Intrauterine Growth Restriction
Bronwen F. Kahn
John C. Hobbins
Henry L. Galan
Approximately 10% of the almost 4 million infants born each year in the United States are classified as low birth weight (LBW). Terminology used to describe the small fetus/newborn can be confusing. The term low birth weight is used clinically by pediatricians postnatally and is defined strictly as a birth weight of <2,500gm. Antenatally, the terms small for gestational age (SGA) and intrauterine growth restriction (IUGR) frequently are used interchangeably. However, the term small for gestational age encompasses a group of fetuses that are small for a variety of reasons that confer varying prognoses. These etiologies include infection, congenital malformations, aneuploidy, multiple gestation, maternal disease, malnutrition, and toxins and the normal or constitutionally small fetus. The term intrauterine growth restriction is a subgroup of SGA and more specifically identifies the fetus that is pathologically small. Placental insufficiency accounts for the majority of IUGR fetuses. It is important to recognize that not all fetuses or newborns classified as SGA are small due to pathologic reasons (i.e., constitutionally small) but simply represent the smaller fetuses/newborns at the lower end of the bell-shaped distribution of the normal population and are small for familial reasons. Conversely, some fetuses or neonates who are average for gestational age (AGA) may suffer from relative growth restriction if they are not achieving their individual, genetic growth potential. These babies may have normal weight percentiles for their gestational age but suffer from differential growth delay and show abnormal body proportions or ponderal indices. The prognosis for a given SGA fetus is dependent on the etiology.
The scope of the problem of IUGR is broad, not just because it increases morbidity and mortality of the fetus but also because it does so for the newborn and adult who the fetus is destined to become. IUGR places the fetus at risk for hypoxemia, acidemia, antepartum death, and intrapartum distress. Perinatal mortality rates in growth-restricted neonates are six to ten times greater than in normally grown age-matched controls. In one large series, 52% of unexplained stillbirths were growth restricted. In that series, suboptimal growth carried an odds ratio (OR) of 7.0 for sudden intrauterine unexplained death (95% confidence interval [CI] 3.3 to 15.1). IUGR places the neonate at risk for a number of metabolic disturbances, including polycythemia, pulmonary transition difficulties, intraventricular hemorrhage (IVH), impaired cognitive function, and cerebral palsy. The threshold of viability is both later in gestational age and larger in birth weight among neonates with severe IUGR compared with normally grown infants who are delivered at extremely preterm ages. Several epidemiologic and animal studies in the early 1990s began to report on long-term sequelae of IUGR, including adult hypertension, heart disease, stroke, and diabetes. The theory of fetal programming as the origin of adult disease is commonly referred to as the “Barker hypothesis.” The challenge in management of the IUGR fetus is to identify the condition and manage it so that adverse sequelae are minimized and balanced against the risks of premature delivery. The use of real-time ultrasound and Doppler velocimetry play pivotal roles in the diagnosis and management of IUGR. This chapter reviews normal placental–fetal growth, etiology of the SGA fetus, screening for growth restriction, and practical uses of ultrasound and Doppler velocimetry in the diagnosis and management of the IUGR fetus.
Determinants of Normal and Aberrant Placental Growth
Normal Placental Development
Normal growth of the fetus is dependent on normal placentation and growth of the placenta. The placenta is a dynamic and multifaceted organ that serves as
an interface between mother and fetus with the critical role of meeting the metabolic and circulatory demands of the growing fetus. The roles of the placenta include:
an interface between mother and fetus with the critical role of meeting the metabolic and circulatory demands of the growing fetus. The roles of the placenta include:
Nutritional: Provides oxygen, glucose, amino acid, and volume (fluid) transfer.
Immunologic: Protects the fetus from pathogens and the maternal immune system.
Endocrinologic: Produces numerous hormones, growth factors, cytokines, and other vasoactive mediators.
Metabolic: Serves as the respiratory organ and the kidney for the fetus and is responsible for elimination of carbon dioxide, metabolic acids, and other waste products from the fetus to maintain acid–base balance.
Research has begun to provide an understanding of the complexity of the implantation and placentation processes, which require the production and coordination of numerous angiogenic growth factors (fibroblast growth factor, hepatocyte growth factor, placental growth factor, vascular endothelial growth factor), cell-adhesion molecules, cytokines, nitric oxide, extracellular matrix metalloproteinases, hormones, and transcription factors (hypoxia-inducible factor). This process of coordination begins very early in pregnancy and can dictate whether the pregnancy grows in a normal or abnormal direction. By day 13, the cytotrophoblast layer has differentiated into invasive and noninvasive components. The invasive cytotrophoblast forms cell columns that anchor the trophoblastic tissue to the uterine epithelium and establish blood flow to the placenta and fetus. During this process, the invasive cytotrophoblast cells (extravillous trophoblast):
Migrate through the syncytiotrophoblast and into the decidualized endometrium and myometrium
Invade the vessel walls of the maternal spiral arteries in these areas
Induce the remodeling of the spiral arteries from high-resistance to low-resistance vessels.
As the invasive cell columns of the cytotrophoblast penetrate the syncytiotrophoblast, spaces called lacunae are created, which subsequently fuse to form the intervillous space with intervening syncytiotrophoblast columns called trabeculae. The process of intervillous space formation and spiral artery transformation directs an increasing maternal cardiac output into the intervillous space. Loss of spiral artery vessel media is the mechanism by which the spiral arteries decrease their resistance to blood flow (Fig. 13.1).
Angiogenesis represents the formation of new blood vessels from endothelial cells and is classified into branching and nonbranching stages. Branching angiogenesis occurs primarily in the first and early second trimesters and leads to the formation of the immature villous tree. Branching angiogenesis continues until the mid second trimester, when there is a transition to nonbranching angiogenesis. During this process, there is a dramatic elongation of the existing placental vascular tree. A dramatic decrease in vascular resistance and an increase in blood flow through the placenta are coincident with this process and occur via progressive loss of the musculoelastic media in the walls of the maternal spiral arterioles. The decrease in resistance is aided on the fetal side by further villous vascular branching, allowing both fetal and maternal circulations to convert to low-resistance, high-capacitance vascular beds. The progressive decline in vascular resistance is reflected in increasing end-diastolic velocities in Doppler flow velocity waveforms (FVW) of both the uterine and the umbilical arteries (Fig. 13.2). In fact, the resistance in the uterine artery has been shown to be lower on the placental side if the placenta is not in the midline, adding further support to the idea of placental-mediated remodeling of the maternal circulation.
Abnormal Placental Development
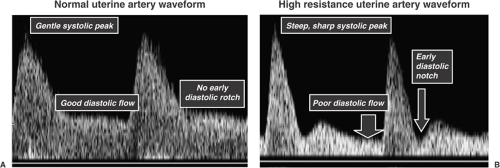
In pregnancies complicated by preeclampsia and IUGR, trophoblast invasion is limited to the decidualized endometrium, which results in failure of the spiral arteries to become low-resistance vessels. This failure can be detected by Doppler velocimetry of the uterine artery, which supplies blood to the spiral arteries. The blood FVWs in the uterine artery obtained with pulsed-wave Doppler velocimetry are reflective of the waveforms downstream at the spiral arteries. These abnormalities are identified on a Doppler FVW profile by a high-resistance pattern (low velocity of flow at end-diastole relative to that at systole) and by a protodiastolic (early diastolic) notch (Fig. 13.3). Failure of this process to occur on the maternal side of the circulation may lead to adverse effects on both the mother and the fetus. Maternal vascular endothelial dysfunction may lead to production of a variety of vasoactive mediators, which could subsequently lead to the development of preeclampsia. Sibai and colleagues have recently published a hypothesis addressing the observation that preeclampsia and IUGR share similar placental pathology and that women who have had a pregnancy complicated by either are at higher risk of cardiovascular disease later in life. They propose that endothelial dysfunction underlies both conditions by predisposing to shallow placentation but that women with metabolic syndrome are prone to preeclampsia. This may be mediated by the action of elevated circulating cytokines. Women with no predisposition to metabolic syndrome, however, may develop IUGR but not preeclampsia. A variety of villous and vascular abnormalities have been described in the placenta of the IUGR fetus. Placentas from IUGR pregnancies have fewer gas-exchanging villi. The villi also are slender, elongated, poorly branched, and poorly capillarized. Vascular abnormalities include reduced branching of stem arteries and disorganized
vascular patterns, including less coiling as depicted by placental vascular cast studies. The reduced branching seen in the villous vasculature creates abnormal blood flow and an increase in vascular resistance to flow that can be likened to that of an electric circuit—the fewer downstream tributaries that exist from the main supply line, the higher the resistance.
vascular patterns, including less coiling as depicted by placental vascular cast studies. The reduced branching seen in the villous vasculature creates abnormal blood flow and an increase in vascular resistance to flow that can be likened to that of an electric circuit—the fewer downstream tributaries that exist from the main supply line, the higher the resistance.
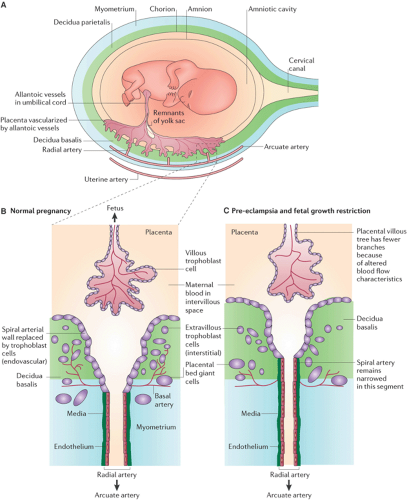 Figure 13.1 (A) Gross anatomy of uterus with placental implantation and blood supply. Remodeling of the spiral arteries in normal pregnancy (B), demonstrating the normal dilation of the artery by means of loss of elastic vascular media and preeclampsia or fetal growth restriction (C) with abnormal invasion and remodeling that impedes the development of low-resistance flow. (Reprinted from Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol 2006;6(8):584–594 . Copyright © Nature Publishing Group, with permission.) (See Color Plate) |
Determinants of Normal and Aberrant Fetal Growth
Normal Fetal Growth
In order for a fetus to grow normally, the placental developmental activities described earlier must proceed
undisturbed. Fetal growth velocity increases across gestation until it peaks at 30 to 35 g per day or 230 to 285 g per week between 32 and 34 weeks. After that, the rate of weight gain decreases, reaching a plateau at 40 weeks and even a decline, or weight loss, at 41 to 42 weeks. At 37 weeks gestation, the placenta has reached maximal villous nutrient exchange via a surface area of 11 m2 and weighs approximately 500 g. Coincident with this is maximal amniotic fluid volume and maximal human placental lactogen levels, suggesting peak placental function. Interestingly, although the fetus grows less quickly at term, calorie acquisition by the fetus continues to be quite high. At this time in pregnancy, the fetus is rapidly accumulating fat that provides thermal stability for the immediate postnatal period. Fat has a high caloric content (9 calories/g) compared with carbohydrates and proteins (4 calories/g). The high metabolic demands of the fetus result in a fetal temperature that is 0.5°C above that of the mother. This difference in temperature is seen in the maternal immediate postpartum shivering, which reflects a compensatory response to the loss of fetal-derived heat.
undisturbed. Fetal growth velocity increases across gestation until it peaks at 30 to 35 g per day or 230 to 285 g per week between 32 and 34 weeks. After that, the rate of weight gain decreases, reaching a plateau at 40 weeks and even a decline, or weight loss, at 41 to 42 weeks. At 37 weeks gestation, the placenta has reached maximal villous nutrient exchange via a surface area of 11 m2 and weighs approximately 500 g. Coincident with this is maximal amniotic fluid volume and maximal human placental lactogen levels, suggesting peak placental function. Interestingly, although the fetus grows less quickly at term, calorie acquisition by the fetus continues to be quite high. At this time in pregnancy, the fetus is rapidly accumulating fat that provides thermal stability for the immediate postnatal period. Fat has a high caloric content (9 calories/g) compared with carbohydrates and proteins (4 calories/g). The high metabolic demands of the fetus result in a fetal temperature that is 0.5°C above that of the mother. This difference in temperature is seen in the maternal immediate postpartum shivering, which reflects a compensatory response to the loss of fetal-derived heat.
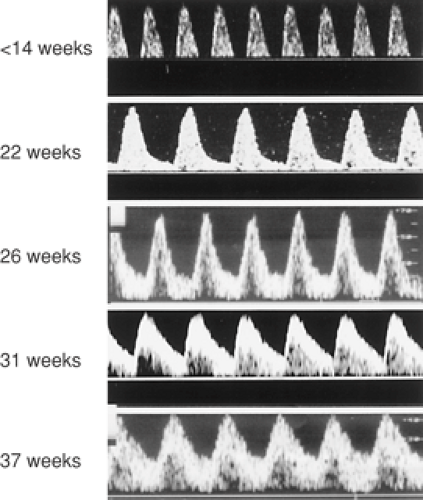 Figure 13.2 Doppler FVW profiles in the umbilical artery across normal gestation. Note the progressive increase in end-diastolic flow. |
For the fetus to achieve maximal growth potential, the uterine–placental–fetal circulation must be normal in order for the fetus to receive a variety of necessary substrates. A key feature of the uterine vascular bed in pregnancy is the lack of responsiveness to changes in blood gas tensions (PO2, PCO2). Thus, oxygen therapy for either maternal disease or for fetal benefit will not cause vasoconstriction. In contrast, the lack of responsiveness and lack of autoregulation in the uterine vascular bed renders these vessels incapable of compensating for maternal hypotension. Animal studies have shown that the volume blood flows (mL per minute) in the uterine and umbilical circulations are unaffected by maternal hyperoxygenation. This is important clinically since the improvement in fetal PO2 by maternal oxygen therapy does not appear to have an adverse affect on fetal blood flow.
Glucose, oxygen, and amino acids are the major substrates needed for normal fetal growth. Glucose freely crosses the placenta by facilitated diffusion into the fetus. The maternal–fetal glucose concentration gradient that exists widens with advancing gestational age in order to accommodate the increasing metabolic demands of the fetus. Under normal circumstances, glucose is metabolized by the fetus to produce energy in the form of adenosine triphosphate (ATP) in the presence of oxygen. Oxygen passes across the placenta to the fetus by simple diffusion and is regulated by concentration gradients and uterine blood flow, as described by the Fick principle. Transplacental transport of all essential and nonessential amino acids
occurs by active transport. Animal and human studies have confirmed that amino acid carrier systems are present on both the maternal and fetal sides of the placenta. The placenta is quite active in amino acid metabolism, contributing significantly to net umbilical–fetus uptake of certain amino acids.
occurs by active transport. Animal and human studies have confirmed that amino acid carrier systems are present on both the maternal and fetal sides of the placenta. The placenta is quite active in amino acid metabolism, contributing significantly to net umbilical–fetus uptake of certain amino acids.
Abnormal Fetal Growth
Failure of the placenta to deliver any of these primary substrates to the fetus will result in diminished protein production by the fetus, reduced glucose metabolism, and reduced glycogen deposition in the liver. If oxygen supply is markedly reduced either from an acute or chronic insult, the fetus will convert from an aerobic to an anaerobic metabolic state in order to meet energy (ATP) requirements. Anaerobic metabolism is far less efficient at producing ATP from a given unit of glucose compared with aerobic metabolism. Furthermore, anaerobic activity will produce “fixed” acids (lactate, urate, etc.), which diffuse slowly across the placenta thus accumulating in the fetal system. If the anaerobic process is not reversed, the accumulation of acids will consume available buffers, and the fetal blood pH will fall, leading to an acidemic and acidotic fetus.
The IUGR fetus attempts to compensate for reduced substrate delivery by several mechanisms. From a metabolic standpoint, the fetus changes the maternal–fetal glucose gradient. The normally wide glucose gradient that exists between the mother and fetus, which is needed for movement of glucose to the fetus, widens further. This compensatory mechanism enhances glucose movement across the placenta to the fetus. The smaller AC noted in the IUGR fetus is a result of less hepatic glycogen formation in order to maximize glucose availability. In a similar fashion to the liver, fat stores, which are normally an important depot site for fat-soluble vitamins and fatty acids, are reduced. This change in body composition is reflected in the ponderal index, which neonatologists use as an index of “scrawniness.” The fetus also adjusts to reduced nutrient delivery by redistributing systemic blood flow to vital organs. The fetus will reduce flow to nonvital organs by reducing vascular resistance and increasing blood flow to the brain, which normally has a relatively high vascular resistance pattern compared with other organ systems. This can be demonstrated with pulsed-wave Doppler velocimetry of the middle cerebral artery (MCA), in which the flow velocity profile shows an increase in end-diastolic velocity. Other organs being “spared” through vascular redistribution include the heart and adrenal glands.
Intrauterine Growth Restriction Defined
A number of definitions of IUGR have been proposed based on percentile, standard deviation (SD), or growth rate. The most commonly used clinical definition of intrauterine growth restriction is an estimated fetal weight (EFW) less than the tenth percentile as determined by ultrasound. This mirrors the definition of small for gestational age, which originally was described by Battaglia and Lubchenco in 1967 as a birth weight less than the tenth percentile for gestational age. They noted that SGA infants were at increased risk for neonatal death. The problem with the tenth percentile as a cutoff for the diagnosis of IUGR is that a number of fetuses with an EFW below that value will be normally small, otherwise referred to as “constitutionally” small and not at risk. Studies have demonstrated that if determinants of birth weight such as maternal ethnicity, parity, maternal weight, and height are considered, up to 50% of fetuses at less than the tenth percentile will be constitutionally small. This has been the basis for using other definitions, including less than the third or fifth percentile or less than two SDs from the mean. Some authors have suggested using an abdominal circumference (AC) of less than two SDs for gestational age. The AC measurement represents a single objective ultrasound measurement rather than a combination of several fetal biometric ultrasound parameters into a formula where each parameter is weighted differently. This is the measure most closely related to pathologic growth restriction, as it reflects loss of hepatic glycogen stores and subcutaneous fat, which is correlated with fetal nutritional status.
While it seems that an EFW less than the tenth percentile is not a sufficiently strict definition of IUGR, there is also a significant problem associated with the use of more strict criteria. If a definition of less than the third percentile is used, there is a reasonable chance that one could miss some fetuses that do not meet their growth potential and could be at risk for adverse events. No receiver operator curves have been established to assess sensitivity and specificity in order to establish a “cutoff” for the diagnosis of IUGR. This has been quite difficult to do, in part because of a wide biologic variation between patients and because of a wide variation of parameters used to diagnose IUGR (EFW, AC <2 SD, etc.). Customized growth curves, such as those envisioned and created by Gardosi, which include variables that impact fetal size, may be the answer to establishing a better cutoff value for IUGR. These growth curves are calculated based on maternal ethnicity, parity, height and weight, and fetal gender. When these curves were used in a large retrospective cohort study and compared with the standard population-based growth curve that is based on gestational age alone, a further 4.1% of infants were identified who were SGA. These babies had perinatal outcomes comparable to infants who were SGA by population-based standards, with three- to eightfold increases in perinatal morbidity and mortality above AGA neonates. This study also identified a population of babies who were SGA by population-based standards but not by customized curves and whose perinatal outcomes were similar to AGA babies, with no increased risks based on their size. Software
is available (Gestation-related Optimal Weight (GROW) at http://www.gestation.net) in which one may enter specific maternal and fetal variables to generate such a customized growth curve.
is available (Gestation-related Optimal Weight (GROW) at http://www.gestation.net) in which one may enter specific maternal and fetal variables to generate such a customized growth curve.
Etiology of Intrauterine Growth Restriction
The type and timing of insult during fetal development will dictate the subsequent development and morphology of the fetus. Fetal growth in the first trimester is characterized primarily by hyperplasia (growth in the number of cells), in the second trimester by a combination of hyperplasia and hypertrophy (growth in the size of existing cells), and in the third trimester primarily by hypertrophy. If an insult occurs in the first half of pregnancy where hyperplasia predominates, all fetal cell numbers can be reduced and lead to a small fetus that is symmetrically proportioned. That is, somatic and cerebral growth will both be similarly reduced. The underlying etiology of symmetric IUGR varies widely and includes karyotypic abnormalities, congenital anomalies, or congenital infections. Maternal medical illness, obstetric conditions, or primary placental pathology place the fetus at risk for uteroplacental insufficiency that may lead to a small fetus that is asymmetrically proportioned. Because there is significant overlap between these two types, body proportion alone cannot be used to determine the etiology. If an insult that typically causes uteroplacental insufficiency occurs sufficiently early in pregnancy, there can be an impact on hyperplasia of cells and a symmetric growth pattern. More commonly, there is an impact on hypertrophy that occurs late in pregnancy and primarily affects fat and hepatic glycogen deposition. The reduction in hepatic glycogen stores reduces liver size and results in an increase in the head circumference to abdominal circumference, which defines asymmetric growth. Asymmetric growth is also characterized by a redistribution of fetal cardiac output to vital organs including the brain, heart, and adrenal glands. The redistribution of blood flow to the head allows the fetal head and brain to be preserved and to maintain a normal growth velocity compared with parameters of somatic growth (abdomen and extremities). This is the basis for the common phrase, “brain sparing.” Thus, the relative proportions of fetal dimensions can provide some insight to the etiology of IUGR based on the symmetric or asymmetric nature of the ultrasound parameters.
Diagnosis of Intrauterine Growth Restriction
Dating the Pregnancy
Accurate dating of pregnancy begins with the establishment of the estimated date of confinement (EDC) based on information gathered on the last menstrual period (LMP). Normal human gestation lasts 280 days from the LMP. The 280-day gestation is, in turn, based on a normal menstrual cycle length of 28 days. However, many patients do not know the date of their LMP, and others will have menstrual cycle lengths that vary from 21 to 35 days, which will shorten or lengthen the gestational dating, respectively. Therefore, the EDC should be adjusted accordingly. Other important questions regarding the LMP include regularity, certainty (calendar recorded, etc.), date of conception, and oral contraceptive use at the time of the LMP. It has been reported previously that the LMP is unreliable up to one third of the time, and it is particularly in these circumstances that ultrasound becomes a valuable resource. Early ultrasound has been shown to be more accurate than most menstrual dating. Because it is more common to ovulate late in a cycle than early, gestational age established by ultrasound is usually younger than menstrual age. Therefore, the new EDC is most often later, meaning that if the first ultrasound is done late in the second trimester, SGA may be overdiagnosed. Well-established dating reduces the incidence of post-term pregnancy as well as false-positive diagnoses of SGA.
After establishment of gestational age by LMP and ultrasound, clinical acumen can lead to a presumptive diagnosis of IUGR. The most commonly used clinical tool for assessing growth of the pregnancy is serial measurement of uterine fundal height during regular clinic visits. Measurement of uterine fundal height (in centimeters) from the symphysis pubis across the uterus to the top of the fundus provides an index of growth for which a nomogram has been reported. In general, the uterine fundus will be within 2 cm of the gestational age in weeks. This simple screening technique has been reported to be up to 75% accurate in diagnosing IUGR. However, the measurement may be erroneous because of several variables that impact uterine size, including interobserver variation, obesity, uterine fibroids, multiple gestation, and polyhydramnios.
Ultrasound
Ultrasound remains the cornerstone for the diagnosis and management of the SGA fetus. The diagnosis of SGA is made by combining ultrasound biometric measurements of the fetus into a formula that calculates the EFW. The most commonly measured fetal biometric parameters include the biparietal diameter (BPD), the head circumference (HC), the AC, and the femur length (FL). As described earlier, SGA is diagnosed when the EFW falls below the tenth percentile for gestational age. If available, it is important to use local standards for the diagnosis, as it has been shown previously that the tenth percentile for EFW can vary depending on the population studied. For example, a fetal weight nomogram constructed by ultrasound in Colorado suggests that consistently, across gestational ages, fetuses in Denver are lighter than fetuses at sea level. The Shepard and Hadlock formulas are the most commonly used
formulas for calculating EFW. Table 13.1 shows the EFW using Hadlock’s formula plotted across gestation, with separate EFWs for the tenth percentile depending on fetal gender. In general, the more parameters included, the more accurate the EFW (Hadlock: 4 parameters; Shepard: 3 parameters). However, as the number of parameters in the formula increases, EFW begins to lose accuracy because of the standard error of the method associated with the measuring of each parameter. Using the Shepard formula, a practitioner will obtain an EFW that will fall within 5% of the true weight 50% of the time and within 10% of the weight 80% of the time.
formulas for calculating EFW. Table 13.1 shows the EFW using Hadlock’s formula plotted across gestation, with separate EFWs for the tenth percentile depending on fetal gender. In general, the more parameters included, the more accurate the EFW (Hadlock: 4 parameters; Shepard: 3 parameters). However, as the number of parameters in the formula increases, EFW begins to lose accuracy because of the standard error of the method associated with the measuring of each parameter. Using the Shepard formula, a practitioner will obtain an EFW that will fall within 5% of the true weight 50% of the time and within 10% of the weight 80% of the time.
TABLE 13.1 In Utero Fetal Weight Standards at Ultrasound | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A commonly encountered clinical scenario is the patient who is sent for evaluation of suspected IUGR in whom clinical dating criteria are poor and gestational age unknown. For example, a fetus measuring 3 to 4 weeks less than expected may be the result of any of the following three possibilities:
The patient is 3 to 4 weeks off on clinical dating.
The fetus is truly 3 to 4 weeks less than clinical dating but is genetically predisposed to be small.
The fetus is small, growth-restricted, and at risk.
Several ultrasound strategies are available to address this problem and to categorize the dating of pregnancy with reasonable accuracy. Ultrasound biometric parameters can be used to calculate ratios and provide some insight into the severity of the IUGR. In the 1970s, Campbell and Thoms first described the HC/AC ratio. In approximately 60% of cases with IUGR, the HC/AC ratio is greater than the 90th percentile for gestational age, which suggests a brain-sparing process. The FL/AC ratio provides information on the amount of muscle and fat mass present on the fetus, providing a picture of “scrawniness” for the fetus. This is analogous to the ponderal index used by neonatologists. Unlike the HC/AC ratio, the FL/AC ratio is gestational-age independent and may be useful when the gestational age is unknown. Other aspects of the fetus that appear to be relatively independent of the IUGR process and that remain consistent throughout gestation include the transcerebellar diameter (TCD), foot length, and epiphyseal centers. These are other strategic tools to help approximate the gestational age when dating criteria are poor.
The TCD measured in millimeters mirrors the gestational age until about 22 weeks and then accelerates. Cerebellar measurements are shown in Table 13.2. If the
gestational age by TCD is greater than that suggested by other biometric parameters and is consistent with the unsure LMP dating criteria, it may be that the fetus is indeed further along and possibly growth restricted. In a similar fashion, Hadlock and colleagues showed that the foot length is gestational-age independent and may be useful in IUGR. Although not useful for estimating gestational age in the second and early third trimester, the appearance of epiphyseal centers of the long bones on ultrasound provides reassurance that the fetus is in the second half of the third trimester of gestation. In general, the distal femoral epiphysis appears at 32 to 34 weeks gestation, the proximal epiphysis at 36 weeks, and the proximal humeral epiphysis at 38 weeks. In a nondiabetic population, the presence of a distal femoral epiphysis of greater than 3 mm and the presence of any proximal tibial epiphysis indicate a mature lung profile in almost all cases. The presence of the tibial and humeral epiphyseal centers are useful markers of pulmonic maturity, while the absence of these centers may suggest a more immature fetus.
gestational age by TCD is greater than that suggested by other biometric parameters and is consistent with the unsure LMP dating criteria, it may be that the fetus is indeed further along and possibly growth restricted. In a similar fashion, Hadlock and colleagues showed that the foot length is gestational-age independent and may be useful in IUGR. Although not useful for estimating gestational age in the second and early third trimester, the appearance of epiphyseal centers of the long bones on ultrasound provides reassurance that the fetus is in the second half of the third trimester of gestation. In general, the distal femoral epiphysis appears at 32 to 34 weeks gestation, the proximal epiphysis at 36 weeks, and the proximal humeral epiphysis at 38 weeks. In a nondiabetic population, the presence of a distal femoral epiphysis of greater than 3 mm and the presence of any proximal tibial epiphysis indicate a mature lung profile in almost all cases. The presence of the tibial and humeral epiphyseal centers are useful markers of pulmonic maturity, while the absence of these centers may suggest a more immature fetus.
TABLE 13.2 A Nomogram of the Transverse Cerebellar Diameter (in millimeters) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||