INTRODUCTION
Hysterectomy is the most common gynecologic procedure performed in the United States with approximately 500,000 being performed annually, although the numbers seem to be trending downward slightly for the past few years. The vast majority of benign hysterectomies (over 65%) are still performed through a laparotomy incision despite the urging of professional societies like the American College (Congress) of Obstetricians and Gynecologists (ACOG) and the American Association of Gynecologic Laparoscopists (AAGL) to employ minimally invasive approaches to hysterectomy, including vaginal and laparoscopic techniques. Laparoscopic hysterectomy was first described by Harry Reich in 1989 and has slowly gained popularity from less than 0.5% of hysterectomies in 1990 to a current rate of approximately 15%. Failure of laparoscopic hysterectomy to ascend to the primary approach for hysterectomy may be due, in part, to the technical challenges associated with the large uterus or concomitant intra-abdominal pathology. Additionally, the yielding of advanced surgical training time in residencies to other necessary components of the general obstetrics and gynecology curriculum (e.g., high-risk pregnancies and primary care) creates what Reich has called “a formidable obstacle to promotion of this technique.” Indeed, most gynecologic surgeons who gain proficiency in laparoscopic hysterectomy have done so, and will likely continue to do so, during fellowship programs or other postresidency training.
The term laparoscopic hysterectomy includes a family of procedures that vary in degree to which the procedure is performed laparoscopically. This ranges from simple treatment of endometriosis or adhesiolysis and division of ovarian vasculature to completion of the entire procedure laparoscopically, including cuff closure. In 2000, the AAGL published a detailed classification system for total (
Table 32C.1) and supracervical (
Table 32C.2) laparoscopic hysterectomy in order to standardize terminology and reporting of outcomes. In this chapter, the techniques of total laparoscopic hysterectomy (TLH) and laparoscopic supracervical hysterectomy (LSH) are addressed. A logical and systematic surgical approach is described, based on “honoring the anatomy” and highlighting similarities to comparable steps of the abdominal hysterectomy approach, further supporting the mantra that laparoscopy is an access, not a procedure. Laparoscopically assisted vaginal hysterectomy (LAVH) is not described here, as it is basically a variant of vaginal hysterectomy that employs the laparoscopic approach simply to mobilize adnexal structures and treat relevant abdominal pathology such as adhesions. Nor is robotic-assisted laparoscopic hysterectomy discussed. Robotic technology applied to laparoscopic surgery has stimulated the adoption of TLH by many gynecologists previously hesitant to perform that procedure. By adding three-dimensional vision and instruments with articulating tips, clearer identification of tissue planes and easier laparoscopic suturing are facilitated. However, roboticassisted laparoscopy is neither a technique nor an access, but rather a tool for performing laparoscopic hysterectomy.
Historical Perspective
Laparoscopy was incorporated into gynecologic practice in the 1950s by Raoul Palmer in France, followed by Kurt Semm in Germany. After gaining popularity in Europe, it was spread to the United States by Melvin Cohen in the 1960s. Although there was increased utilization over the ensuing decades, early descriptions of laparoscopic appendectomy, ovarian cystectomy, and treatment of ectopic pregnancy were often refused publication in American journals as they were considered “unethical” surgical adventures. It was not until the development of microchip video cameras enabled projection of images onto television monitors in the mid-1980s, ushering in the so-called era of videolaparoscopy, that the techniques of laparoscopy became more integrated into gynecologic surgery.
Harry Reich and John DeCaprio published their landmark description of the first TLH in 1989. They used bipolar current to coagulate blood vessels, monopolar current to amputate the uterus from the vagina, and running Vicryl to close the vaginal cuff after delivering the specimen through the vagina. Viewing it as a substitute for abdominal hysterectomy and not for vaginal hysterectomy, they noted that this approach “may avoid the increased morbidity associated with abdominal surgery while retaining the surgical advantages of the abdominal approach, that is, thorough visualization and easy access to the vascular pedicles.” Shortly thereafter, Kurt Semm described the classic intrafascial supracervical hysterectomy (CISH) procedure that involved coring out the endocervical canal including the squamocolumnar junction. About this same time, Thomas Lyons described a technique for LSH that was more similar to its laparotomy counterpart. This, in part, stimulated a renewed interest in supracervical hysterectomy. Although LSH was touted initially as preserving pelvic support and improving posthysterectomy sexual satisfaction, subsequent studies have shown no difference in either sexual function or pelvic support between supracervical and total hysterectomy. However, the technique of LSH did gain increased popularity among many
gynecologic surgeons as it was easier and quicker to perform, was associated with less blood loss, allowed the surgeon to stay farther away from the ureter while securing vascular pedicles, decreased infectious complications by avoiding vaginal entry, and did not require suturing the vaginal cuff.
Today’s high definition and even 3D video cameras, advanced electrosurgical instruments with interactive generators, innovative devices for safe colpotomy while protecting the ureters, and enabling tools for laparoscopic suturing should continue to enhance the acceptance of laparoscopic hysterectomy as part of our surgical armamentarium.
Advantages and Disadvantages of Laparoscopic Hysterectomy
The decision for appropriateness of hysterectomy as a therapeutic intervention is the same regardless of the approach being considered, although the access for hysterectomy is generally a function of patient pathology and surgeon skill and preference. A 2006 Cochrane database systematic review including over 3,600 patients in 27 randomized studies pointed to significant advantages of laparoscopic hysterectomy (LH) over abdominal hysterectomy (AH), including less blood loss, fewer wound infections or fevers, smaller incisions with less pain, shorter hospital stay, and speedier recovery. However, LH was associated with longer operating time and greater likelihood of urinary tract injuries. The eVALuate trial is one of the largest randomized trials comparing different approaches to hysterectomy. Conclusions pointed to LH as being associated with less pain, quicker recovery, and better quality of life compared with AH but as taking longer to perform. The report also concluded that total vaginal hysterectomy (TVH) was the preferred approach, when possible, as it offered similar benefits as LH with less cost and shorter operating times.
While TVH may be the preferred hysterectomy route for a variety of reasons, there are definitely patients in whom this approach is less than ideal. Specifically, a laparoscopic approach may be favored in patients who are morbidly obese, who have a constricted pelvic anatomy, who have no uterine descensus, or who have known or suspected concomitant pelvic disease (e.g., adhesions, endometriosis, etc). Indeed, there are few contraindications to laparoscopic hysterectomy, and most are relative contraindications related to the patient’s comorbidities, including deficiencies in main physiologic functions and elevated body mass index. These would include the following:
Medical conditions that would limit pneumoperitoneum, adequate ventilation, or Trendelenburg positioning (e.g., morbid obesity, increased intracranial pressure, ventriculoperitoneal shunt, portal or pulmonary hypertension, hemorrhagic shock)
Severe abdominal or pelvic adhesive disease or other conditions that preclude safe entry or adequate operating space (e.g., advanced pregnancy, bulky uterine or fibroid size that precludes access to uterine vessels)
Malignancy or other tumors in which a large specimen needs to be removed intact (e.g., ovarian cancer, dermoid, leiomyoma with necrotic degeneration or other findings suspicious for leiomyosarcoma)
On the other hand, there are recognizable challenges to performing laparoscopic hysterectomy, including the following:
Reduced range of motion through laparoscopic ports and with conventional (straight) laparoscopic instruments resulting in reduced dexterity
Reduced field of view in which only the tissues actively being manipulated are generally seen by the surgeon
Reduced depth perception in converting a 3D surgical field to a 2D video image
Reduced haptics and difficulty in assessing degree of force needed or being applied to tissues
Reduced intuitive movements due to the fulcrum effect in which the tool tips move in the opposite direction as the surgeon’s hands
In all cases, a decision regarding route of hysterectomies is largely dependent on the surgeon’s capabilities. Insufficient knowledge, skill, and experience of the surgeon remain the most common reasons for assigning or converting any hysterectomy to the abdominal route.
Supracervical Versus Total Hysterectomy
Supracervical hysterectomy was first performed by Wilhelm Alexander Freund in 1878 and remained the leading technique of hysterectomy for over 80 years. At that time, there was a recognized association between cervicectomy and complications such as peritonitis, fistula, hemorrhage, ureteral injury, cystotomy, and enterotomy. In fact, the mortality rate associated with total hysterectomy through the 1930s was up to 50% higher compared with the supracervical approach. By the late 1940s, supracervical hysterectomy was largely abandoned in favor of total hysterectomy. This trend reflected the considerable refinement of surgical instruments and techniques, introduction of safer and more effective antibiotics, progression of blood banking technology with transfusions becoming more routine, and subsequent occurrence of cervical cancer in almost 2% of patients following supracervical hysterectomy.
In the early 1990s, after descriptions of successful laparoscopic techniques for supracervical hysterectomy by Kurt Semm and Thomas Lyons, there was a resurgence of interest in supracervical hysterectomy. Some have suggested this was driven equally by surgeon interest in a laparoscopic hysterectomy that was easier, quicker, and safer to perform than TLH as well as industry supply of instruments and devices to facilitate advanced laparoscopic procedures and tissue extraction. Conventional wisdom would assume that preservation of the cervix during LSH reduces the potential for intraoperative injury to the ureter, bladder, and rectum that would more likely occur during the transection of the cardinal ligament complex required to isolate and remove the cervix. Furthermore, hemorrhage most often occurs below the level of the uterine isthmus. Despite a plethora of studies comparing laparoscopic, vaginal, and abdominal approaches to hysterectomy, comparatively, few studies have focused on the role of the cervix. A meta-analysis of 47 studies published in 2006 reported the rate of urinary tract injury in all gynecologic surgery to be approximately 0.33%. In this report, the incidence in TLH was noted to be approximately 1.3% but fell to under 0.1% with LSH. More recent studies have demonstrated that, when performing concomitant laparoscopic sacrocolpopexy, LSH has been shown to offer an almost fivefold decrease in mesh erosion compared with TVH. Accordingly, there is objective evidence that leaving the cervix does indeed offer protection from complications in selected patients.
Another driver of LSH has been patient perception that pelvic support and sexual satisfaction would be preserved or enhanced over TLH. However, a systematic review of randomized trials comparing LSH with TLH documented no differences in rates of incontinence, prolapse, dyspareunia, sexual satisfaction, transfusion rate, recovery times, and readmission rates. Interestingly,
a 2-year prospective study demonstrated significant overall improvement in sexual functioning, including increased libido, coital activity, and orgasm with decreased dyspareunia after TLH. Although several other studies have suggested increased orgasmic frequency after LSH, subsequent studies have failed to confirm these findings. Nonetheless, perception is a powerful motivating force. Many women contemplating elective hysterectomy consider preservation of the cervix a pivotal factor in the decision whether to undergo this procedure.
During the past decade, many gynecologists have reassessed the value of supracervical hysterectomy. The rationale for routine cervicectomy to prevent cervical cancer has been largely eliminated by the effectiveness of present-day cytologic and molecular screening for cervical disease, the natural history of human papillomavirus (HPV) infections in immunocompetent patients, and changes in treatment algorithms for preinvasive disease. Further, development of cervical dysplasia is rare in appropriately selected low-risk patients, and there is no evidence that supracervical hysterectomy increases the risk of cervical cancer. The incidence of cancer of the cervical stump appears to be equal to that of an intact uterus, as is the prognosis.
Finally, removal of the uterine corpus alone is often adequate treatment for women suffering from abnormal uterine bleeding or benign uterine fibroids. Nonetheless, many surgeons remain reluctant to offer this approach to women requiring hysterectomy for benign disease, citing the risk for persistent pain and cyclic vaginal bleeding necessitating subsequent trachelectomy. The incidence of persistent cyclic bleeding or spotting remains low (between 2% and 12%) and may be associated more with younger patients and those having preexisting endometriosis. In fact, some have considered the presence of extensive endometriosis to be a relative contraindication as these women may have persistence of dyspareunia if the cervix is retained.
The only absolute contraindication to supracervical hysterectomy is the presence of a malignant or premalignant condition of the uterine corpus or cervix. Supracervical hysterectomy is indicated for select patients who choose this procedure after appropriate counseling, and occasionally in surgical emergencies. Supracervical hysterectomy should not be performed simply because of the surgeon’s lack of comfort with removing the cervix. Instead, assistance from more skilled surgeons should be sought.
PREPARING FOR HYSTERECTOMY
Preoperative Preparation
Prior to any elective surgical intervention, patients should be optimized with respect to comorbidities such as diabetes, hypertension, pulmonary compromise, and underlying disease processes that may affect performance of the procedure and postoperative healing; this is no different with laparoscopic hysterectomy. Special attention should be made to coordinate care with the anesthesia team in light of physiologic stresses that abdominal insufflation and prolonged Trendelenburg positioning can have on critical cardiopulmonary physiology, including peak inspiratory pressures and cardiac preload. Few objective criteria guide this assessment; it is the product of the surgeon’s experience in consultation with the anesthesiologist.
In consideration of the Surgical Care Improvement Project (SCIP) protocol of the Joint Commission, plans for prophylactic antibiotics and deep vein thrombosis prophylaxis should be addressed, the latter of which may extend into the postoperative interval. Although there is no compelling objective evidence that a cathartic bowel preparation is indicated for benign gynecologic surgery, many surgeons find that decompression of the bowel through clear liquid diet or a mild laxative on the day prior to surgery is beneficial in keeping the bowel out of the surgical field.
Any discussion of patient preparation would be incomplete without mentioning the importance of informed consent. Notably, this is a process of patient counseling that is usually documented by a consent form. The process of informed consent should include a discussion of those risks that will be incurred by the patient related to any surgical procedure in general (e.g., complications of anesthesia, infection, bleeding, pain, scarring), related to the specific surgical procedure being performed (e.g., damage to the bowel, bladder, or ureters), and related to the instrumentation that will be used (e.g., thermal injury from electrosurgical devices or intraperitoneal dissemination of benign or previously undetected malignant tissue from the use of a morcellator). Further, the downstream consequences of those risks should be discussed (e.g., possible need for transfusion in cases of excessive blood loss or need for additional surgical procedures to address a ureteral injury). Finally, the potential need for conversion to a laparotomy should always be a part of the consent process for any laparoscopic procedure. In summary, the informed consent process is an educational process that should inform the patient, in terms she can understand, of all the relevant information a reasonable patient would want to know in order to make decisions related to the planned procedure.
Positioning and Port Placement
There are no specific variants from the basic principles of patient positioning for laparoscopic surgery that must be adapted for laparoscopic hysterectomy. However, a few key elements merit special attention. First, the patient should be situated sufficiently far down on the table. I find this critical concept to be underestimated often, only to encounter challenges with maneuvering the uterus when the table interferes with range of motion of the uterine manipulator after the procedure is well underway. One useful trick that can help address this is to rock the patient’s hips forward to reduce lordosis, which can stabilize the patient’s pelvis. Secondly, it is imperative that arm and leg position and padding relieve pressure points that could lead to nerve injury. A draw sheet placed across the operating table wrapped under well-padded arms and tucked back under the patient’s back can provide excellent arm stability, maintain good function of iv’s and blood pressure cuff, and allow for maximum mobility of the surgeon throughout the procedure. Third, extra measures should be taken to prevent patients from sliding cranially on the operating table with steep Trendelenburg positioning. A gel or egg crate foam pad, or a surgical beanbag, can stabilize even larger patients. Although shoulder braces are discouraged due to the increased potential for brachial plexus injury, placing them laterally over the acromioclavicular joint rather than medially on the shoulder may minimize that risk. Finally, prior to the surgical scrub, the patient should be placed in maximum Trendelenburg position to confirm adequate positioning and stability prior to starting the surgical procedure (tilt test).
Objective guidelines for optimal laparoscopic port placement are limited; the decision is usually influenced more by habit than logic. Importantly, although a surgeon may be comfortable with a particular port distribution, there needs to be flexibility in number and location of ports to accommodate procedure, pathology, and preference. Adding 1 to 2 more strategically positioned ports does not contribute significant morbidity, but may dramatically improve the ergonomics of the procedure while reducing surgical time.
The camera port is typically placed through the umbilicus in uncomplicated patients with a smaller uterus. This can be placed using a traditional closed technique before or after abdominal insufflation, with or without an optical trocar to visualize passing through abdominal wall layers. Alternatively, an open (Hasson) technique or a left upper quadrant (Palmer point) entry might be chosen. While these alternative modes of initial entry are often recommended in patients with prior surgery where periumbilical adhesions are suspected, there is no evidence that any specific entry technique always prevents injury to underlying viscera. However, the Hasson method has been associated with decreased incidence of vascular injury. A 1999 study of 814 patients undergoing left upper quadrant entry for laparoscopy described the incidence of infraumbilical adhesions depending on prior surgical history, including no prior incision (0.68%), prior laparoscopic procedure (1.6%), prior Pfannenstiel incision (19.8%), and prior vertical midline incision (51.7%). They also described the rates of “severe adhesions with potential risk for bowel injury” during trocar insertion in these same four groups as 0.42%, 0.80%, 6.87%, and 31.46%, respectively. However, they did not report the incidence of upper abdominal adhesions, nor did they include patients with prior splenectomy or bariatric surgery in whom the incidence of left upper quadrants adhesions may be increased. There is strong evidence to support using the surgeon’s most common initial entry method regardless of the underlying pathology.
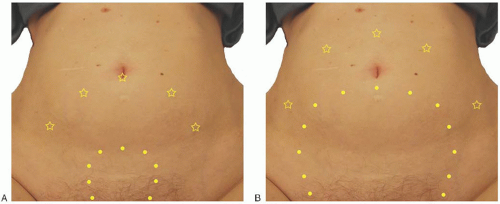
All subsequent accessory ports should be placed under laparoscopic guidance. Typical port placement is illustrated in
Figure 32C.1. It is usually helpful for at least one port to be a 10-to 12-mm size (often the midline port) to facilitate use of a 10-mm laparoscope, passing suture, introducing adhesion barriers, or passing specimen bags for tissue removal as needed. In most cases, all other ports can be 5 mm to minimize postoperative pain and reduce the chance of trocar site hernia. With a small uterus less than 10 weeks in size, a three-port technique is often adequate with umbilical placement of a camera port and a primary accessory port in the left and right lower abdominal quadrant. If the uterus is slightly larger, up to about 14 weeks in size, umbilical placement of a port for the camera still usually permits adequate visualization. However, additional (secondary) accessory ports placed higher and slightly more medial than the lower (primary) accessory ports may aid significantly with uterine manipulation and access to intended operative sites (
Fig. 32C.1A). In addition to using 10-mm angled telescopes, using a 5-mm straight, angled, or flexible telescope through well-positioned accessory ports at different times during the procedure can be very useful for optimal visualization of the operative field. The primary lower lateral ports should be positioned to provide a good angle for access to ipsilateral pelvic structures and to provide retraction for access to the contralateral pelvic structures. Caution should be taken not to place these ports too low on the abdominal wall close to the pelvic bones. Such a choice of port placement, which is often driven by concern for cosmesis rather than functionality, usually results in an insufficient angle to access to the ipsilateral deep pelvic structures and increases the chance of injury to the ilioinguinal, iliohypogastric, and superficial circumflex vessels. A point at least 2-cm cephalad to the anterior superior iliac spine and at least 2 cm lateral to the rectus sheath is generally a safe starting point for placement choice. Secondary accessory ports become more important contributors to the surgical procedure as uterine size increases. When used, they should be placed cephalad and medial enough to assist with both ipsilateral and contralateral surgical maneuvers and/or retraction and to provide adequate triangulation for suturing. With a larger uterus approximating 20 weeks’ size or more, port placement, including the midline camera port, will typically need to be displaced more cephalad but not necessarily more lateral as illustrated in
Figure 32C.1B.