Background
There is growing interest in uterine conservation at the time of surgery for uterovaginal prolapse, but limited data compare different types of hysteropexy.
Objective
We sought to compare 1-year efficacy and safety of laparoscopic sacral hysteropexy and vaginal mesh hysteropexy.
Study Design
This multicenter, prospective parallel cohort study compared laparoscopic sacral hysteropexy to vaginal mesh hysteropexy at 8 institutions. We included women ages 35–80 years who desired uterine conservation, were done with childbearing, and were undergoing 1 of the above procedures for stage 2–4 symptomatic anterior/apical uterovaginal prolapse (anterior descent at or beyond the hymen [Aa or Ba ≥ 0] and apical descent at or below the midvagina [C ≥ –TVL/2]). We excluded women with cervical elongation, prior mesh prolapse repair, cervical dysplasia, chronic pelvic pain, uterine abnormalities, and abnormal bleeding. Cure was defined as no prolapse beyond the hymen and cervix above midvagina (anatomic), no vaginal bulge sensation (symptomatic), and no reoperations. Pelvic Organ Prolapse Quantification examination and validated questionnaires were collected at baseline and 12 months including the Pelvic Floor Distress Inventory Short Form, Female Sexual Function Index, and Patient Global Impression of Improvement. In all, 72 subjects/group were required to detect 94% vs 75% cure (80% power, 15% dropout). Intention-to-treat analysis was used with logistic regression adjusting for baseline differences.
Results
We performed 74 laparoscopic sacral hysteropexy and 76 vaginal mesh hysteropexy procedures from July 2011 through May 2014. Laparoscopic patients were younger ( P < .001), had lower parity ( P = .006), were more likely premenopausal ( P = .008), and had more severe prolapse ( P = .02). Laparoscopic procedure (174 vs 64 minutes, P < .0001) and total operating time (239 vs 112 minutes, P < .0001) were longer. There were no differences in blood loss, complications, and hospital stay. One-year outcomes for the available 83% laparoscopic and 80% vaginal hysteropexy patients revealed no differences in anatomic (77% vs 80%; adjusted odds ratio, 0.48; P = .20), symptomatic (90% vs 95%; adjusted odds ratio, 0.40; P = .22), or composite (72% vs 74%; adjusted odds ratio, 0.58; P = .27) cure. Mesh exposures occurred in 2.7% laparoscopic vs 6.6% vaginal hysteropexy ( P = .44). A total of 95% of each group were very much better or much better. Pelvic floor symptom and sexual function scores improved for both groups with no difference between groups.
Conclusion
Laparoscopic sacral hysteropexy and vaginal mesh hysteropexy had similar 1-year cure rates and high satisfaction.
Introduction
Surgery for uterovaginal prolapse traditionally involves a hysterectomy even though the uterus is anatomically normal. Many women prefer uterine conservation. In general, hysteropexy is safe, and effective, with shorter operating time, less blood loss, and faster recovery compared to prolapse repairs with hysterectomy. Described procedures include sacrospinous hysteropexy, vaginal mesh hysteropexy (VMHP), and abdominal sacral hysteropexy. The overall number and quality of hysteropexy studies is increasing, but most are retrospective and lack controls. Existing controlled trials compare hysteropexy to vaginal hysterectomy; however, no trials compare different uterine suspension procedures. Patient counseling regarding the optimal type of hysteropexy is therefore challenging.
Sacrospinous and abdominal sacral hysteropexy provide good apical support, but were associated with recurrent anterior prolapse (51–61%) in 2 randomized controlled trials. Sacral hysteropexy graft configuration varies widely and often only includes a posterior graft. Both abdominal and laparoscopic sacral hysteropexy (LSHP) have high anatomic cure similar to sacral colpopexy, and the addition of anterior mesh should improve anterior support. Newer lightweight, trocarless vaginal mesh kits, such as Uphold (Boston Scientific, Marlborough, MA), support the apex and anterior wall and can be used for VMHP. At study onset, there was 1 published case series involving Uphold showing high success and low risks among 115 subjects at 11.8 months. Given the paucity of data comparing hysteropexy techniques, our goal was to compare 1-year efficacy and safety of LSHP and VMHP.
Materials and Methods
This multicenter, prospective parallel cohort study compared LSHP to VMHP using Uphold or Uphold Lite at 8 institutions. The Strengthening the Reporting of Observational Studies in Epidemiology guidelines were followed for this study. Institutional review board approval was obtained at each site ( ClinicalTrials.gov identifier NCT01377142 ). We included women ages 35-80 years who desired uterine conservation and were undergoing 1 of the above procedures for stage 2–4 symptomatic anterior/apical uterovaginal prolapse. This was defined as anterior descent at or beyond the hymen (Aa or Ba ≥ 0); apical descent at or below the midvagina (C ≥ -TVL/2); and a positive response to Pelvic Floor Distress Inventory Short Form (PFDI-20) question 3 (“Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?”). Subjects had completed childbearing or were practicing reliable contraception and had a normal size uterus (<10 cm) on examination or ultrasound. We excluded women with cervical elongation (surgeon discretion), prior mesh prolapse repair, current foreign-body complications, increased risk or recent history of cervical dysplasia, chronic pelvic pain, significant uterine abnormalities, and abnormal menstruation. We also excluded women with postmenopausal bleeding in the past 12 months.
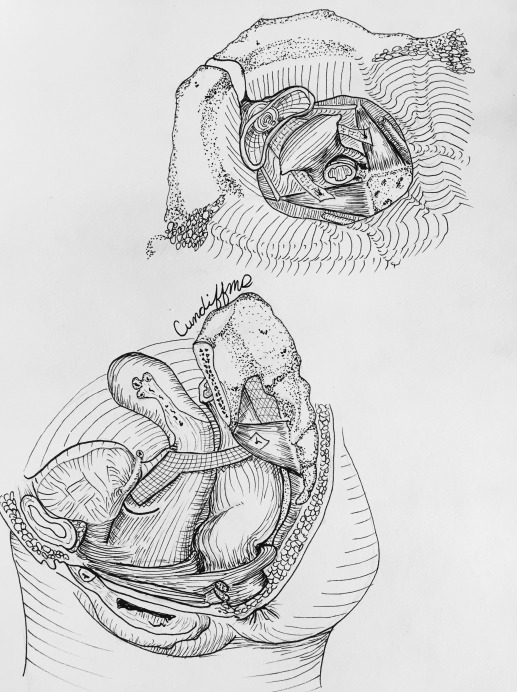
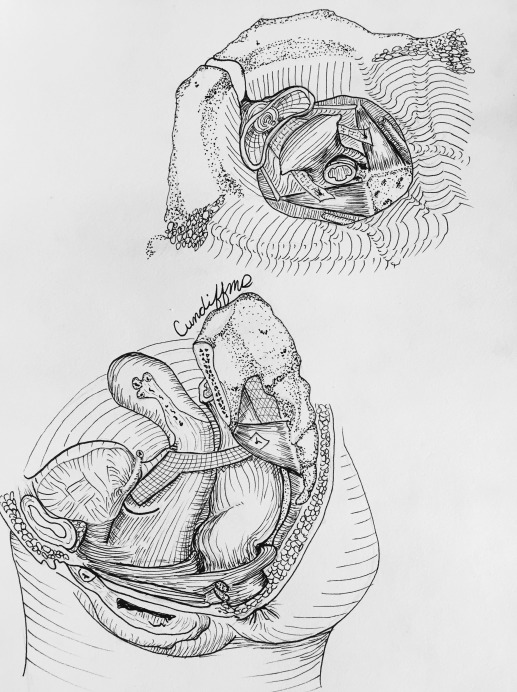
Hysteropexy approach was determined through shared decision-making between the patient and surgeon. Investigators were required to view videos demonstrating the 2 standardized hysteropexy techniques and to document completion of at least 10 trocarless anterior vaginal mesh repairs and 10 laparoscopic mesh repairs prior to participating. LSHP ( Figure 1 ) was performed using an anterior and posterior lightweight, type 1, polypropylene mesh. Each mesh strap had at least a 4-cm long attachment to the proximal vagina and cervix with permanent or delayed absorbable sutures. The posterior mesh was secured to the anterior longitudinal ligament with at least 2 permanent sutures and a longer vaginal extension was permitted toward but not to the perineal body. The anterior mesh contained a central portion that attached to the vagina with permitted extension to the urethrovesical junction and lateral arms that travelled through windows in the broad ligament and were secured to the anterior longitudinal ligament or the posterior mesh with at least 2 permanent sutures.
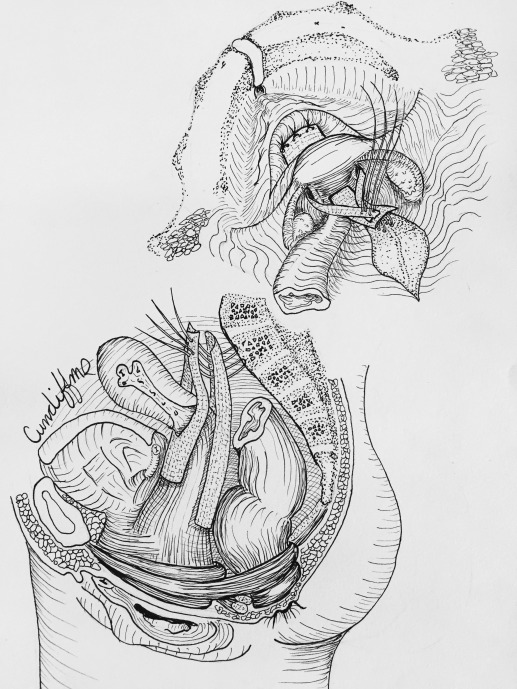
VMHP ( Figure 2 ) was performed using the Uphold or Uphold Lite system. During the first 9 months of enrollment, we transitioned to Uphold Lite after it was Food and Drug Administration (FDA) approved. An inverted U-shaped anterior vaginal incision was made proximal to the urethrovesical junction and the fibromuscular layer was split with a small amount left on the bladder side. The dissection continued to the anterior cervix and the residual fibromuscular layer adherent to the bladder muscularis was usually plicated (not required) in the midline using interrupted 2–0 absorbable or delayed absorbable sutures. The arms of the mesh were inserted into the sacrospinous ligament via the anterior approach. The graft was secured to the cervix with at least 1 permanent or delayed absorbable suture and could be attached to the fibromuscular layer on the bladder side using absorbable sutures.
Cystoscopy was required after the study procedures, and all subjects received antibiotic and thromboembolism prophylaxis. Additional procedures were performed at the discretion of the surgeon. Retropubic or transobturator full-length midurethral slings were permitted through a separate anterior incision. Posterior repair was performed for posterior prolapse (Bp ≥ –1) following apical suspension or at the surgeon’s discretion. Anterior colporrhaphy was performed after LSHP for anterior prolapse (Ba ≥ –1) following apical suspension or at the surgeon’s discretion.
Written informed consent was obtained and demographic data collected including age, race, parity, body mass index, hormonal status, Charlson Comorbidity Index medical history, tobacco use, and prior prolapse or incontinence surgery. Pelvic Organ Prolapse Quantification (POP-Q) examination was performed and validated quality-of-life measures were collected including the PFDI-20 and Female Sexual Function Index (FSFI). Perioperative and postoperative data collection included operative time, blood loss, complications, and length of hospitalization. Patients were seen at 6 weeks, 3 months, and 12 months postoperatively with POP-Q examinations performed by an independent examiner. Complications were collected at each visit and severe study-related adverse events for each patient were categorized using the modified Clavien-Dindo surgical complication grading scale. Patients with mesh, sutures, or granulation tissue excised or trimmed in the office received a Clavien-Dindo grade I, similar to wound infections opened at the bedside.
Our primary outcome was surgical success, a dichotomous composite outcome of anatomic and symptomatic cure at 12 months. Anatomic cure was defined as no anterior or posterior prolapse beyond the hymen (Aa, Ba, Ap, Bp all ≤ 0), cervix above midvagina (C < –TVL/2), and no prolapse reoperation or pessary use. Symptomatic cure was defined as no bulge sensation (negative PFDI-20 question 3). Secondary outcomes consisted of individual POP-Q and quality-of-life measures (PFDI-20, FSFI, and Patient Global Impression of Improvement for Prolapse ) at 3 and 12 months. We collected information regarding subsequent treatment for prolapse and uterine/cervical disease. Pain was assessed using the modified Surgical Pain Scale and medication diary at baseline; daily for 2 weeks after surgery; 6 weeks; 3 months; and 12 months. Functional activity was assessed using the Activity Assessment Scale preoperatively, and 2 weeks, 6 weeks, and 6 months (telephone) after surgery. Activity Assessment Scale scores range from 0-100 with higher numbers associated with better functioning.
We determined that 61 women per group provides 85% power for detecting a difference in 1-year cure rates of 75% vs 94%, based on a 2-sided Z test with pooled variance with a .05 significance level (PASS 11.0, NCSS). To compensate for a 15% dropout rate, the initial sample size needed was 72 per group or a total of 144 subjects.
Baseline measures were compared between treatment groups to identify imbalances and participants were analyzed in the preoperatively assigned groups for the primary analysis. For our primary aim, a logistic regression model was used to model the dependent measure (surgical cure). Covariates included in the model included study site and baseline demographic, medical, and physical examination (eg, POP-Q) variables that were statistically different ( P ≤ .05) between study groups. For the primary analyses, participants with missing data at 12 months were not included. To evaluate the impact of missing data on the primary aim, sensitivity analysis was performed whereby subjects with missing data that did not allow an assessment of the surgical cure were considered first as all failures, in a second analysis as all successes, and in a third with missing data in one group a success and in the other as a failure (ie, worst case scenario). For the quality-of-life measures, linear regression analysis was performed with 12-month scale score as the dependent variable and baseline scale score and baseline differences between groups as covariates. Other secondary outcomes were compared with the Pearson χ 2 test for categorical data and the Student t test for parametric continuous data or Wilcoxon rank sum test for ordinal or nonparametric continuous data. Statistical analysis was performed using JMP Pro 12.1 (SAS Institute, Cary, NC).
An independent safety monitor reviewed all adverse events at routine intervals and evaluated enrollment and study conduct. Serious and unanticipated adverse events underwent expedited evaluation. A predetermined reoperation stopping criteria of 7.5% was set for mesh complications or other adverse events directly related to the study procedure.
Materials and Methods
This multicenter, prospective parallel cohort study compared LSHP to VMHP using Uphold or Uphold Lite at 8 institutions. The Strengthening the Reporting of Observational Studies in Epidemiology guidelines were followed for this study. Institutional review board approval was obtained at each site ( ClinicalTrials.gov identifier NCT01377142 ). We included women ages 35-80 years who desired uterine conservation and were undergoing 1 of the above procedures for stage 2–4 symptomatic anterior/apical uterovaginal prolapse. This was defined as anterior descent at or beyond the hymen (Aa or Ba ≥ 0); apical descent at or below the midvagina (C ≥ -TVL/2); and a positive response to Pelvic Floor Distress Inventory Short Form (PFDI-20) question 3 (“Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?”). Subjects had completed childbearing or were practicing reliable contraception and had a normal size uterus (<10 cm) on examination or ultrasound. We excluded women with cervical elongation (surgeon discretion), prior mesh prolapse repair, current foreign-body complications, increased risk or recent history of cervical dysplasia, chronic pelvic pain, significant uterine abnormalities, and abnormal menstruation. We also excluded women with postmenopausal bleeding in the past 12 months.
Hysteropexy approach was determined through shared decision-making between the patient and surgeon. Investigators were required to view videos demonstrating the 2 standardized hysteropexy techniques and to document completion of at least 10 trocarless anterior vaginal mesh repairs and 10 laparoscopic mesh repairs prior to participating. LSHP ( Figure 1 ) was performed using an anterior and posterior lightweight, type 1, polypropylene mesh. Each mesh strap had at least a 4-cm long attachment to the proximal vagina and cervix with permanent or delayed absorbable sutures. The posterior mesh was secured to the anterior longitudinal ligament with at least 2 permanent sutures and a longer vaginal extension was permitted toward but not to the perineal body. The anterior mesh contained a central portion that attached to the vagina with permitted extension to the urethrovesical junction and lateral arms that travelled through windows in the broad ligament and were secured to the anterior longitudinal ligament or the posterior mesh with at least 2 permanent sutures.
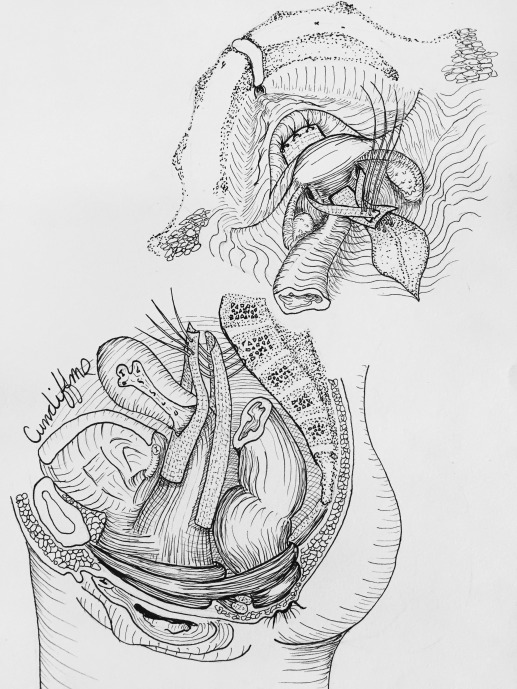
VMHP ( Figure 2 ) was performed using the Uphold or Uphold Lite system. During the first 9 months of enrollment, we transitioned to Uphold Lite after it was Food and Drug Administration (FDA) approved. An inverted U-shaped anterior vaginal incision was made proximal to the urethrovesical junction and the fibromuscular layer was split with a small amount left on the bladder side. The dissection continued to the anterior cervix and the residual fibromuscular layer adherent to the bladder muscularis was usually plicated (not required) in the midline using interrupted 2–0 absorbable or delayed absorbable sutures. The arms of the mesh were inserted into the sacrospinous ligament via the anterior approach. The graft was secured to the cervix with at least 1 permanent or delayed absorbable suture and could be attached to the fibromuscular layer on the bladder side using absorbable sutures.
Cystoscopy was required after the study procedures, and all subjects received antibiotic and thromboembolism prophylaxis. Additional procedures were performed at the discretion of the surgeon. Retropubic or transobturator full-length midurethral slings were permitted through a separate anterior incision. Posterior repair was performed for posterior prolapse (Bp ≥ –1) following apical suspension or at the surgeon’s discretion. Anterior colporrhaphy was performed after LSHP for anterior prolapse (Ba ≥ –1) following apical suspension or at the surgeon’s discretion.
Written informed consent was obtained and demographic data collected including age, race, parity, body mass index, hormonal status, Charlson Comorbidity Index medical history, tobacco use, and prior prolapse or incontinence surgery. Pelvic Organ Prolapse Quantification (POP-Q) examination was performed and validated quality-of-life measures were collected including the PFDI-20 and Female Sexual Function Index (FSFI). Perioperative and postoperative data collection included operative time, blood loss, complications, and length of hospitalization. Patients were seen at 6 weeks, 3 months, and 12 months postoperatively with POP-Q examinations performed by an independent examiner. Complications were collected at each visit and severe study-related adverse events for each patient were categorized using the modified Clavien-Dindo surgical complication grading scale. Patients with mesh, sutures, or granulation tissue excised or trimmed in the office received a Clavien-Dindo grade I, similar to wound infections opened at the bedside.
Our primary outcome was surgical success, a dichotomous composite outcome of anatomic and symptomatic cure at 12 months. Anatomic cure was defined as no anterior or posterior prolapse beyond the hymen (Aa, Ba, Ap, Bp all ≤ 0), cervix above midvagina (C < –TVL/2), and no prolapse reoperation or pessary use. Symptomatic cure was defined as no bulge sensation (negative PFDI-20 question 3). Secondary outcomes consisted of individual POP-Q and quality-of-life measures (PFDI-20, FSFI, and Patient Global Impression of Improvement for Prolapse ) at 3 and 12 months. We collected information regarding subsequent treatment for prolapse and uterine/cervical disease. Pain was assessed using the modified Surgical Pain Scale and medication diary at baseline; daily for 2 weeks after surgery; 6 weeks; 3 months; and 12 months. Functional activity was assessed using the Activity Assessment Scale preoperatively, and 2 weeks, 6 weeks, and 6 months (telephone) after surgery. Activity Assessment Scale scores range from 0-100 with higher numbers associated with better functioning.
We determined that 61 women per group provides 85% power for detecting a difference in 1-year cure rates of 75% vs 94%, based on a 2-sided Z test with pooled variance with a .05 significance level (PASS 11.0, NCSS). To compensate for a 15% dropout rate, the initial sample size needed was 72 per group or a total of 144 subjects.
Baseline measures were compared between treatment groups to identify imbalances and participants were analyzed in the preoperatively assigned groups for the primary analysis. For our primary aim, a logistic regression model was used to model the dependent measure (surgical cure). Covariates included in the model included study site and baseline demographic, medical, and physical examination (eg, POP-Q) variables that were statistically different ( P ≤ .05) between study groups. For the primary analyses, participants with missing data at 12 months were not included. To evaluate the impact of missing data on the primary aim, sensitivity analysis was performed whereby subjects with missing data that did not allow an assessment of the surgical cure were considered first as all failures, in a second analysis as all successes, and in a third with missing data in one group a success and in the other as a failure (ie, worst case scenario). For the quality-of-life measures, linear regression analysis was performed with 12-month scale score as the dependent variable and baseline scale score and baseline differences between groups as covariates. Other secondary outcomes were compared with the Pearson χ 2 test for categorical data and the Student t test for parametric continuous data or Wilcoxon rank sum test for ordinal or nonparametric continuous data. Statistical analysis was performed using JMP Pro 12.1 (SAS Institute, Cary, NC).
An independent safety monitor reviewed all adverse events at routine intervals and evaluated enrollment and study conduct. Serious and unanticipated adverse events underwent expedited evaluation. A predetermined reoperation stopping criteria of 7.5% was set for mesh complications or other adverse events directly related to the study procedure.
Results
We performed 74 LSHP and 76 VMHP from July 2011 through May 2014. Flow of participants can be seen in Figure 3 . Table 1 displays the baseline demographic and POP-Q measurements between groups. LSHP patients were younger, had lower parity, and were more commonly premenopausal with more advanced prolapse involving the cervix (POP-Q point C) and posterior wall (POP-Q point Bp). There were no other baseline differences. Table 2 contains perioperative outcomes. Half of LSHP used robotic assistance. All patients underwent general anesthesia except 9% of VMHP used regional. LSHP procedure time (174 vs 64 minutes, P < .0001) and total operating time (239 vs 112 minutes, P < .0001) were longer. Concomitant surgical procedures were similar except for more perineorrhaphy and adnexal surgery with LSHP. Most of the LSHP adnexal surgery (salpingectomy, unilateral or bilateral salpingo-oophorectomy) was performed for ovarian cancer risk reduction purposes or contraception. Two LSHP converted to VMHP and 1 to abdominal sacral hysteropexy. One VMHP was aborted following cystotomy and underwent anterior and posterior colporrhaphy and sling. The surgeon withdrew this patient from the study. No hysterectomies were performed and no differences observed for blood loss and hospital stay. Despite similar number of slings, VMHP subjects were more likely to be discharged with a catheter.