Fig. 11.1
Uterine manipulators (From Nezhat et al. [11]. © 2013 Cambridge University Press, reproduced with permission)
The procedure is facilitated by the use of four ports, the sizes of which depend on the suturing technique employed by the surgeon, and the type of morcellation/extraction technique planned. Generally, one can perform most laparoscopic myomectomies with a 5–10 mm umbilical port, and 1–2 ports, each 5 mm, on each side. One side port is typically at the level of the umbilicus or slightly caudad to it, while the other is medial to the anterior–superior iliac spine (ASIS). A lateral port may be placed above the umbilicus for larger uteri. A suprapubic 11–12-mm port may be placed to later facilitate morcellation.
Following exam under anesthesia, diagnostic hysteroscopy is a critical step in order to assess the uterine cavity for any pathology, such as intracavitary myoma or polyp or even synechias which need to be addressed in the same setting, especially if the myomectomy is performed for fertility reasons. Prior to the placement of the accessory ports, the size and position of the myomas are carefully assessed. If there is any doubt about safely performing the myomectomy via laparoscopy, a conventional or minilaparotomy should be performed. The threshold for conversion depends on the surgeon’s skills and experience.
Pelvic cavity should be evaluated for any associated pathology, such as endometriosis, adhesions and tubo-ovarian pathology. Nezhat et al., reported that most patients with endometriosis have leiomyomas which we believe should be treated [12].
After proper placement of the trocars and complete survery of the pelvis and abdomen, dilute vasopressin solution is injected into the subserosa and myometrium overlying the myoma(s) until the tissue blanches. This is easily accomplished using a control-top syringe and a 4-in. 22-gauge spinal needle, which can be inserted percutaneously over the uterus, or using a laparoscopic needle tip device. The use of vasopressin for gynecologic surgery has been controversial, but is widely accepted in the US. In two prospective randomized studies, dilute vasopressin solution was found to decrease blood loss at time of myomectomy via laparotomy compared with placebo or a tourniquet [13]. An alternative to the use of vasopressin is to inject 0.25 % bupivacaine with epinephrine into the sub-serosa and myometrium in a similar fashion. This also has a vasoconstrictive effect and has been found in one randomized study to decrease the need for postoperative pain medication in women undergoing laparoscopic myomectomy as well as to decrease blood loss [14].
Another alternative, that requires advanced surgical skills, is the laparoscopic uterine artery occlusion prior to performing the enucleation of the myomas. This can be accomplished by either using a permanent or reversible clip, or energy coagulation of the uterine arteries at their origin from the hypogastric artery [15]. This technique has especially been found more helpful in challenging cases, such as the ones involving cervical myomas [16].
Pedunculated myomas are the least difficult to manage laparoscopically. Following the administration of dilute vasopressin into the stalk, the myoma is removed by cutting and coagulating the stalk. Care must be taken to stay close to the myoma and avoid thermal damage to the normal myometrium from which the stalk arises. An alternative, is to place one or two ties around the pedicle such as an Endoloop (Ethicon) and to excise the myoma with electrosurgery around its base approximately 1–2 cm above the insertion of the pedicle. The pedicle can then be over sewn to assure hemostasis. This technique minimizes the risk of later uterine rupture, which has been reported during pregnancy following laparoscopic removal of a pedunculated myoma [17, 18].
The removal of subserousal myomas is less challenging than the removal of deep intramural myomas. Dilute vasopressin is injected in multiple sites between the myometrium and the fibroid capsule. An incision is made on the serosa overlying the leiomyoma, using the CO2 laser (superpulse or ultrapulse mode), a monopolar electrode (hook or scissors), a fiber laser, or harmonic scalpel or spatula. Traditionally, the incision has been oriented in the vertical direction, along the axis of the uterus. Some authors have found that incising transversely instead facilitates both the dissection and the repair. Once the incision is made, it is extended until it reaches the capsule. The myometrium retracts as the incision is made and the myoma bulges outward. Two grasping, toothed forceps can be used to hold the edges of the myometrium, and the suction–irrigator, or Kittner dissector or a Maryland dissector or the harmonic scalpel, can be used to shell the leiomyoma from its capsule. A myoma screw is inserted into the tumor or a single tooth tenaculum can be used to grasp the myoma and to apply traction during blunt dissection. An alternative is to insert a finger through a 12-mm suprapubic port site incision and manually dissect the myoma free from the myometrium. Visible vessels are electrocoagulated before being cut. Following complete removal of the myoma(s), the uterine defect is irrigated. Bleeding points are identified and controlled, preferably with bipolar electrosurgery. Point coagulation of identifiable vessels can be accomplished by using short bursts of cutting or coagulation monopolar or bipolar current, however we highly advise against this, since excessive use of electrosurgery not only makes the repair of the myometrium more difficult but it also impedes tissue healing with subsequent risk of utero-peritoneal fistula and rupture during subsequent pregnancies. Instead, hemostatic agents such as Floseal (Baxter, Deerfield, IL) or Surgiflo (Ethicon Inc) can be used to decrease bleeding. The edges of the uterine defect are approximated by superficial suturing. Figures 11.2, 11.3, 11.4, 11.5 and 11.6 depict key steps of laparoscopic myomectomy.
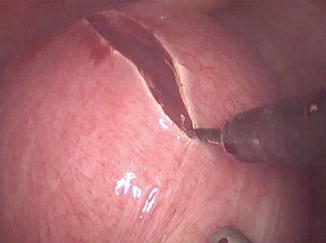
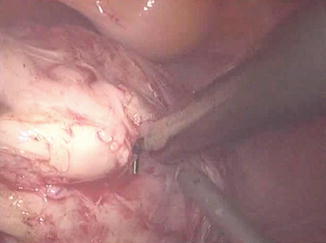
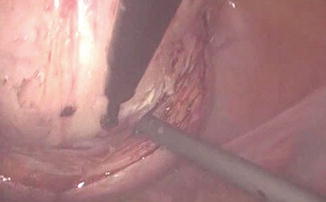
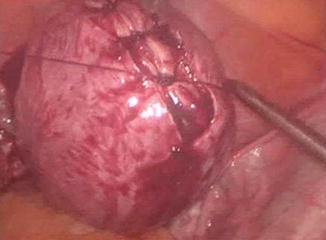
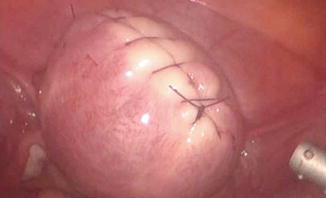

Fig. 11.2
Uterine incision

Fig. 11.3
Dissection of the myoma

Fig. 11.4
Enucleation of the myoma

Fig. 11.5
Closure of the myometrial defect

Fig. 11.6
Hemostatic closed incision
Deep intramural or broad ligament intramural myomas are the most difficult to properly manage laparoscopically and should be done only by surgeons skilled in laparoscopic suturing. This is especially true if the patient plans future childbearing. The myometrial closure traditionally recommended following open myomectomy is a three-layered closure beginning at the base of the defect, to obliterate the dead space, with figure of- eight or horizontal mattress sutures. A second layer of continuous suture is then placed to further approximate the myometrium and finally ending with a continuous imbricating “baseball” stitch on the serosa. Synthetic absorbable polyglactin sutures (Vicryl, Ethicon, Somerville, NJ; Polysorb, USSC, Norwalk, CT) are recommended because they produce less inflammatory reaction than catgut. True comparisons of alternative suturing methods have not been performed. Intraligamentous and broad ligament myomas require careful identification of the course of the ureters, the bladder, and pelvic sidewall blood vessels. Depending on the location of the myoma, an incision is made on the anterior or posterior leaf of the broad ligament. The myoma is removed with the techniques described above for subserosal and intramural tumors. Throughout the procedure, the location of the ureters is noted. Hemostasis is obtained with sutures, clips, or bipolar forceps. The broad ligament and peritoneum are not closed but allowed to heal spontaneously. Drains are used infrequently.
Stringer and associates [19] described a simplified way of closing deep myometrial defects using the Endo Stitch automatic suturing device (United States Surgical, Norwalk CT) [17]. Multiple layers of continuous interlocking sutures are placed using this device, which captures the 9-mm needle in the opposite jaw when the handles are squeezed. The suture is held taut by the assistant, and finally an intracorporeal knot is tied with the device. The Endo Stitch must be used through a 10-mm port. Its limitations are a semi-straight needle that is relatively short, making it a challenge to grasp adequate amounts of uterine tissue in each bite. Some surgeons feel this can be faster than conventional open suturing techniques.
The introduction of the Quill™ bidirectional barbed suture (Angiotech Pharmaceuticals, Inc., Vancouver, BC, Canada) and later the “V-LOC” (Covidien, Mansfield, M) (Fig. 11.7) have changed the way laparoscopic myomectomies are performed. The barbs minimize tissue recoil and do so with accurate soft tissue approximation, achieving hemostasis without the use of locking and figure eight sutures. Barbed suture allows for a shorter operative time, as there is an ease of suturing without the complication of knot tying. The advantages of using barbed sutures in this setting include the ability to perform knotless suturing and rapid suture deployment, which may result in decreased operative time and blood loss. In addition, the tensile strength of the suture is maintained by the barbs, which facilitates the operative procedure and may potentially lead to a more even distribution of tension along the closure [15, 20–22]. If these types of suture were to be used on the serosa of the uterus, one should take care not to leave extra suture behind, since this raises the risk of post-operative bowel obstruction.
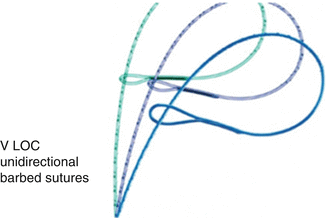

Fig. 11.7
Barbed suture (From Nezhat et al. [11]. © 2013 Cambridge University Press, reproduced with permission)
Specimen Removal Techniques
Other than laparoscopic suturing, the greatest challenge and often the most frustrating step in laparoscopic myomectomy is specimen removal. For multiple small myomas, the enucleated specimens can be placed in a specimen retrieval bag, brought up to the largest port site, morcellated in the bag at the skin line using an 11 scalpel blade or scissors. While the introduction of power morcellators, in the last decade, has revolutionized the laparoscopic myomectomy procedure, due to its ease of use, there have recently been serious concerns regarding the safety of its use. The most widely used devices in the US are made by Gynecare (Johnson & Johnson, Somerville, NJ), Karl Storz, WISAP, and Richard Wolf. Seeding of port sites with malignant cells or endometriosis is a well-known sequela of laparoscopic procedures. This may also occur with morcellated myoma tissue, as was shown in a case report by Ostrzenski [23]. Intra-abdominal seeding of benign leiomyoma could lead to parasitic leiomyomas that can become symptomatic or require further surgery [24, 25]. However, more concerning is the morcellation of an unsuspected leiomyosarcoma, that can lead to seeding of malignant tissue in the abdominal cavity, significantly altering the stage and prognosis of the disease [26]. Since there is no reliable test that could predict the presence of leiomyosarcoma in a leiomyomatous uterus, and based on recent reported cases of disseminated leiomyosarcoma following power morcellation [27], the US Food and Drug Administration (FDA) had issued a warning that discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids, as of April 2014 (http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm). This has followed same warning issued by multiple societies including the Society of Gynecologic Oncologists (SGO), the American Association of Gynecologic Laparoscopists (AAGL) and American College of Obstetrics and Gynecology (ACOG). As a result, multiple hospital systems throughout the United States have banned the use of power morcellators. However, there are multiple alternatives available to power morcellation. One alternative includes the removal of the specimen through a posterior and occasionally anterior colpotomy incision. The posterior colpotomy incision can be done vaginally below the cervix between the uterosacral ligaments. Once the peritoneum is opened, the myoma is grasped vaginally in a bag with a tenaculum or Leahy clamp and removed intact or progressively morcellated using a coring technique, while enclosed in a laparoscopic bag. Alternatively, the vagina can be identified laparoscopically with the help of the uterine manipulator, vaginal probe, or a sponge stick placed in the posterior fornix. An incision is then made laparoscopically using an electrosurgical needle, scissors, harmonic scalpel, or CO2 laser. The disadvantage of this approach is that the pneumoperitoneum is rapidly lost, making it difficult to bring the myoma into the cul-de-sac. A wet lap pad or a surgical glove containing 2 wet sponges (also known as Ceana’s glove), may be placed in the vagina to restore the pneumoperitoneum and view the pelvis. Recently, the development of automatic insufflators, such as the AirSeal system (SurgiQuest, Connecticut), that re-fills the pneumoperitoneum when the vagina is open or gas leaks are present, make the procedure easier. Multiple small myomas can be removed with a specimen retrieval bag placed through the colpotomy incision. The colpotomy incision can be sutured laparoscopically or vaginally.
Another alternative method that we prefer is the use of laparoscopically assisted myomectomy/minilap myomectomy (LAM). This technique was first reported by Nezhat et al. in 1994 [28] and is less difficult and requires less time to complete than other modes of myomectomy. The decision to perform a LAM is usually made intraoperatively following completion of exploratory laparoscopy and treatment of other pelvic abnormalities. Cases most suitable for a LAM include a myoma greater than 8 cm, multiple myomas requiring extensive morcellation, a deep, large, intramural myoma that requires uterine repair in multiple layers. A combination of laparoscopy and a 2–4 cm abdominal incision may put laparoscopic myomectomy within the technical reach of more gynecologists. Better pelvic exposure during the laparoscopy allows the gynecologist to diagnose and treat associated endometriosis or adhesions, and the traditional access allows for fast morcellation and conventional suturing preserving myometrial integrity. In addition, LAM with morcellation and conventional suturing may reduce the mean operative time. Similar benefits may be seen with the introduction of barbed suture. Further support for the LAM technique is found in a study of in a study by Glasser that detailed 139 minilaparotomy myomectomies [29]. Out of the 139 cases presented, 66 underwent LAM, during which the laparoscope was used to identify and mark the incision site or to perform adhesiolysis. The authors concluded that myomectomy performed through a 3–6 cm minilaparotomy incision affords the advantage of same-day discharge as well as the ability to palpate the uterus and close the defect using a standard three-layered suturing technique. The most prominent myoma is injected at its base with 3–7 mL of diluted vasopressin. An incision is made over the uterine serosa at the surface of the tumor and extended until the capsule of the leiomyoma is reached. A corkscrew manipulator is inserted into the leiomyoma and used to elevate the uterus toward the midline suprapubic puncture. Alternatively a laparoscopic single tooth tenaculum can be used for the same purpose. With the trocar and manipulator attached to the myoma, a 4–6 cm transverse incision is made through skin and fascia and the rectus muscle separated in the midline. After opening the peritoneum transversely, an Alexis wound retractor (Applied medical) or a Mobius abdominal retractor (Cooper surgical) (Fig. 11.8) is introduced for better exposure. The leiomyoma is grasped with two Lahey tenacula (Fig. 11.9). The tumor is shelled and morcellated sequentially (Fig. 11.10), and after its complete removal, the uterine wall defect shows through the incision. When multiple leiomyomas are found, as many as possible are removed through one uterine incision if it can be accomplished without excessive tunneling. When other myomas are located and cannot be removed through the initial uterine incision, the 4-cm abdominal opening is approximated temporarily with two or three Allis clamps, or an inflated latex glove (placed over the self-retaining retractor while the insufflator is turned ON). The laparoscope is reintroduced, and the remaining myomas are identified, and brought to the incision and removed in a similar fashion. The uterus is then repaired in two to three layers (Fig. 11.11). The abdominal incision is then closed in routine fashion, ideally with a subcuticular skin closure. The laparoscope is used to evaluate hemostasis. The pelvis is observed to detect and treat endometriosis and adhesions that may have been obscured previously by myomas. Copious irrigation is used, blood clots are removed, and Interceed (Gynecare, Somerville, NJ) may be applied over the uterus to help prevent adhesions if the incision is completely dry. Nezhat and associates evaluated 143 charts from their practice [30], including those of patients who had myomectomy by laparotomy (15.3 %), laparoscopic myomectomy (44.7 %), or LAM (39.8 %). Mean operating times, estimated blood loss, hospital stay (after eliminating the first 15 LAM patients), and time to resume work or regular activities were the same for laparoscopic myomectomy and LAM despite larger myomas, difficult locations of intramural myomas, and adjunctive laparoscopy in the latter group. The leiomyoma weights were greater in the LAM group than in the laparoscopic myomectomy group (P ≤ 0.05). Time to 100 % recovery was slightly longer in the LAM group. Most patients are observed in an outpatient unit and discharged the morning after the operation, although some can leave the hospital on the afternoon or evening of the procedure.

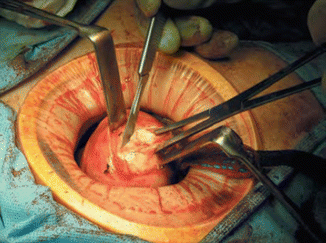
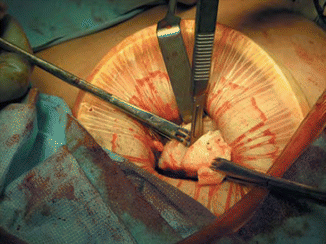
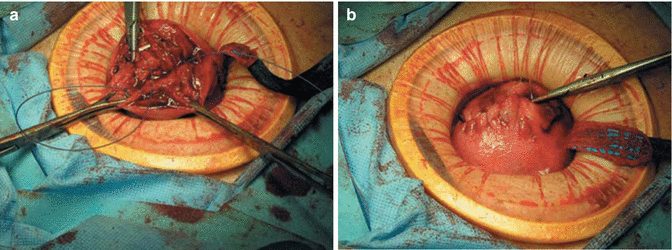

Fig. 11.8
Self-retaining wound retractor (From Nezhat et al. [11]. © 2013 Cambridge University Press, reproduced with permission)

Fig. 11.9
Laparoscopic assisted myomectomy

Fig. 11.10
Laparoscopic assisted myomectomy (Shelling and morcellation of leiomyoma)

Fig. 11.11
(a, b) Laparoscopic assisted myomectomy (Closure of myometrium)
Role of GNRH Prior to Laparoscopic Myomectomy
Pre-treatment with GnRH agonists before myomectomy remains controversial. In a systematic review of randomized controlled trials of the use of GnRH analogues before hysterectomy and myomectomy, it was found that pre- and postoperative blood counts were improved, uterine and myoma volume decreased, and operative time and blood loss reduced by GnRH agonist therapy [31]. Reduction in leiomyoma size makes the procedure less time-consuming because a smaller uterine incision can be made and less morcellation is required. The individual myomas, however, are softer, which often results in the clamps tearing through the tissue, resulting in the loss of upward traction, which is counterproductive and may result in increased bleeding. Thus, although GnRH agonist treatment may soften the myoma (facilitating morcellation) and shorten operative times, it also may increase the duration of laparoscopic or minilaparotomy myomectomy [32]. For anemic patients, preoperative GnRH treatment may enable restoration of a normal hematocrit, decrease the size of the myoma, and reduce the need for transfusion [33]. However, not all studies have shown the benefits described above. Increased difficulty in identifying and dissecting the cleavage planes and shrinking small myomas to the point at which they cannot be seen or palpated may increase recurrence [34]. In a recent meta-analysis by Chen et al. looking at the effect of preoperative GnRH use prior to laparoscopic myomectomy, GnRH agonist did not reduce operative time [35]. However, intraoperative blood loss was statistically lowered (mean difference, 60 mL; 95 % confidence interval [CI], 39–82). Statistical difference was also observed in postoperative hemoglobin concentration (mean difference, 1.15 g/dL; [95 % CI, 0.46–1.83]) and red blood cell count (mean difference, 0.65 × 10(6) cells/mL; [95 % CI, 0.16–1.14]) but not serum iron concentration. In our practice, we do not use routinely preoperative GnRH agonists, unless the patient is anemic.
Adhesion Prevention
Post-myomectomy adhesions have long been thought to possibly compromise fertility, although sound data is lacking. Single, vertical, anterior and midline uterine incisions may cause fewer adhesions. Although sutures predispose patients to adhesions [36] they are necessary to close the uterine defect. Both Seprafilm [37] (HAL-F) bioresorbable membrane (Genzyme Corporation, Cambridge, MA) and Interceed (oxidized regenerated cellulose) [38], have been shown to reduce postoperative adhesions in this setting. However, the impact on fertility was not assessed in these studies, and their routine use cannot be advocated for this purpose [39, 40]. Regardless of the adhesion prevention material used, most are ineffective in the absence of hemostasis. This was shown very early in a study on animal models by Linsky and associates [41]. Using an imbricating baseball-type closure on the uterine serosa is both hemostatic and minimizes the presence of raw edges, which could possibly predispose to adhesion formation.
Uterine Rupture Following Laparoscopic Myomectomy
Spontaneous uterine rupture of the gravid uterus following laparoscopic myomectomy has been reported in all types of leiomyomas including pedunculated, subserosal or intramural types, mainly in the 2nd and 3rd trimesters of pregnancy [42, 43]. In a review of 19 case reports of uterine rupture following laparoscopic myomectomy, published between 1992 and 2004 [42], looking at risk factors for uterine rupture, the authors concluded that in order to decrease the risk of this complication surgeons need to adhere to techniques developed for abdominal myomectomy including limited use of electrosurgery and multilayered closure of the myometrium. The necessity for complete closure of the defect cannot be emphasized enough. It is inadequate to place two or three large through-and through interrupted sutures in a deep myometrial defect if the patient plans future childbearing. Other reports of uterine rupture following laparoscopic myomectomy emphasize the importance of adequate closure of the myometrial defect [44–46]. A recent study reported the appearance of myomectomy scars in 15 women undergoing elective cesarean delivery because of previous myomectomy [47]. The scars in the five women who had myomectomies performed laparoscopically were all described as “strained with thinned tissue and illdefined edges” despite being sutured. In the 10 women who had their myomectomy performed by laparotomy, the majority of scars were symmetric, with the tissue being of uniform thickness compared with the surrounding myometrium. Certainly the liberal use of electrocautery for hemostasis might be the etiologic factor in uterine rupture. In summary, patients must be thoroughly counseled about the possibility of uterine rupture at any time during the gestation, and made aware of the possibility of fetal loss and even hysterectomy, if hemorrhage cannot be controlled. During pregnancy, these patients should be followed carefully and all complaints of abdominal pain addressed immediately. Despite the lack of evidence, the most common practice among obstetricians is to recommend cesarean delivery in future pregnancies for patients with a history of myomectomy regardless of the approach, especially if the uterine cavity has been penetrated during the myomectomy [48]. In our practice, we recommend cesarean delivery when there is deep myometrial invasion, regardless whether or not the uterine cavity was entered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


