Leiomyomata
Arthur F. Haney
Uterine leiomyomata, commonly termed fibroids, are by far the most common benign tumors of the female genital tract and likely are the most common soft tissue tumors of all. Approximately 200,000 hysterectomies and 20,000 myomectomies are performed annually in the United States because of symptoms caused by leiomyomata. The symptoms are location dependent: very large leiomyomata may have few if any symptoms, and alternatively, small leiomyomata may cause life-threatening uterine bleeding and disabling dysmenorrhea. The incidence of leiomyomata far exceeds the frequency of clinical problems, with as many as 50% of women having identifiable fibroids at menopause.
Clinical Presentation
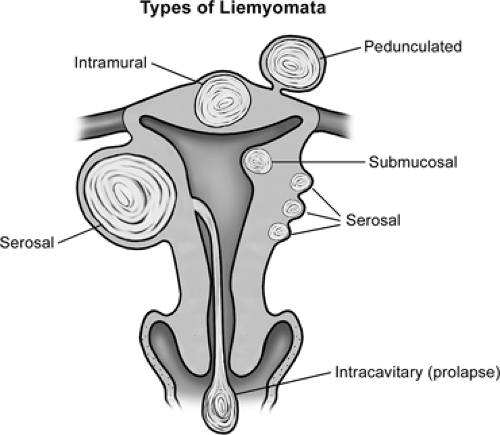
Leiomyomata come to clinical attention for a variety of symptoms (Table 55.1) depending on tumor size and location (Fig. 55.1). It is not unusual for fibroids to become symptomatic before age 30, but all occur after puberty. Excessive menstrual bleeding (menorrhagia) is the most frequent symptom. Fibroids may cause debilitating dysmenorrhea as well as compress the adjacent pelvic viscera (frequent urination, constipation, or hydronephrosis) and cause pelvic pain with physical activities or intercourse. The symptoms caused by leiomyomata vary depending on the size, number, and location of the tumors. Irregular vaginal bleeding (oligomenorrhea), regardless of the amount, or intermenstrual bleeding (metrorrhagia) does not suggest fibroids but rather an underlying endocrine abnormality (e.g., anovulation). Furthermore, the typical scenario encountered with fibroids is not a sudden heavy bleeding episode but rather gradually increasing menstrual bleeding, paralleling tumor growth. Leiomyomata may undergo rapid enlargement during pregnancy, outstripping their blood supply and resulting in central avascular necrosis, the so-called red degeneration. The pain may be severe, requiring hospitalization and narcotics, but rarely puts a pregnancy at risk. As the size and number of the leiomyomata increase, the adjacent pelvic viscera may be compressed, resulting in urinary frequency, constipation, and occasionally dyspareunia. Rarely, when the fibroids are large and fill the pelvis or grow laterally from the midportion of the uterine body, they compress the ureters, causing hydronephrosis (Fig. 55.2). Intracavitary fibroids often are on a vascular pedicle and may be even be extruded through the cervix, presenting as a necrotic mass. Rarely, large uterine leiomyomata will become incarcerated in the pelvis when rapidly expanding tumors are entrapped by the promontory of the sacrum, causing the cervix to descend and occasionally present at the introitus. This is particularly true when a woman with large serosal leiomyomata conceives and both the tumors as well as the gravid uterus enlarge rapidly. While fibroids can cause symptoms at any age after puberty, they typically do so in the early to mid 30s. With the rising age at which women first attempt pregnancy, this increasingly represents a difficult management problem that did not exist a generation ago when simple hysterectomy was the frequent curative choice. Preserving reproductive function while relieving symptoms is the most pressing current challenge.
Anatomic Features
Leiomyomata are benign, sex steroid–responsive, smooth muscle tumors of the uterus originating as clonal expansions of individual myometrial cells. The histology is virtually indistinguishable from normal myometrium except for a discrete circular whorling pattern with the cellularity and mitotic activity being highly variable. The number of mitoses per high powered field is usually low (<5 to 8 per high-power field), with mitotic activity utilized to predict the risk of malignancy. Leiomyosarcomas do not arise from preexisting leiomyomata and present much later in life, well after menopause. There are often areas of fibrosis interspersed with the smooth muscle and occasional calcification,
especially after pregnancy-induced degeneration and in postmenopausal women. Leiomyomata typically grow in a spherical or nodular fashion with a relatively distinct demarcation from the surrounding normal myometrium, reflecting their clonal origin.
especially after pregnancy-induced degeneration and in postmenopausal women. Leiomyomata typically grow in a spherical or nodular fashion with a relatively distinct demarcation from the surrounding normal myometrium, reflecting their clonal origin.
TABLE 55.1 Symptoms of Leiomyomata | |
|---|---|
|
Leiomyomata can arise from cells located anywhere in the myometrium (Fig. 55.1), with women often having very large numbers of fibroids. When the cell of origin is near the serosal surface, the path of least resistance to expansion is to grow outward into the peritoneal cavity, termed a serosal or subserosal fibroid. Serosal tumors can grow very large with few or minimal symptoms, as they do not cause bleeding. Occasionally, a pedunculated fibroid will result with a pedicle narrower than the tumor diameter that contains the vascular supply. These may become detached from the uterus completely and reestablish blood supply from an adjacent organ. This is likely the result of pressure necrosis of the interface between the tumor and the adjacent viscera or torsion of the pedicle and attachment at the new site during healing. When the myometrial cell of origin is within the myometrium, this forms an intramural fibroid. These are associated with menorrhagia and dysmenorrhea, with failure to constrict the vessels supplying the endometrium during menses. If the myometrial cell of origin is near the endometrium, the tumor will find the path of least resistance to growth toward the endometrium, and a submucosal fibroid will result. These are most frequently associated with menorrhagia and dysmenorrhea, even when of relatively small size. If the fibroid originates from a cell immediately adjacent to the endometrial layer, the tumor may completely protrude into the endometrial cavity, and an intracavitary fibroid on a pedicle may develop. These intracavitary tumors can cause the most severe symptoms despite their small size. With uterine contractions increasing the intracavitary pressure, the stalk may elongate, extruding the fibroid through the cervix, typically associated with a sanguineous vaginal discharge and erosion of the surface of the fibroid. Aseptic necrosis may be present, and the degenerating tumor becomes secondarily infected, often making it difficult to distinguish from a necrotic cervical cancer. While cervical fibroids can occur, they are very infrequent, paralleling the small number of myometrial cells within the cervix. There is virtually no neovascularity within fibroids, and they derive their vascular supply from the vessels on the periphery of the tumor at the interface with the normally vascularized myometrium. While fibroids are stimulated to grow by sex steroids but the vascular supply is not, this is the limiting factor for the growth of individual tumors. Once the uterine vasculature
is maximally enlarged, the fibroids will cease growing and the center may even undergo necrosis, limiting their ultimate size. This is initially described as “red degeneration” and later, as the devitalized central tissue is replaced by fibrosis, described as “hyalinized degeneration” and may ultimately become calcified. It is not unusual to find marked calcium deposits in leiomyomata that have long ago undergone degeneration and were never symptomatic. Collateral vascular channels are comparably maximally engorged and may represent a surgical challenge. Not surprisingly, the blood loss with any extirpative surgery may be large, even if performed by experienced surgeons.
is maximally enlarged, the fibroids will cease growing and the center may even undergo necrosis, limiting their ultimate size. This is initially described as “red degeneration” and later, as the devitalized central tissue is replaced by fibrosis, described as “hyalinized degeneration” and may ultimately become calcified. It is not unusual to find marked calcium deposits in leiomyomata that have long ago undergone degeneration and were never symptomatic. Collateral vascular channels are comparably maximally engorged and may represent a surgical challenge. Not surprisingly, the blood loss with any extirpative surgery may be large, even if performed by experienced surgeons.
Influence of Sex Steroids
There is little doubt that the growth of leiomyomata is dependent on sex steroids as they: (a) are not noted prior to puberty, (b) typically regress after menopause, (c) possess sex steroid receptors (estrogen and progesterone), (d) often dramatically enlarge during pregnancy when estrogen and progesterone levels are very high, and (e) can be made to shrink with medically induced hypogonadism. Clearly, the uterus, like other secondary sex organs, initially develops to its adult size with exposure to the levels of ovarian steroids produced at puberty. However, this growth is programmed and should cease once reaching the appropriate development, despite continued exposure to sex steroids throughout the reproductive life span.
There is no evidence that higher or aberrant patterns of ovarian steroid secretion of estrogens, progestins, or androgens contribute to the development of leiomyomata. However, myomatous tissue has the same number of estrogen receptors but a higher number of progesterone receptors than the adjacent normal myometrium. This coupled with the observations that mitoses within myomas are more frequent in the luteal phase of the cycle and that progesterone up-regulates several growth factors suggests that progesterone is somehow causally involved in either myomatous development or continued growth. Since estrogen stimulates the synthesis of progesterone receptors in other reproductive tissues, a more complex relationship between the two dominant female sex steroids and leiomyomata is likely. Despite these observations, there is no consensus as to the specific roles of the various sex steroids aside from being necessary but not sufficient to develop leiomyomata. Situations that increase lifetime exposure to estrogen such as obesity and early menarche are associated with increased risk with the interval from the last delivery inversely related to risk.
The use of oral contraceptives has not been demonstrated to impact the likelihood of developing or enhancing the growth of fibroids. Treatment of postmenopausal women with hormonal replacement therapy may allow continued growth of previously existent but quiescent leiomyomata. There is no evidence to suggest that if leiomyomata are not present they will develop in response to hormone replacement therapy after menopause. In clinical decision making, there is no rationale for withholding hormone replacement therapy for fear of stimulating leiomyomata in otherwise appropriate candidates for postmenopausal hormonal replacement. Special note should be made of postmenopausal women with breast cancer treated with tamoxifen, as this compound has mixed estrogen agonist and antagonist properties and has the potential to influence the pattern of fibroid growth. Undoubtedly, gonadal steroids are important in the growth of leiomyomata.
Genetic Inheritance Pattern
It is estimated that more than 40% of first-degree female relatives of women with leiomyomata will develop fibroids sometime during their lifetime. These will not necessarily be symptomatic, and the number and location are not predictable. While leiomyomata are common in all races, black women appear to have a somewhat higher incidence than women of other ethnicities, despite being a common disease in all ethnicities. Black women who are undergoing hysterectomies have increased numbers of fibroids that are larger in size. Leiomyomata are by far the commonest genital tract tumors for all women and remain the most frequent indication for gynecologic surgery. This familial pattern seems most consistent with a multifactorial genetic inheritance pattern, which is modified by confounding cofactors such as the impact of gonadal steroids. Aside from noting that the disease has apparent familial, age, and ethnic associations, there is little clinical predictability for an individual woman.
Molecular Mechanisms and Genetic Dysregulation
Leiomyomas represent monoclonal neoplasms, a situation in which etiologic genetic mutations in individual tumors are likely. Cytogenetic studies of individual leiomyomas reveal that approximately one third have some type of chromosomal aberration, but these are not consistent between individual tumors in the same woman, further supporting their clonal nature. The most common aberrant patterns are translocations between chromosomes 12 and 14, deletions of the short arm of chromosome 7, and rearrangements of the long arm of chromosome 6. It is not clear whether there is clinical relevance to these differences in terms of the rate of tumor growth, recurrence rates, and responses to the various therapies. Interestingly, when tumors have translocations between chromosomes 12 and 14, they are more likely to be larger myomas, whereas deletions of the long arm of chromosome 7 are found more often in smaller tumors. While there are no consistent alterations in gene expression noted in leiomyomata, the transcription factor high-motility group A2 is up-regulated
in leiomyomata with expressing the 12;14 chromosome translocation. These observations imply that despite similar histologic appearance and benign growth characteristics, there may be several molecular mechanisms by which these tumors develop.
in leiomyomata with expressing the 12;14 chromosome translocation. These observations imply that despite similar histologic appearance and benign growth characteristics, there may be several molecular mechanisms by which these tumors develop.
Undoubtedly, individual myometrial cells become neoplastic as a result of a complex interaction between ethnicity, genetic mutations, endogenous sex steroids, and reproductive patterns. Molecular geneticists have noted abnormal expression of a variety of genes leading to altered growth factors and steroid receptors in individual leiomyomata, but there remains no single predominant molecular mechanism or group of mechanisms underlying their development and growth. In the coming years, this molecular puzzle will undoubtedly be better understood but will just as likely prove to be very complex without a single abnormality identified.
Impact of Leiomyomata on Reproduction
The impact of leiomyomata on pregnancy remains difficult to define for individual patients (Table 55.2). Clearly, the most logical approach to achieving pregnancy is for a woman with leiomyomata to attempt to conceive and elect treatment only if difficulty is encountered. There is general agreement that intracavitary and submucosal leiomyomata can be causally related to infertility on the basis of implantation failure. The presence of fibroids within the endometrial cavity or impinging on the cavity contour can be detected by a hysterosalpingogram, sonohysterography, or hysteroscopy, and their removal should improve fertility. With intramural leiomyomata, a relationship to infertility is less certain, and all other potential etiologies should be considered before considering leiomyomata etiology. Intramural leiomyomata have been associated with a reduced pregnancy rate following assisted reproductive technology (ART), suggesting that they may have an impact on implantation. Given the time and expense of ART, it is prudent to remove intramural fibroids in selected infertile women prior to undergoing ART. Postoperative adhesions that may form after myomectomy are of lesser importance when undergoing ART, as they will not affect oocyte retrieval. While fibroids can be found anywhere in the myometrium, when they arise adjacent to the tubal ostia, they may occlude a tube or impede its function. For this to be seriously considered a problem related to infertility, however, both fallopian tubes would need to be affected, which is an infrequent circumstance.
TABLE 55.2 Mechanisms of Infertility with Leiomyomata | |
|---|---|
|
Whether leiomyomata are associated with a higher risk of first-trimester pregnancy loss, preterm labor, or intrauterine growth restriction is much more controversial. The impact of fibroids in individual patients is critically dependent on location, not simply size or number. Clearly, many women with large myomatous uteri deliver infants without difficulty, whereas in others, fibroids may compromise the ability of the endometrial cavity to accommodate a growing fetus or the maternal vascular adaptation necessary for normal placental function. Complicating this picture is our inability to predict which women with leiomyomata will experience rapid enlargement of their fibroids during pregnancy. An abruption can occasionally occur when the placental bed overlies an enlarging fibroid. Lower-segment fibroids have the potential to obstruct labor, and a classic cesarean delivery is occasionally required when the presenting part is unable to be directly applied to the cervix. If the leiomyomata are extremely large and intramural in location, preconception removal may be considered, but the potential benefit must be carefully weighed against the complications of the procedure. Clinical judgment will be sorely taxed to make correct decisions in the absence of a previous adverse clinical outcome. Overall, term delivery rates following myomectomy for symptomatic leiomyomata in an unselected patient population vary from 40% to 50%. The need to also perform a cesarean following myomectomy needs to be considered in any risk–benefit analysis.
Leiomyosarcoma
Rarely, a leiomyosarcoma is encountered instead of leiomyomata, but these are typically in older postmenopausal women with an average age well over 60. The diagnosis is often suggested by enlargement of the uterus after menopause in the absence of any hormone replacement therapy. Uterine fibroids known to be present prior to menopause may enlarge when the gonadal steroids are replaced, but this does not represent a frequent problem.
While leiomyomata are not thought to transform into malignant smooth muscle tumors, a small proportion of women with fibroids have the same pattern of chromosome deletions and transcriptional profiles that are observed in leiomyosarcomas. However, clinical transformation has not documented this, so it remains an interesting but not clinically relevant observation. Occasionally, lung metastasis of histologically benign-appearing smooth muscle is observed. These can be from the so-called benign metastasizing leiomyomata or another condition called lymphangioleiomyomatosis, characterized by the
proliferation of leiomyoma-like cells in the lungs that have been observed. These are extremely rare and should not alter the recommendations to women with typical uterine leiomyomata.
proliferation of leiomyoma-like cells in the lungs that have been observed. These are extremely rare and should not alter the recommendations to women with typical uterine leiomyomata.
Rapid growth alone is not indicative of malignancy, as this is a common occurrence in premenopausal women. No physical findings or unique imaging characteristics can reliably distinguish leiomyomata from leiomyosarcoma. Leiomyosarcoma are diagnosed by histology and not gross appearance, as degenerating leiomyomata may have unusual and varied features, including necrosis and central liquefaction. The histologic changes suggesting malignancy include increased numbers of mitoses, cellular pleomorphism, and thrombotic degeneration within the tumor. Many fibroids are very cellular but without other characteristics suggestive of malignant potential. Typically, >10 mitoses per high-power field suggests a risk that the tumor is malignant, with between 5 and 10 representing an actively growing fibroid and <5 more typical for leiomyoma. Because of the difficulty in accurately characterizing the number of mitoses by frozen section microscopy, the intraoperative diagnosis of a leiomyosarcoma is difficult to make with confidence and permanent sections are required. Leiomyosarcomas are estimated to comprise 0.1% of all uterine tumors, and 1.7% of women undergoing hysterectomy for leiomyomata are in their seventh decade of life.
Diagnostic Studies
The majority of leiomyomata are detected on pelvic examination performed because of gynecologic symptoms. The uterus is typically noted to be enlarged and irregular on bimanual examination. It is important to distinguish leiomyomata from other pelvic masses, and it may be difficult to do so in the presence of a large uterus. This is most easily done with an endovaginal or abdominal ultrasound scan, as the leiomyomata appear echogenic with similar acoustic impedance to the normal myometrium. Computerized tomography and magnetic resonance imaging (MRI) may prove useful in selected circumstances (Table 55.3, Fig. 55.2), but they are much more expensive and yield little more useful information than office sonography.
The proximity of the leiomyomata to the endometrial cavity can usually be demonstrated by taking advantage of the acoustic differences between normal myometrium, fibroid tumors, and the endometrial cavity. The endometrial stripe is a reliable marker of the endometrial cavity, and finding a smooth, continuous endometrial stripe with normal underlying myometrium between the cavity and any fibroids suggests that they are not submucosal. Simultaneously injecting saline into the endometrial cavity while performing an endovaginal ultrasound examination (sonohysterography) improves the ability to delineate submucous and intracavitary leiomyomata. However, it is not possible to distinguish an endometrial polyp from an intracavitary myoma by virtually any imaging technique.
TABLE 55.3 Diagnostic Imaging Techniques | |
|---|---|
|
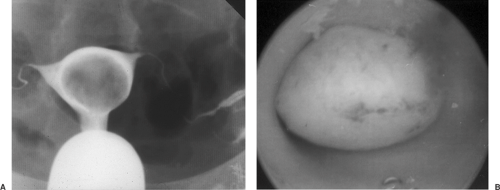
The closer the fibroid is to the endometrial cavity, the greater the likelihood and severity of dysmenorrhea and menorrhagia. Additionally, distortion of the endometrial cavity increases the probability that difficulty in achieving and maintaining a pregnancy will be encountered. Hysterosalpingography is often undertaken if infertility is present concurrently, as this technique can identify intracavitary tumors or a large but otherwise normal endometrial cavity caused by the stretching the normal myometrium around leiomyomata (Fig. 55.3). This radiographic technique has the added advantage of determining tubal patency as well. Increasingly, office hysteroscopy is being used when tubal patency is not an issue, as this technique allows clear differentiation between leiomyomata and other intracavitary pathology such as endometrial adhesions, uterine septae, and endometrial polyps.
Adenomyosis, which is another disease involving the myometrium, can occasionally be difficult to distinguish clinically from leiomyomata, and imaging studies may not be helpful. This is a process wherein functional endometrial glands and stroma infiltrate the myometrium. Frequently, a marked fibrotic reaction is present around the nests of endometrial cells, presumably because of irritation caused by menstrual shedding. When the process is localized, these fibrotic areas may be difficult to distinguish from leiomyomata and the true diagnosis only made at surgery. Leiomyomata typically have a clear demarcation from the underlying myometrium whereas adenomyosis has a very indistinct infiltrating border, making complete surgical excision problematic. MRI has been reported to be useful in differentiating adenomyosis from leiomyomata, as it better delineates the borders of the intramyometrial pathology, but this is not routinely utilized preoperatively because of the expense involved. Like endometriosis, adenomyosis may be associated with an elevation in serum CA125, but this is a very nonspecific serum marker.
Preventing Development, Progression, and Recurrence
The ultimate goal of understanding the pathophysiology of leiomyomata is to prevent their occurrence in both clearly genetically susceptible women as well as women without a familial history. With further insight regarding the
molecular mechanism(s) of growth, it may be possible to identify medicinal approaches that will interfere with the molecular pathway(s) and reduce the risk of development, progression, and recurrence of leiomyomata. This will be particularly useful for women at risk for development of leiomyomata because of a strong familial history, those with previous myomectomy, and women with leiomyomata approaching menopause who can anticipate spontaneous regression once ovarian function ceases. There are few, if any, predictors of the development of leiomyomata aside from a family history. Endocrine markers such as early puberty, late menopause, parity, oral contraceptive use, and hormone replacement therapy have not been correlated with the recurrence of fibroids after myomectomy.
molecular mechanism(s) of growth, it may be possible to identify medicinal approaches that will interfere with the molecular pathway(s) and reduce the risk of development, progression, and recurrence of leiomyomata. This will be particularly useful for women at risk for development of leiomyomata because of a strong familial history, those with previous myomectomy, and women with leiomyomata approaching menopause who can anticipate spontaneous regression once ovarian function ceases. There are few, if any, predictors of the development of leiomyomata aside from a family history. Endocrine markers such as early puberty, late menopause, parity, oral contraceptive use, and hormone replacement therapy have not been correlated with the recurrence of fibroids after myomectomy.