Background
Whereas advances in minimally invasive surgery have made laparoscopic staging technically feasible in stage I epithelial ovarian cancer, the practice remains controversial because of an absence of randomized trials and lack of high-quality observational studies demonstrating equivalent outcomes.
Objective
This study seeks to evaluate the association of laparoscopic staging with survival among women with clinical stage I epithelial ovarian cancer.
Study Design
We used the National Cancer Data Base to identify all women who underwent surgical staging for clinical stage I epithelial ovarian cancer diagnosed from 2010 through 2012. The exposure of interest was planned surgical approach (laparoscopy vs laparotomy), and the primary outcome was overall survival. The primary analysis was based on an intention to treat: all women whose procedures were initiated laparoscopically were categorized as having had a planned laparoscopic procedure, regardless of subsequent conversion to laparotomy. We used propensity methods to match patients who underwent planned laparoscopic staging with similar patients who underwent planned laparotomy based on observed characteristics. We compared survival among the matched cohorts using the Kaplan-Meier method and Cox regression. We compared the extent of lymphadenectomy using the Wilcoxon rank-sum test.
Results
Among 4798 eligible patients, 1112 (23.2%) underwent procedures that were initiated laparoscopically, of which 190 (17%) were converted to laparotomy. Women who underwent planned laparoscopy were more frequently white, privately insured, from wealthier ZIP codes, received care in community cancer centers, and had smaller tumors that were more frequently of serous and less often of mucinous histology than those who underwent staging via planned laparotomy. After propensity score matching, time to death did not differ between patients undergoing planned laparoscopic vs open staging (hazard ratio, 0.77, 95% confidence interval, 0.54–1.09; P = .13). Planned laparoscopic staging was associated with a slightly higher median lymph node count (14 vs 12, P = .005). Planned laparoscopic staging was not associated with time to death after adjustment for receipt of adjuvant chemotherapy, histological type and grade, and pathological stage (hazard ratio, 0.82, 95% confidence interval, 0.57–1.16).
Conclusion
Surgical staging via planned laparoscopy vs laparotomy was not associated with worse survival in women with apparent stage I epithelial ovarian cancer.
Ovarian cancer is the fifth leading cause of cancer-associated death among women. Although most patients with epithelial ovarian cancer present with advanced-stage disease, patients with early-stage ovarian cancer have a good prognosis; the 5 year survival for patients with surgically evaluated stage I disease is approximately 90%. Patients with ovarian cancer that appears confined to the ovary require surgical staging, a procedure traditionally performed via exploratory laparotomy, which includes salpingo-oophorectomy, pelvic and paraaortic lymph node dissection, omentectomy, peritoneal washings, and peritoneal biopsies. Hysterectomy and removal of apparently uninvolved adnexa are also recommended for patients not seeking future fertility.
Laparoscopic surgery has been proposed as an alternative to laparotomy for surgical staging and the treatment of apparently early stage ovarian cancer. Compared with laparotomy, minimally invasive surgery is associated with fewer complications and a shorter recovery period and has been adopted in early-stage endometrial cancer. Although advances in minimally invasive surgery have made laparoscopic staging technically feasible in stage I epithelial ovarian cancer, the practice remains controversial because of an absence of randomized trials and lack of high-quality observational studies demonstrating equivalent outcomes.
Most published studies are small and many lack comparison groups, whereas others do not control for possible confounding. A recent systematic review for the Cochrane Library concluded that a paucity of high-quality evidence precluded the assessment of the safety of laparoscopic staging in stage I ovarian cancer.
The present study uses a national tumor registry database to compare outcomes between women who underwent laparoscopic staging and those who underwent staging via laparotomy for apparent stage I epithelial ovarian cancer.
Materials and Methods
We used data from the National Cancer Data Base, a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, which aggregates tumor registry data from more than 1500 hospitals and includes 70% of all incident cancer diagnosis in the United States. The National Cancer Data Base includes information about patient demographics, tumor characteristics, cancer-directed therapies (surgery, radiation, and chemotherapy), treating facility, and overall survival. This investigation was exempt from institutional review board oversight.
The study cohort flow diagram is illustrated in Figure 1 . We identified all patients diagnosed with epithelial ovarian cancer from 2010 to 2012 in the 2013 National Cancer Data Base public use data file. We defined women who underwent surgery for apparent stage I ovarian cancer as those with American Joint Committee on Cancer, seventh edition, clinical stage I, IA, IB, or IC. We also included patients with missing American Joint Committee on Cancer clinical stage who were American Joint Committee on Cancer pathological stage I, IA, IB, or IC.
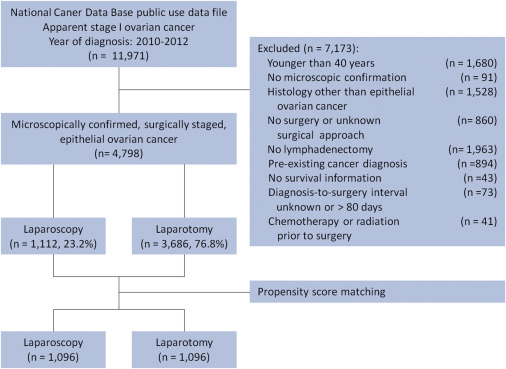
We excluded women with cancer diagnoses that were not microscopically confirmed and those with histological codes that did not correspond to serous, mucinous, clear cell, endometrioid, or other adenocarcinoma of the ovary ( Appendix Table 1 ). Because the National Cancer Data Base suppresses facility type for women younger than 40 years, precluding adjustment for this variable, these women were excluded. We also excluded women who did not undergo lymphadenectomy, had surgery more than 80 days after diagnosis, had missing data on surgical approach, received chemotherapy or radiation prior to surgery, or had a cancer diagnosis in addition to ovarian cancer.
The exposure of interest was a planned surgical approach. In the primary analysis, we considered all subjects whose staging procedure was initiated with a minimally invasive approach (laparoscopy or robot-assisted laparoscopy) as having undergone a planned laparoscopic staging procedure, irrespective of whether the procedure was completed laparoscopically or via laparotomy.
The primary outcome was overall survival defined as months from cancer diagnosis to death or the date of last contact as recorded by the cancer registry. Secondary outcomes included cumulative survival at 3 and 4 years after diagnosis and frequency of death within 90 days of surgery. We also compared the frequency of unplanned readmission within 30 days of surgical discharge, length of stay after surgery, and pathological lymph node count.
Control variables included patient age at the time of diagnosis (categorized as 40–49, 50–59, 60–69, 70–79, and 80 years or older), race-ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or other), tumor histology ( Appendix Table 1 ), and tumor grade. Receipt of any adjuvant chemotherapy was categorized as yes, no, or unknown. We grouped tumors as smaller than 1 cm, 1.0–4.9 cm, 5.0–9.9 cm, and 10 cm or lager in tables and by 1 cm increments for the calculation of propensity scores. Extent of comorbidity was categorized as 0, 1, or more than 1 comorbidity, using the Deyo adaptation of the Charlson comorbidity index.
The location of the treating facility was categorized by US Census region (Northeast, South, Midwest, and West). We identified rural residence based on ZIP code when the US Department of Agriculture classified the county as “completely rural or less than 2500 urban population, not adjacent to a metro area.”
The median household income, as estimated by the 2012 American Community Survey, in the subject’s ZIP code of residence was used as a proxy for subjects’ income and was categorized according to quartiles across all US ZIP codes. Insurance status was categorized as uninsured, privately insured, insured by Medicare or having another type of government insurance (military or Medicaid). The treating facility was categorized according to the Commission on Cancer accreditation program as a community cancer program, comprehensive community cancer program, academic/research program, integrated network cancer program, or other.
Analyses
We compared the frequency of 30 day readmission, death within 90 days of surgery, lymph node count, and postoperative length of stay using the Wilcoxon rank-sum and χ 2 tests. We used propensity score matching to create a cohort in which subjects who underwent planned laparoscopy and laparotomy were balanced on measured covariates that might confound the effect of planned surgical approach on overall survival.
We estimated the propensity to undergo planned laparoscopic staging with a logistic regression model with variables selected a priori based on their potential to influence the likelihood of a subject undergoing laparoscopic surgery. The independent variables included age, race-ethnicity, tumor size, treating facility type, insurance status, income, number of comorbidities, census region, rural status, and year of diagnosis.
We used a 1:1 optimal matching algorithm with caliper set to 0.2 SD of the logit of the propensity score to identify matches. We evaluated the success of propensity score matching using absolute standardized differences, with dummy variables used in the case of nominal/ordinal covariates, considering covariates well balanced when the absolute standardized difference was less than 10% ( Appendix Figure ).
We plotted survival functions for women who underwent planned laparoscopic staging and those who underwent planned staging laparotomy in the propensity score matched cohort using the Kaplan-Meier method and compared these using the log rank test. We estimated the hazard ratio for overall mortality after planned laparoscopic staging using a Cox proportional hazard model. We also estimated survival rates and associated 95% confidence intervals, at 3 and 4 years after diagnosis, and compared these with the Z-test for independent proportions.
To adjust for potential confounding introduced by factors that may have been evident only after surgical staging, we also calculated the hazard ratio for overall mortality after planned laparoscopic staging using a Cox proportional hazard model that included pathological stage, receipt of adjuvant chemotherapy, tumor grade, and histological type as covariates.
Sensitivity analyses
To evaluate whether the effect of planned laparoscopy was sensitive to receipt of adjuvant chemotherapy, we stratified the propensity-matched cohort by chemotherapy status and repeated the primary survival analysis in each group. To confirm that our findings persisted among patients whose procedures were planned and completed laparoscopically, we repeated all analyses after excluding women whose procedures were initiated laparoscopically but completed by laparotomy.
Because propensity score matching and Cox regression control only for observed characteristics, we investigated the robustness of the estimated treatment effect to the presence of potential unmeasured confounders. We considered the possibility that 2 unobserved confounders, elevated serum CA-125, and suboptimal surgical staging were associated with both the planned surgical approach and inferior survival. We calculated the hazard ratio (with associated 95% confidence interval) for death after planned laparoscopic staging (relative to laparotomy) after adjustment for the presence of each potential confounder.
Using published data, we estimated that the preoperative CA-125 level of ≥30 U/mL was associated with an increased risk of death (hazard ratio, 1.65) and that undergoing suboptimal staging was associated with an increased risk of death (hazard ratio, 2.31) among patients who did not receive chemotherapy but had no effect among those who did.
We considered a range of scenarios, with the assumptions that elevated CA-125 was more prevalent among patients who underwent laparotomy and that suboptimal staging was more prevalent among subjects who underwent laparoscopy (see Appendix for details).
All statistical analysis was performed using R 3.0.3 ( R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 4798 patients who met study criteria, 1112 (23.2%) underwent planned laparoscopic surgery for clinical stage I epithelial ovarian cancer ( Figure 1 ). Of these, 190 (17.1%) had conversion to laparotomy. Among the 922 patients whose staging surgery was completed laparoscopically, 367 (39.4%) underwent robotic-assisted laparoscopy. Patients who underwent planned laparoscopic surgery were more likely to be white, receive treatment in nonacademic facilities, live in higher-income areas, reside in the Northeast or South, have private insurance, and have fewer comorbidities than patients who underwent laparotomy ( Table 1 ). Laparoscopically staged patients had smaller tumors that were more frequently serous, and less frequently mucinous, than women who underwent open surgery ( Table 1 ).
| Characteristic | All subjects (n = 4798) | Propensity score–matched cohort a (n = 2192) | ||||
|---|---|---|---|---|---|---|
| Laparoscopy (n = 1112) | Laparotomy (n = 3686) | P value | Laparoscopy (n = 1096) | Laparotomy (n = 1096) | P value | |
| Median age at diagnosis, y (interquartile range) | 56.5 (49–65) | 57 (50–64) | .68 b | 57 (49–65) | 57 (50–65) | .38 b |
| Race/ethnicity | < .001 d | .98 d | ||||
| Black | 44 (4.0) c | 207 (5.6) | 44 (4.0) | 45 (4.1) | ||
| Hispanic | 37 (3.3) | 204 (5.5) | 37 (3.4) | 38 (3.5) | ||
| White | 960 (86.3) | 2984 (81.0) | 944 (86.1) | 949 (86.6) | ||
| Other | 59 (5.3) | 253 (6.9) | 59 (5.4) | 53 (4.8) | ||
| Unknown | 12 (1.1) | 38 (1.0) | 12 (1.1) | 13 (1.2) | ||
| Facility type | .01 d | .59 d | ||||
| Academic/research program | 470 (42.3) | 1773 (48.1) | 469 (42.8) | 451 (41.1) | ||
| Community cancer program | 45 (4.0) | 115 (3.1) | 43 (3.9) | 36 (3.3) | ||
| Comprehensive community cancer program | 502 (45.1) | 1479 (40.1) | 491 (44.8) | 497 (45.3) | ||
| Integrated network cancer program | 92 (8.3) | 310 (8.4) | 91 (8.3) | 109 (9.9) | ||
| Other | 3 (0.3) | 9 (0.2) | 2 (0.2) | 3 (0.3) | ||
| Area of residence | .34 d | .88 d | ||||
| Urban | 1071 (96.3) | 3512 (95.3) | 1055 (96.3) | 1055 (97.2) | ||
| Rural | 12 (1.1) | 52 (1.4) | 12 (1.1) | 10 (0.9) | ||
| Unknown | 29 (2.6) | 122 (3.3) | 29 (2.6) | 31 (2.8) | ||
| Region | < .001 d | .88 d | ||||
| Northeast | 281 (25.3) | 754 (20.5) | 272 (24.8) | 259 (23.6) | ||
| Midwest | 254 (22.8) | 995 (27.0) | 253 (23.1) | 253 (23.1) | ||
| South | 378 (34.0) | 1208 (32.8) | 372 (33.9) | 388 (35.4) | ||
| West | 199 (17.9) | 729 (19.8) | 199 (18.2) | 196 (17.9) | ||
| Median household income of ZIP code of residence | < .001 d | .98 d | ||||
| Less than $38,000 | 96 (8.6) | 484 (13.1) | 96 (8.8) | 95 (8.7) | ||
| $38,000–$47,999 | 212 (19.1) | 738 (20.0) | 209 (19.1) | 217 (19.8) | ||
| $47,000–$62,999 | 308 (27.7) | 1018 (27.6) | 303 (27.6) | 310 (28.3) | ||
| $63,000 or more | 490 (44.1) | 1431 (38.8) | 483 (44.1) | 469 (42.8) | ||
| Unknown | 6 (0.5) | 15 (0.4) | 5 (0.5) | 5 (0.5) | ||
| Insurance status at diagnosis | < .001 d | .94 d | ||||
| Private | 744 (66.9) | 2,309 (62.6) | 731 (66.7) | 733 (66.9) | ||
| Medicare | 277 (24.9) | 875 (23.7) | 274 (25.0) | 280 (25.5) | ||
| Other government | 34 (3.1) | 245 (6.6) | 34 (3.1) | 28 (2.6) | ||
| None | 44 (4.0) | 202 (5.5) | 44 (4.0) | 41 (3.7) | ||
| Unknown | 13 (1.2) | 55 (1.5) | 13 (1.2) | 14 (1.3) | ||
| Number of comorbidities | .041 d | .961 d | ||||
| 0 | 939 (84.4) | 2990 (81.1) | 923 (84.2) | 924 (84.3) | ||
| 1 | 151 (13.6) | 609 (16.5) | 151 (13.8) | 148 (13.5) | ||
| 2 or more | 22 (2.0) | 87 (2.4) | 22 (2.0) | 24 (2.2) | ||
| Histological type | < .001 d | .05 d | ||||
| Endometrioid carcinoma | 1352 (28.2) | 324 (29.1) | 321 (29.3) | 303 (27.6) | ||
| Serous carcinoma | 319 (28.7) | 892 (24.2) | 314 (28.6) | 301 (27.5) | ||
| Mucinous carcinoma | 123 (11.1) | 645 (17.5) | 121 (11.0) | 169 (15.4) | ||
| Clear cell carcinoma | 195 (17.5) | 682 (18.5) | 193 (17.6) | 192 (17.5) | ||
| Other adenocarcinoma | 151 (13.6) | 439 (11.9) | 147 (13.4) | 131 (12.0) | ||
| Histological grade | .36 d | .92 d | ||||
| 1 | 201 (18.1) | 689 (18.7) | 198 (18.1%) | 193 (17.6%) | ||
| 2 | 210 (18.9) | 756 (20.5) | 207 (18.9%) | 206 (18.8%) | ||
| 3 | 632 (56.8) | 1987 (53.9) | 623 (56.8%) | 621 (56.7%) | ||
| Unknown | 69 (6.2) | 254 (6.9) | 68 (6.2%) | 76 (6.9%) | ||
| Tumor size | < .001 d | .83 d | ||||
| Smaller than 1 cm | 60 (5.4) | 160 (4.3) | 60 (5.4) | 62 (5.7) | ||
| 1.0–4.9 cm | 224 (20.1) | 440 (11.9) | 218 (19.9) | 222 (20.2) | ||
| 5.0–9.9 cm | 302 (27.2) | 605 (16.4) | 292 (26.6) | 308 (28.1) | ||
| 10 cm or larger | 382 (34.4) | 2096 (56.9) | 382 (34.6) | 376 (34.3) | ||
| Unknown | 144 (12.9) | 385 (10.4) | 144 (13.1) | 128 (11.7) | ||
| Adjuvant chemotherapy | .37 d | .27 d | ||||
| No | 427 (38.4) | 1429 (38.8) | 419 (38.2) | 404 (36.9) | ||
| Yes | 608 (54.7) | 2044 (55.5) | 600 (54.7) | 630 (57.5) | ||
| Unknown | 77 (6.9) | 213 (5.8) | 77 (7.0) | 62 (5.7) | ||
a Propensity score model included age, race/ethnicity, facility type, year of diagnosis, area of residence, region, median household income of ZIP code of residence, insurance status, comorbidities, and tumor size
c All cells are counts with percentages in parentheses unless otherwise stated
We matched 1096 subjects (98.6%) who underwent planned laparoscopic staging to subjects who had staging by laparotomy. Associations between demographic, socioeconomic, and histopathological covariates and receipt of laparoscopic surgery, seen in the total cohort, were not evident after propensity score matching ( Table 1 ), suggesting that the matched cohort was well balanced on observed covariates. Balance of covariates was confirmed with absolute standardized differences, which were less than 10% for all variables after propensity score matching ( Appendix Figure ).
The frequency of surgical complications as measured by death within 90 days of surgery (0.3% vs 0.9%; P = .10) and unplanned readmission within 30 days of surgical discharge (2.8% vs 3.9%; P = .37) did not differ between subjects who had planned laparoscopy and propensity-matched patients who had planned laparotomy ( Table 2 ). However, subjects who underwent planned laparoscopic surgery had higher lymph node counts (median, 14 vs 12 nodes; P = .005) and shorter postoperative hospital stays (median, 3 vs 4 days; P < .001).
| Outcome | Laparoscopy (n = 1096) | Laparotomy (n = 1096) | P value |
|---|---|---|---|
| Median follow-up, mo (95% CI) a | 28.7 (20.4–38.9) | 29.3 (20.6–39.3) | .77 b |
| Survival probability, % (95% CI) a | |||
| 3 years | 94.1 (92.4–95.8) | 92.4 (90.4–94.3) | .19 c |
| 4 years | 91.5 (89.0–94.1) | 88.5 (85.3–91.9) | .17 c |
| Length of postoperative admission, d, median (IQR) | 3 (1–4) | 4 (3–5) | < .001 d |
| Unplanned readmission within 30 d of surgical discharge, % (95% CI) e | 2.8 (2.0–4.0) | 3.9 (2.9–5.2) | .37 f |
| Death within 90 d of surgery, % (95% CI) g | 0.3 (0–0.9) | 0.9 (0.4–1.7) | .10 f |
| Lymph node count, median (IQR) | 14 (7–22) | 12 (6–20) | .005 d |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


