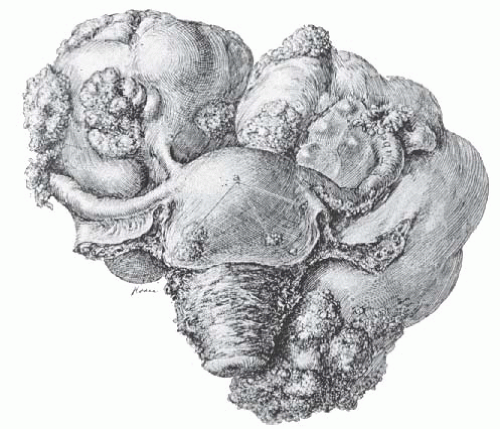
The typical uterine leiomyoma is a firm multinodular structure of variable size. The largest tumor, reported by Hunt in 1888, weighed more than 65 kg. Tumors of 4 to 5 kg are not rare, but most are smaller. In the operating room, leiomyomata appear as nodular tumors of different sizes that distort the uterus in various ways, depending on their size, location, and direction of growth. Growth between the leaves of the broad ligament and origin from the cervix may make surgical removal difficult. Subserous and subserous pedunculated tumors, as well as intraligamentous tumors, may create problems in diagnosis because they are difficult to distinguish from tumors arising from the adnexal organs (Fig. 31.2). When tumors cause symmetric enlargement of the uterus, they may be mistaken for a pregnant uterus on bimanual examination.
The “normal” intramural leiomyoma on section protrudes from the surrounding compressed myometrium. Ordinarily, there is a clear distinction between the myoma and the myometrium so that dissection between the two is easy to accomplish. Myomata usually can be removed from surrounding myometrium with ease. Although these tumors are not encapsulated, a clear distinction can usually be made between a myoma and the myometrium that surrounds it. The cut surface appears as glistening pinkish white and gray. It is firm, and there is a whorllike arrangement of the muscle and the fibrous tissue. In contrast to this typical appearance, the myometrium may be thickened by a diffuse, ill-defined nodularity of smooth muscle. This so-called diffuse leiomyomatosis usually involves all parts of the myometrium and causes symmetric enlargement of the uterine corpus. The nodules of smooth muscle are not distinct, contain little collagen, and merge with one another and the surrounding hypertrophied myometrium.
The extracellular matrix of leiomyomata is composed mostly of collagen but also contains proteoglycans and fibronectin. According to Fujita, myomata contain 50% more collagen than does normal myometrium, and the ratio of collagen type I to collagen type III is increased in myomata. Proteoglycans provide hydrated spaces between myoma cells. Fibronectin is a glycoprotein that mediates adhesion between myoma cells and extracellular matrix.
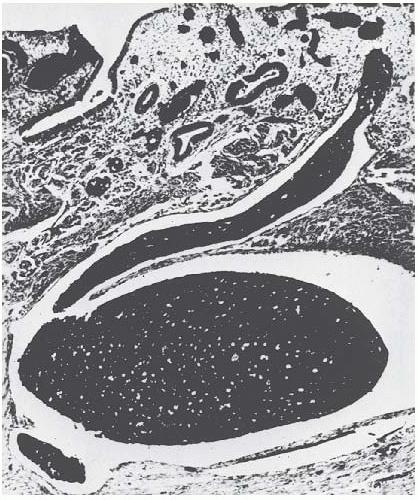
The most common change in leiomyomata is hyaline degeneration. The cut surface of a hyalinized area is smooth and homogeneous and does not show the whorllike arrangement of the rest of the leiomyoma. Almost all leiomyomata, except the smallest, have scattered areas of hyaline degeneration. Eventually, these may become liquefied and form cystic cavities filled with clear liquid or gelatinous material
(Fig. 31.3). Sometimes, the cystic change is so great that the leiomyoma becomes a mere shell and is truly a cystic tumor. Softness of a tumor does not necessarily indicate cystic degeneration. Fleshy leiomyomata may be equally soft.
(Fig. 31.3). Sometimes, the cystic change is so great that the leiomyoma becomes a mere shell and is truly a cystic tumor. Softness of a tumor does not necessarily indicate cystic degeneration. Fleshy leiomyomata may be equally soft.
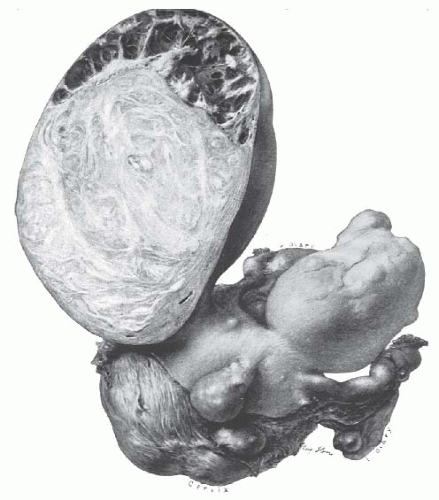 FIGURE 31.3 Multiple leiomyomata are present. A large subserous myoma has undergone partial cystic degeneration. |
Over time, with continued diminished blood supply and ischemic necrosis of tissue, calcium phosphates and carbonates are deposited in myomata. Their presence is evidence of a continuum of degenerative changes. The calcium may be deposited in varying amounts. If it is deposited at the periphery of the tumor, the leiomyoma may resemble a calcified cyst. Other calcified leiomyomata may show an irregular or diffuse distribution throughout with a honeycomb or mulberry appearance. When the degenerative change is advanced, the leiomyoma may become solidly calcified. Such calcified tumors have been called “womb stones.” Calcified leiomyomata are seen most often in elderly women, in African American women, and in women who have pedunculated subserous tumors. They are easily seen radiographically (Fig. 31.4).
Leiomyomata may undergo changes as a result of infection. Submucous leiomyomata are most commonly infected when they protrude into the uterine cavity, or especially into the vagina (Fig. 31.5). The pedunculated submucous leiomyoma thins out the endometrium as it grows inward, and eventually, the surface becomes ulcerated and infected (Fig. 31.6). An intramural leiomyoma in an involuting puerperal uterus can also become infected when endometritis is present. Microscopic abscesses can be found, and gross abscesses occasionally occur, particularly if the leiomyoma descends as low as the cervical canal. Such infections are usually streptococcal and may be virulent. Bacteroides fragilis infections also occur. Parametritis, peritonitis, and even septicemia may result.
Necrosis of a leiomyoma is caused by interference with its blood supply. Occasionally, a pedunculated subserous leiomyoma twists, and if an operation is not done immediately, infarction results. Necrosis sometimes occurs in the center of
a large tumor simply as a result of poor circulation. Necrotic leiomyomata are dark and hemorrhagic in the interior. Eventually, the tissue breaks down completely. So-called red or carneous degeneration is seen occasionally, especially in association with pregnancy. This condition is thought to result from poor circulation of blood through a rapidly growing tumor. Thrombosis and extravasation of blood into the myoma tissue are responsible for the reddish discoloration (Fig. 31.7).
a large tumor simply as a result of poor circulation. Necrotic leiomyomata are dark and hemorrhagic in the interior. Eventually, the tissue breaks down completely. So-called red or carneous degeneration is seen occasionally, especially in association with pregnancy. This condition is thought to result from poor circulation of blood through a rapidly growing tumor. Thrombosis and extravasation of blood into the myoma tissue are responsible for the reddish discoloration (Fig. 31.7).
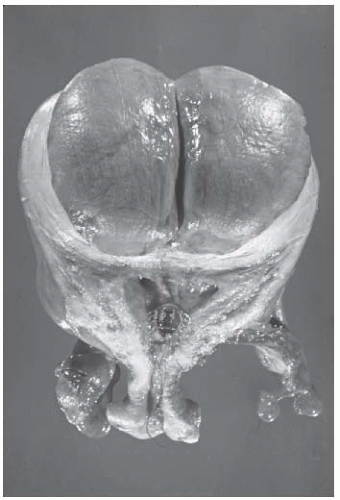 FIGURE 31.7 Degenerating leiomyoma showing carneous discoloration caused by thrombosis and extravasation of blood into the myoma tissue. A Dalkon shield can be seen in the endometrial cavity. |
A subserous and especially a subserous pedunculated myoma may gradually outgrow its blood supply (Fig. 31.8). To keep the myoma tissue from undergoing complete ischemic necrosis, the omentum becomes adherent to the peritoneal surface of a pedunculated subserous myoma and provides whatever blood supply is needed. Eventually, the pedicle may disappear or twist, and the myoma will become completely free from the uterus, wander in the upper abdomen, and receive its “parasitic” blood supply from the omentum and other sources.
On occasion, fat occurs in leiomyomata as true fatty degeneration. The cut surface may have a yellowish discoloration. Infrequently, a deposit of true fat may form a fibrolipoma; however, the presence of fat in a leiomyoma is rare. Indeed, if fat is seen grossly or microscopically in a curettage specimen, one should not assume that it represents fatty degeneration of a leiomyoma. One should assume that the uterus has been perforated and that fragments of fat have been curetted from the mesentery or omentum.
The most important, but rare, change in a leiomyoma is sarcomatous degeneration. There is much variation in the reported incidence of sarcoma in leiomyomata. The incidence given by
Novak is 0.7%. However, a review of 13,000 myomata by Montague and associates at Johns Hopkins Hospital revealed 38 cases of malignant change, the incidence of sarcoma thus being 0.29%. Corscaden and Singh indicated by their study that the true incidence of sarcoma developing within uterine leiomyomata is no higher than 0.13% and is probably as low as 0.04%. It should be remembered that because most women with uterine leiomyomata do not undergo surgical removal, the true incidence of sarcoma in leiomyomata is probably much lower than 1 per 1,000 (0.1%).
Novak is 0.7%. However, a review of 13,000 myomata by Montague and associates at Johns Hopkins Hospital revealed 38 cases of malignant change, the incidence of sarcoma thus being 0.29%. Corscaden and Singh indicated by their study that the true incidence of sarcoma developing within uterine leiomyomata is no higher than 0.13% and is probably as low as 0.04%. It should be remembered that because most women with uterine leiomyomata do not undergo surgical removal, the true incidence of sarcoma in leiomyomata is probably much lower than 1 per 1,000 (0.1%).
After hysterectomy in 1,429 patients with presumed benign leiomyomata, the histologic diagnosis of leiomyosarcoma was made in seven (0.49%), according to a study by Leibsohn and coworkers. There was no evidence of malignancy in the endometrial sampling of any of these seven patients, and the diagnosis was suspected intraoperatively in only three. Uterine weights ranged from 120 to 1,100 g. In a woman between 41 and 50 years of age with presumed symptomatic leiomyomata, there is a 1 in 112 chance of a leiomyosarcoma being present, according to these authors. This information has important implications in the consideration of conservative or delayed treatment for these women. Parker and associates found that the total incidence of uterine sarcomas (leiomyosarcoma, endometrial stromal sarcoma, and mixed mesodermal tumor) among patients operated on for presumed benign uterine leiomyoma is lower (0.23%) than the 0.49% reported by Leibsohn and coworkers.
The difficulty in defining the true incidence of sarcomatous change is understandable if one is familiar with the histology of leiomyomata. Abundantly, cellular leiomyomata are relatively common, and at first glance, they suggest sarcoma; however, they lack a significant number of mitotic figures, and patients from whom such tumors are removed all remain well. Misinterpretation of the histologic picture of this type of cellular leiomyoma undoubtedly accounts for the increased incidence of leiomyosarcoma reported by some. When cutting into leiomyomata in the operating room, the surgeon finds that sarcomatous areas have a somewhat characteristic appearance, although the histologic diagnosis certainly cannot be made by gross examination. A sarcoma is likely to occur in a rather large leiomyoma and toward the center of the tumor, where the blood supply is poorest. Instead of being firm fibrous tissue that grates when scraped with a knife blade, the tissue is soft and homogeneous and is described as resembling raw pork. Later, as necrosis of the malignant tissue occurs, it becomes more friable and hemorrhagic.
It has been difficult to understand uterine leiomyosarcoma, because pathologists do not agree on the criteria necessary for diagnosis. Some pathologists rely on the mitotic count. All tumors with less than 5 mitotic figures per 10 high-power fields are considered benign. All tumors with more than 10 mitotic figures per 10 high-power fields are called malignant. Those in between can be called smooth muscle tumors of uncertain malignant potential.
Other pathologists believe the mitotic count may have some significance but choose to rely instead on the presence of nuclear hyperchromatism, nuclear pleomorphism, or giant cells and other bizarre cell forms to make the diagnosis. Corscaden and Singh believe that no combination of histologic features is reliable and that only smooth muscle tumors that metastasize or recur are definitely malignant. We believe that all of these features should be taken into consideration for diagnosis and prognosis. When the tumor is confined to the uterus, both mitotic grade and histologic grade are important in the diagnosis and prognosis. A poor prognosis is associated with high mitotic counts and extremely atypical and anaplastic cytologic features. Bell and colleagues at Stanford University Medical Center assessed a variety of histopathologic features of 213 problematic smooth muscle neoplasms for which there were at least 2 years of clinical follow-up data. From the wide variety of light microscopic features assessed, the important predictors that emerged were mitotic index, the degree of cytologic atypia, and the presence or absence of coagulative tumor cell necrosis. Previously, the mitotic index was relied on exclusively to determine whether a uterine smooth muscle tumor was benign or malignant, but currently, an approach is used that incorporates additional histopathologic features.
A normal chromosome complement (46,XX) was observed by Meloni and coworkers in about 50% of leiomyoma cases. About 50% showed clonal abnormalities, such as those of chromosomes 1, 7, and 13, and t(12;14). Interstitial deletions of chromosome 7 were the ones most often involved, suggesting that this abnormality may be of primary importance in the cellular proliferation of leiomyomata. A relation between more aggressive histology and chromosomal abnormalities was also suggested.
Tumors that show obvious evidence of blood vessel invasion or spread to contiguous organs are rarely cured. The extent of the disease at the time of initial diagnosis is of even greater significance. In other words, when the diagnosis is suspected for the first time by the pathologist when he or she examines routine sections from a uterine leiomyoma, the patient almost always survives. However, if the diagnosis is made preoperatively by the gynecologist or is suspected during the operative procedure because of invasion of surrounding organs, the prognosis is grave.
An unusual atypical smooth muscle tumor was first described in the stomach by Martin and associates in 1960. Variously called bizarre leiomyoma, leiomyoblastoma, clear cell leiomyoma, and plexiform tumorlet, these atypical smooth tumors probably all belong together. The term epithelioid leiomyoma was adopted by the World Health Organization. Kurman and Norris have proposed that this term be used for all atypical leiomyomata. Histologically, the characteristic feature is the mixture of rounded polygonal cells and multinucleated giant cells present in epithelioid clear cell and plexiform patterns. Clinically, in the uterus most of these tumors are benign. They may rarely exhibit malignant potential. Malignancy is difficult to predict from histologic criteria because some metastases occurred from tumors that demonstrated very few mitoses. Kurman and Norris have suggested, however, that epithelioid neoplasms having more than 5 mitotic figures per 10 highpower fields should be called epithelioid leiomyosarcomas and that the term epithelioid leiomyoma should be applied when there is a lower level of mitotic activity. Although combination therapy (surgery plus radiation therapy or chemotherapy) may not be indicated for a patient with an epithelioid leiomyoma, follow-up should be considered essential, as emphasized by Klunder and colleagues.
An unusual benign form of leiomyomata uteri, intravenous leiomyomatosis, was first recognized at the turn of the 20th century and has been reported sporadically since then. Before 1982, about 50 cases had been reported, according to Bahary and coworkers. Probably, at least that many have been reported since. Marshall and Morris presented the first detailed report of this entity in the American literature in 1959. The characteristic feature of this peculiar smooth muscle tumor is the extension of the polypoid intravascular projections into the veins of the parametrium and broad ligaments. Although there may be some difficulty in distinguishing such lesions from lowgrade sarcoma, they are distinctly different histologically from stromatosis uteri because the intravenous plugs are mainly smooth muscle in origin. In 1966, Edwards and Peacock collected 32 cases of intravenous leiomyomatosis, including two cases of their own, and reviewed the clinical experience with this condition. In approximately 50% of the cases, the intravenous tumor was confined to the parametrium; in 75%, it extended no further than the veins of the broad ligament. The observations of Edwards and Peacock suggest that the severed intravenous extensions are probably incapable of independent parasitic existence and remain dormant after removal of the uterus. However, the cases presented by Bahary and associates tend to refute this idea. Total surgical excision of the tumor should be attempted for successful therapy. Some patients have survived for many years after incomplete resection of the tumor. A review of 14 cases of this rare uterine tumor from the file of the Armed Forces Institute of Pathology has been reported by Norris and Parmley. In this series, two of three patients with incomplete resection had a recurrence; the recurrent tumor was excised surgically, and the patients were alive and free of disease 5 and 11 years after operation. The authors concluded that this tumor behaves clinically like a benign neoplasm, although its wormlike extensions may involve uterine, vaginal, ovarian, and iliac veins. The uterine veins in the broad ligaments are the most common sites of extension. The mitotic index is quite low, with the most active lesions showing only one mitosis per 15 high-power fields. The material from the Armed Forces Institute of Pathology provides histologic evidence consistent with both theories of origin of intravenous leiomyomatosis, namely, that it may be the result of unusual vascular invasion from a leiomyoma or may arise de novo from the wall of veins within the myometrium.
Extension of benign leiomyomatosis up the vena cava and into the right atrium has been reported in several cases, with a fatal outcome in some. Before 1994, approximately 27 cases of intravenous leiomyomatosis extending to the heart were reported. Several recent cases requiring open heart surgery to remove the intracardiac tumor thrombosis have been successful and without recurrence. All reported cases occurred in women. Tierney and colleagues reported that substantial quantities of cytoplasmic estradiol and progesterone receptors were found in the right atrial tumor removed from a patient with intravenous leiomyomatosis. Their patient was treated with the antiestrogen tamoxifen because of residual tumor in the vena cava that could be estrogen dependent. Irey and Norris have presented evidence that female reproductive steroids can produce intimal proliferation of veins in predisposed persons. Interestingly, of the 30 patients with leiomyomata and leiomyosarcomas of the vena cava reviewed by Wray and Dawkins, 80% were female. Both intravenous leiomyomatosis and benign metastasizing leiomyoma have been reported to metastasize to the lung. As suggested by Banner and coworkers, by Horstmann and associates, and by Evans and colleagues, oophorectomy may be indicated in patients with these conditions, again because of the possibility that these tumors may be estrogen dependent or that estrogens may have the ability to stimulate their development, whether in a uterine or extrauterine location and whether they appear to be endothelial or mesenchymal in origin.
The possibility of metastases from a histologically benign uterine leiomyoma has been discussed by Idelson and Davids and by Clark and Weed. When such a case occurs, it is usually settled by finding a sarcomatous component in the leiomyoma or by finding evidence of intravenous leiomyomatosis. However, multiple cases have now been reported in which a benign uterine leiomyoma metastasized. Idelson and Davids’ case showed metastases to the aortic lymph nodes. The patient reported by Cramer and associates had metastatic tumor to the omentum, ovary, periaortic lymph node, and lung. In each location, the histology and estrogen receptor content of the tumor resembled those of a benign leiomyoma. The recommended treatment consists of surgical removal with castration and little or no estrogen replacement.
Leiomyomatosis peritonealis disseminata is sometimes confused with intravenous leiomyomatosis. However, only subperitoneal surfaces of the uterus and other pelvic and abdominal viscera are involved with leiomyomatosis peritonealis disseminata, and invasion of the lumen of blood vessels does not occur. Only about 15 cases have been reported, according to Pearce. All occurred in patients in the reproductive years who often had large uterine leiomyomata and were usually pregnant or taking oral contraceptives. The condition is likely to be confused with a disseminated intraabdominal malignancy, but it is entirely benign histologically and clinically. Parmley and colleagues have demonstrated the histologic similarities between this peritoneal lesion and the decidual change of the mesothelium in the pelvis, and they propose that the condition represents a benign reparative process in which fibroblasts replace soft peritoneal decidua. They suggest that this fibrotic reaction occurs during pregnancy and especially in the postpartum period, resulting in nodules with a pseudoleiomyomatous pattern. Similar findings have been noted in patients with endometriosis treated with prolonged Enovid therapy. These findings indicate that prolonged and continuous stimulation of subperitoneal decidua by either endogenous or exogenous estrogen and progesterone is important in the pathogenesis of this condition. Parmley and coworkers suggest that the condition is more appropriately called disseminated fibrosing deciduosis. Goldberg and associates, on the other
hand, on the basis of electron microscopy studies, believe that the tumors arise from smooth muscles of small blood vessels. This has been confirmed by Ceccacci and colleagues. It has been possible to show a continuum from fibroblastic cells through myofibroblasts to leiomyocytes. Although the cell of origin of this tumor is still controversial, the tumor is benign, and the acceptable treatment to date is total abdominal hysterectomy and bilateral salpingo-oophorectomy. If this tumor occurs in the omentum, an omentectomy should also be performed to define more clearly the histologic nature of the lesion.
hand, on the basis of electron microscopy studies, believe that the tumors arise from smooth muscles of small blood vessels. This has been confirmed by Ceccacci and colleagues. It has been possible to show a continuum from fibroblastic cells through myofibroblasts to leiomyocytes. Although the cell of origin of this tumor is still controversial, the tumor is benign, and the acceptable treatment to date is total abdominal hysterectomy and bilateral salpingo-oophorectomy. If this tumor occurs in the omentum, an omentectomy should also be performed to define more clearly the histologic nature of the lesion.
In attempting to distinguish between benign and malignant disease in a patient with uterine leiomyomata who also has unusual clinical findings, it is appropriate to keep the entities mentioned earlier (intravenous leiomyomatosis, atypical bizarre leiomyoma, benign metastasizing leiomyoma, and disseminated intraperitoneal leiomyomatosis) in mind. Although they all have features similar to those of malignant disease, they are almost always benign and amenable to treatment. One should also remember that benign uterine leiomyomata have been associated with pseudo-Meigs syndrome in a few cases. Meigs reported five cases in 1954. In these cases, the ascites did not reappear after removal of the uterine leiomyomata.
There is a high frequency of endometrial hyperplasia when the uterus contains leiomyomata. Degligdish and Loewenthal reported that cystic glandular hyperplasia is often found in the endometrium at the margin of the leiomyoma. Yamamoto and coworkers have reported high concentrations of estrone and estrone sulfatase activity in the endometrium overlying a myoma. They suggest that the local hyperestrogenism in the endometrium overlying a leiomyoma may assist in the genesis or enlargement of these tumors.
Gynecologic surgeons are especially concerned about the vascularity of individual leiomyomata and about the blood flow to the uterus in the presence of multiple and sometimes very large leiomyomata. These considerations are pertinent when surgery, especially myomectomy, is contemplated.
According to Vollenhoven and associates, the vascularization of leiomyomata was studied by Vasserman and colleagues, and the findings were presented to the World Congress of Gynecology and Obstetrics in 1988. Using femoral arteriography, selective intraoperative angiography, radiography, and injection of surgical specimens, these investigators showed that leiomyomata have a rich vascular supply, including blood lakes within tumors. They found more than one nutrient vessel per myoma. Venous channels were predominantly peripheral, whereas the arterial supply was both internal and peripheral. Farrer-Brown and coworkers, using radiologic methods, demonstrated that myomata in various locations within the myometrium can cause congestion and dilatation of endometrial venous plexuses by obstructing venous return. These obstructions can result in ectasia of endometrial and myometrial venules (Fig. 31.9). The degree of vascularity of leiomyomata was also studied by Karlsson and Persson. Vascularity varied from many, to few, to no intrinsic vessels demonstrable. Generally, the sum of the width of the uterine arteries increases with the size of the uterus, but the diameter of the two sides sometimes differs markedly. A rich vascularity was found in 22 of 34 uteri with leiomyomata, but with increasing size, there is a tendency to less vascularity. In none of five cases with very large (20 cm or more) leiomyomata uteri was the vascularity rich. The intrinsic vessels were few in two cases and absent in three cases.
CLINICAL FEATURES OF UTERINE LEIOMYOMATA
Asymptomatic Leiomyomata
Most leiomyomata are asymptomatic. Untold numbers of such symptomless leiomyomata are removed surgically by either hysterectomy or myomectomy when they would have been better left undisturbed. The incidence of malignancy in leiomyomata is less than 0.1%, which is lower than the operative mortality rate of hysterectomy in the average hospital; therefore, unless there is some reason to suspect malignant change, the risk of the operation for asymptomatic leiomyomata may exceed the danger of malignancy. A history of rapid growth, however, particularly postmenopausal growth, does indicate removal, even when the tumor produces no symptoms. Signs of rapid enlargement are important in all patients but are even more ominous in older patients. In younger patients, the most common reason for rapid enlargement of a uterus with leiomyomata is pregnancy. If pregnancy can be ruled out, a leiomyosarcoma may be suspected but is rarely found.
Small leiomyomata that are asymptomatic need only to be observed from time to time, with pelvic examinations perhaps every 6 to 12 months and pelvic ultrasonography (US) when indicated. In the beginning, frequent examination may be indicated to determine the growth rate. Such tumors may remain remarkably constant in size for years. If small leiomyomata are discovered late in menstrual life, it is unusual for symptoms to appear or for surgical treatment to be required. Larger tumors can also be watched safely, but if a policy of watchful waiting is adopted, one should be very sure of the nature of the tumors. If there is uncertainty of the uterine or ovarian origin of a tumor, as may well be the case when the tumor fills the whole pelvis or when a pedunculated tumor is felt in the adnexal region (Fig. 31.10), special diagnostic procedures may be indicated. Pelvic examination by an experienced gynecologist can usually clear up the uncertainty. In difficult cases, an examination under anesthesia may be necessary. Laparoscopy may be of great value in determining the nature of an adnexal mass. Before invasive techniques are used, however, noninvasive diagnostic evaluation should be performed. These include radiographic studies of the abdomen and pelvis, US, and computed tomography (CT). The characteristic calcification in a leiomyoma may be seen on radiographs. The US and CT features of uterine leiomyomata have been well described. However, mistakes in the interpretation can still be made. Tada and associates reported that 5% of patients given the diagnosis of uterine leiomyomata by CT actually had an ovarian tumor at operation. Therefore, if uncertainty about the diagnosis persists, laparoscopy or laparotomy should still be performed.
When large asymptomatic leiomyomata occur in premenopausal women who have had their families or in whom future childbearing is not desired, a recommendation for removal may be made. It is impossible to predict which patients will become symptomatic in the remaining years before menopause. However, such tumors, with additional years to grow, are likely to require surgical removal eventually. Therefore, it is better to remove them when the patient is a good operative candidate of relatively low operative risk and when conservation of normal ovaries with a good blood supply can be easily accomplished. Such tumors should usually be 12 to 14 weeks in gestational size or larger. Depending on a variety of factors, either myomectomy or hysterectomy can be recommended to the patient. GnRH agonists may be useful in women approaching menopause to control symptoms or asymptomatic uterine myoma growth until menopause. The regrowth of tumors after the cessation of treatment limits the usefulness of these agents, however. Nakamura and Yoshimura reported their experience with GnRH agonists in the treatment of uterine leiomyomata in perimenopausal women. One third of patients reached menopause after 16 weeks of treatment, thus avoiding the need for surgery.
Reiter and colleagues studied 93 consecutive patients undergoing hysterectomy for leiomyomata. When the uterus was larger than 12 weeks gestational size, there was no increased incidence of surgical complications compared with women with smaller uteri. On the basis of this small series, the authors concluded that hysterectomy need not be recommended to women with large asymptomatic uterine leiomyomata to avoid a possible increased risk of surgical complications.
There is no uniform size of an asymptomatic leiomyomatous uterus that can be used as an indication for hysterectomy or myomectomy. When size is the only significant indication for surgery in an asymptomatic patient, the location of the tumors is more important than the total uterine mass. When the leiomyomata are located in the cornual area or in the lateral wall of the uterus and obscure the anatomy of the adnexa and broad ligament, the risk of error in the early recognition of an ovarian tumor is greater. In such cases, one must carefully weigh the advantages and disadvantages of the conservative approach to the management of uterine leiomyomata. When adnexal tumors are present, it is critical that the origin of these tumors be confirmed. The diagnostic studies mentioned earlier should be performed to establish clearly that the tumors are of uterine origin before a decision is made to follow up the patient rather than operate. It is unacceptable to wait to see whether an adnexal tumor enlarges before identifying the site of origin of the mass as either uterine or ovarian. Ovarian carcinoma remains the most lethal disease of the female reproductive tract and the most difficult to diagnose early. Every diagnostic and therapeutic effort must be made to avoid errors in the clinical evaluation of pelvic neoplasms (Fig. 31.10). In women who are approaching menopause, relatively large uterine leiomyomata can be kept under observation with the knowledge that after menopause, they will not increase in size and may actually regress somewhat. Still, one must be certain
that the entire central pelvic mass is a leiomyomatous uterus. Patient management is largely dependent on knowledge of the exact location and size of leiomyomas. Imaging modalities play an important role in determining patient management, especially when differentiating a benign leiomyoma from other pathologic conditions that may require different therapies.
that the entire central pelvic mass is a leiomyomatous uterus. Patient management is largely dependent on knowledge of the exact location and size of leiomyomas. Imaging modalities play an important role in determining patient management, especially when differentiating a benign leiomyoma from other pathologic conditions that may require different therapies.
Uterine size as an indication for surgical intervention in women with leiomyomata has been thoughtfully discussed by Friedman and Haas. These authors point out that many gynecologists advocate surgical removal of leiomyomata when the uterus reaches 12 weeks gestational size or greater, regardless of the presence or absence of significant symptoms. Historical reasons given for surgical intervention include the following:
The inability to accurately assess the ovaries by examination
The possible malignancy of the pelvic mass
The potential for compromise of adjacent organ function if the mass continues to enlarge
The greater risk of surgical complications if the mass grows to a larger size
The potential for better fertility if myomectomy is performed when the uterus is smaller
The possibility of continued growth of uterine leiomyomata if hormone replacement therapy is given after menopause
Friedman and Haas find very little in the literature to support these indications for surgical intervention and believe the availability of modern high-resolution US and magnetic resonance imaging (MRI) allows for expectant management in many patients with large asymptomatic uterine leiomyomata. They prefer to give primary consideration to the presence and severity of myoma-related symptoms in deciding whether surgical intervention is indicated. We believe that such a course of expectant management is appropriate only when there is relative certainty regarding the benign nature of the central pelvic mass and all of its components and when it is possible to get the patient to return for periodic assessment of gynecologic symptoms and findings on pelvic examination. Repeat MRI may also be required occasionally.
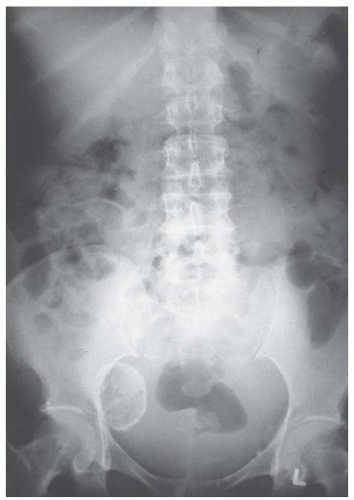
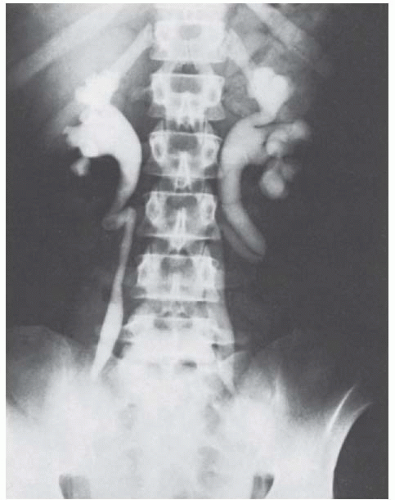
If one elects to observe a patient with a relatively large asymptomatic uterine leiomyoma, it is a good rule to obtain an excretory urogram or renal ultrasound. Everett and Sturgis showed many years ago that ureteral compression at the pelvic brim may occur so that hydroureter and hydronephrosis develop (Fig. 31.11). It is usually the symmetrically enlarged uterus with intramural leiomyomata that extends near or above the umbilicus and rests on the pelvic brim that compresses the ureters, in the same way as a symmetrically enlarged gravid uterus. The process is usually slow and painless even when moderate to severe hydronephrosis has occurred. Pyelographic evidence of kidney damage may be the determining factor in a decision to operate on a patient with an entirely asymptomatic leiomyoma. The irregularly and asymmetrically enlarged uterus with subserous tumors usually does not produce pressure on the ureters.
After menopause, asymptomatic leiomyomata generally should be left undisturbed. Again, the gynecologist must be absolutely certain that an ovarian neoplasm can be ruled out. In the postmenopausal years, shrinkage of myomata and the myometrium occurs. However, the myometrial shrinkage may be disproportionately greater than the myoma shrinkage. Therefore, a myoma in an intramural location before menopause may become a submucous myoma after menopause and then become symptomatic for the first time, usually with postmenopausal bleeding.
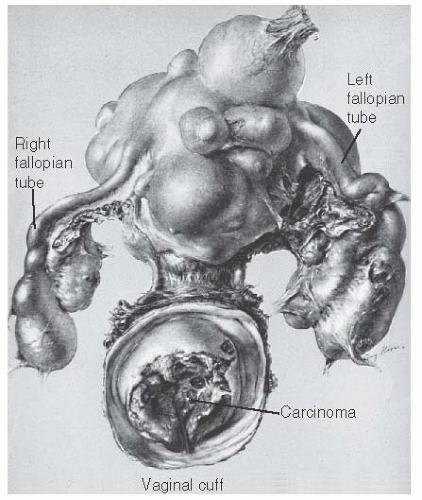
In menopausal women, the appearance of even the slightest trace of vaginal bleeding should make one suspect cervical or endometrial malignancy or the possibility of sarcomatous change in the leiomyoma (Figs. 31.12 and 31.13). Careful pelvic examination, Papanicolaou smear, and evaluation of the cervix by colposcopy or biopsy, pelvic US, fractional curettage, and perhaps hysteroscopy should be done. If the bleeding remains unexplained and the presence of atrophic vaginitis or the use of exogenous estrogens has been excluded, the leiomyomatous uterus should be removed because of the risk of sarcomatous change.
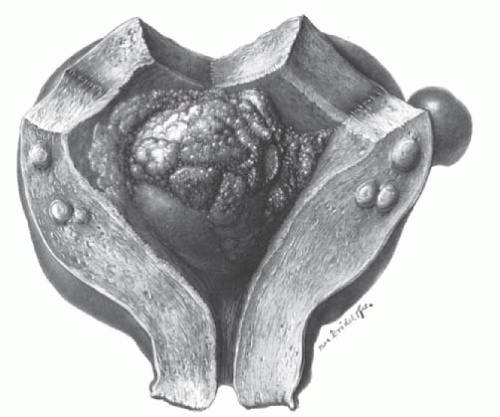 FIGURE 31.12 Adenocarcinoma of the endometrium is present in a symmetrically enlarged leiomyomatous uterus. |
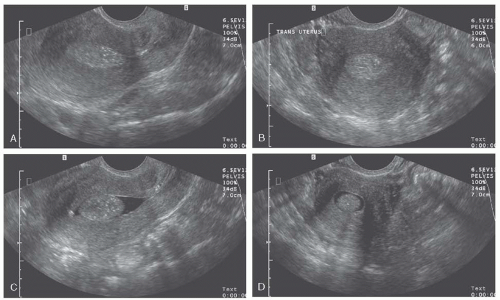
Transabdominal and endovaginal US are the standard imaging modalities for the detection of leiomyomas. Pelvic US by transvaginal and transabdominal techniques is most useful because of good patient tolerance, relatively low cost, availability, and accuracy when performed by well-trained and experienced ultrasonographers (Fig. 31.14). Ultrasonography is the most cost-effective screening mechanism for uterine masses suggestive of myomata. Sonographic criteria for diagnosis have been well described. Generally, abdominal US is unable to detect myomas less than 2 cm in diameter. Transvaginal probes have allowed for improved visualization of both the uterus and adnexa. With higher frequencies, sensitivity in the detection of small myomas has substantially increased. In a series evaluated by Fedele and colleagues using endovaginal ultrasound before hysterectomy, submucous leiomyomas were identified with a sensitivity of 100%. Difficulties may arise, however, if myomas are small or pedunculated, patients are obese, or the uterus is retroverted.
Transvaginal fluid-enhanced vaginal probe sonography (sonohysterography) is a useful technique to assess myomata that distort the endometrial cavity. This technique has little to no complications and is generally well tolerated with only mild cramping described by patients. The limitation of detection of leiomyomas with this modality is 0.5 cm diameter. In a study by Hoetzinger, the majority of intrauterine myomata (14 of 16, or 88%) were detected by sonohysterography. Ultrasound transducing catheters have been suggested as a potential tool to supplement abdominal and endovaginal sonography. Threedimensional data display has recently undergone development and application in sonography; however, the role in evaluation and management of leiomyoma remains unclear (Fig. 31.9).
There is no technique that reliably identifies a leiomyosarcoma. Plain abdominal or pelvic radiographs and hysterosalpingography are older, standard techniques that are still useful in assessing uterine size, calcification in myomata, intrauterine filling defects caused by submucous myomata, and tubal patency. These techniques, combined with US, are the most useful for assessing patients with a central pelvic mass thought to be a leiomyomatous uterus. CA-125 levels may be elevated in women with uterine leiomyomata, but the levels are generally lower than those in patients with ovarian cancer.
Symptomatic Leiomyomata
Less than 50% of patients with uterine leiomyomata have symptoms. Symptoms may be single or multiple and depend on the location, size, and number of tumors present. A clinical and pathologic study of 298 patients with uterine leiomyomata by Persaud and Arjoon revealed no significant relation between the presenting symptoms and the presence of degenerative changes in the tumors. Some form of degeneration was demonstrated in 65% of the specimens, with hyaline degeneration accounting for 63% of all types of degeneration. Hyaline degeneration produces no characteristic symptoms. Symptoms, especially pain and fever, may be present in some patients with red degeneration of a leiomyoma during pregnancy, with torsion and infarction of a subserous pedunculated leiomyoma, or with an infected leiomyoma. A discussion of the signs and symptoms caused by uterine leiomyomata follows.
Abnormal Bleeding
It is surprising but not unusual that even patients with large uterine leiomyomata may have a history of normal menstruation. Such patients should be questioned carefully about recent slight increases in the amount, duration, and frequency of menstruation. Some patients with a history of normal menstruation are found to have iron deficiency anemia from a gradual increase in menstrual blood loss that even the patient has not recognized. If a case of uterine leiomyomata is to be followed, the patient should be asked to monitor her menstrual blood loss carefully and should be given instructions to keep a menstrual calendar and monthly record of the number of pads or tampons used each day. A more objective measurement of the amount of menstrual blood loss using the method of Hallberg and Nilsson may be helpful in doubtful cases. Iron depletion may not be evident by laboratory determination unless one checks serum ferritin levels. In the early months of increased menstrual blood loss, the hemoglobin and hematocrit values are normal. Heavy menstruation does not cause anemia until iron stores are first depleted.
Abnormal bleeding occurs in about one third of patients with symptomatic uterine leiomyomata and commonly indicates that treatment is necessary. The menstrual flow is usually heavy (menorrhagia) but can also occur for prolonged periods
of time at irregular intervals (metrorrhagia). It may also be both heavy and prolonged (menometrorrhagia). Abnormal bleeding may be associated with submucous, intramural, and subserous tumors, but there is a distinct clinical impression that bleeding is more common and more severe in the presence of submucous tumors. The submucous leiomyoma bleeds freely at menstruation and may also bleed between periods as a result of passive congestion, necrosis, and ulceration of the endometrial surface over the tumor and ulceration of the contralateral uterine surface. If the submucous myoma is pedunculated, there is usually a constant, thin, blood-tinged discharge in addition to the menorrhagia. An intramural tumor that is just beginning to encroach on the uterine cavity can also be responsible for menorrhagia. Intramural leiomyomata near the serosal surface and pedunculated subserous tumors can also be associated with abnormal bleeding. When bleeding occurs with such tumors, however, one should search for some other lesions to account for it. The mere presence of leiomyomata in a woman who has abnormal uterine bleeding is not proof that the leiomyomata are causing the bleeding. This fact is important, particularly when there is intermenstrual bleeding. When a patient with leiomyomata has intermenstrual bleeding, it is a rule in our practice to examine and study the cervix carefully with special diagnostic procedures and to sample and evaluate the uterine cavity before we proceed with treatment of the leiomyomata. If an endometrial or cervical malignancy is detected, the treatment of the leiomyomata may need to be altered.
of time at irregular intervals (metrorrhagia). It may also be both heavy and prolonged (menometrorrhagia). Abnormal bleeding may be associated with submucous, intramural, and subserous tumors, but there is a distinct clinical impression that bleeding is more common and more severe in the presence of submucous tumors. The submucous leiomyoma bleeds freely at menstruation and may also bleed between periods as a result of passive congestion, necrosis, and ulceration of the endometrial surface over the tumor and ulceration of the contralateral uterine surface. If the submucous myoma is pedunculated, there is usually a constant, thin, blood-tinged discharge in addition to the menorrhagia. An intramural tumor that is just beginning to encroach on the uterine cavity can also be responsible for menorrhagia. Intramural leiomyomata near the serosal surface and pedunculated subserous tumors can also be associated with abnormal bleeding. When bleeding occurs with such tumors, however, one should search for some other lesions to account for it. The mere presence of leiomyomata in a woman who has abnormal uterine bleeding is not proof that the leiomyomata are causing the bleeding. This fact is important, particularly when there is intermenstrual bleeding. When a patient with leiomyomata has intermenstrual bleeding, it is a rule in our practice to examine and study the cervix carefully with special diagnostic procedures and to sample and evaluate the uterine cavity before we proceed with treatment of the leiomyomata. If an endometrial or cervical malignancy is detected, the treatment of the leiomyomata may need to be altered.
There are several mechanisms by which leiomyomata can cause abnormal bleeding, although a single specific mechanism may not be apparent in a particular patient. According to Sehgal and Haskins, the surface area of the endometrial cavity in a normal uterus is 15 cm2. The surface area of the endometrial cavity in the presence of leiomyomata may exceed 200 cm2. These authors demonstrated a correlation between the severity of the bleeding and the area of endometrial surface. In addition to an increased surface area from which to bleed, the endometrium may demonstrate local hyperestrogenism in areas immediately adjacent to submucous tumors, and endometrial hyperplasia and endometrial polyps are commonly found. Degligdish and Loewenthal noted a broad spectrum of histologic abnormalities in the endometrium associated with leiomyomata, ranging from atrophy to hyperplasia. Thinning and ulceration of the endometrial surface may be present over large submucous tumors; smaller ones may show slight thinning without ulceration. The presence of leiomyomata may interfere with myometrial contractility as well as contractility of the spiral arterioles in the basalis portion of the endometrium. Miller and Ludovici suggested that anovulation and dysfunctional uterine bleeding are more common in the presence of uterine leiomyomata.
Sampson in 1913 was the first to study the blood supply of uterine leiomyomata and its effect on uterine bleeding. More recent studies have been performed by Faulkner and by Farrer-Brown and associates. The most prominent and important change is the presence of endometrial venule ectasia. Tumors that are strategically located in the myometrium may cause obstruction and proximal congestion of veins in the myometrium and endometrium. Thrombosis and sloughing of these large dilated venous channels within the endometrium produce heavy bleeding (Fig. 31.15).
Makarainen and Ylikorkala have presented evidence that further supports the concept that prostanoids play a role in primary menorrhagia. They found that the production of 6-keto-prostaglandin F1 alpha (6-keto-PGF1α), a metabolite of prostacyclin (PGI2), and thromboxane B2 (TXB2), a metabolite of thromboxane A2 (TXA2), was normal in menorrhagic endometrium. However, the balance between TXA2 and PGI2 shifted to a relative TXA2 deficiency and was negatively related to blood loss in patients with menorrhagia. Although ibuprofen decreased the blood loss in patients with primary menorrhagia, it failed to reduce myoma-associated menorrhagia. The authors suggest that uterine factors other than prostanoids are more important in causing menorrhagia associated with uterine leiomyomata.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree