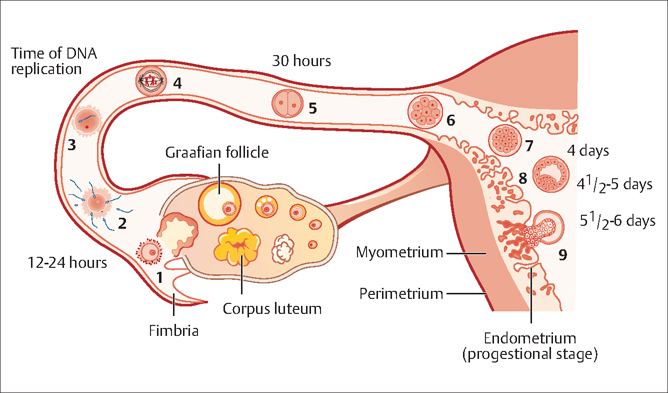
42 Infertility Robert L. Barbieri Fertility is defined as the capacity to conceive and produce offspring. Infertility is the state of a diminished capacity to conceive and bear offspring. In contrast to sterility, infertility is not an irreversible state. The current clinical definition of infertility is the inability to conceive after 12 months of frequent coitus. Infertility prevalence among women aged 15–44 years was 8.5% in 1982 and 7.4% in 2002. Infertility prevalence is greater in older women and possibly in older men. Since the fertility potential of the female partner decreases after 35 years of age, some authorities recommend initiating an infertility evaluation after 6 months of attempting conception in women 35–40 years of age, and immediately in women over 40 years of age. In most mammals, including humans, pregnancy requires the effective function of at least five systems or cell types: Abnormalities in any of these five systems can result in infertility. Based on our current understanding of the causes of infertility, and the most contemporary diagnostic approach to infertility, the most common single causes of infertility are: Fig. 42.1 Schematic representation of the transport of the oocyte, sperm and embryo in the female reproductive tract (1) Ovulation of competent oocyte (2) Fertilization of oocyte, (3, 4) movement of single cell prezygote through the fallopian tube, (5) multicellular embryo in fallopian tube, (6, 7) arrival of the multicellular embryo into the uterus 4–5 days after ovulation, (8) formation of blastocyst, and (9) implantation into endometrium 5–6 days after ovulation In an in vitro fertilization cycle, it is common to observe a 2-cell embryo form, about 1 day after fertilization, a 4-cell embryo form about 2 days after fertilization, and a blastocyst about 5 days after fertilization Three diagnostic tests should be performed in almost all couples with infertility: In some couples, especially where the female partner is greater than 35 years of age, a test should be performed to assess the size of the remaining ovarian oocyte pool. Women who have monthly menses and report moliminal symptoms—such as breast tenderness and dysmenorrhea—are typically ovulatory. In one study, women who reported regular monthly menses were demonstrated to be ovulatory in over 95% of cases. Conversely, women with amenorrhea are likely to be anovulatory (or have uterine adhesions or cervical obstruction preventing menstrual fow). Oligomenorrhea (cycle length >35 days) is typically associated with oligo-ovulation. The least expensive laboratory method for detecting ovulation is the measurement of the basal body temperature. For most women, the morning basal temperature obtained prior to rising from bed is less than 36.7°C (98°F) before ovulation and more than 36.7°C after ovulation. Progesterone production from the ovary appears to raise the hypothalamic set-point for basal temperature by approximately 0.6°F. The normal luteal phase is typically associated with a temperature rise, above 36.7°C, for at least 10 days in length. Occasionally, basal body temperature recordings may appear monophasic, even in the presence of ovulation. A biphasic pattern is almost always associated with ovulation. If the pattern is biphasic, coitus can be recommended every other day for a period including the five days prior to, and the day of, ovulation. The main source of progesterone secretion in women is the corpus luteum, which develops from the dominant ovarian follicle after ovulation. Prior to ovulation, the ovary secretes small quantities of progesterone. A serum progesterone level greater than 3 ng/mL is diagnostic of ovulation. A mid-luteal progesterone concentration less than 10 ng/mL is associated with a lower per-cycle pregnancy rate than progesterone levels above 10 ng/mL. A urine luteinizing hormone (LH) surge is detectable with a home immunoassay kit about 1 day before ovulation. In about 90% of cases, the detection of a urine LH surge is associated with a subsequent ovulation, as demonstrated by an appropriate rise in serum progesterone. The oocytes with the best potential for developing into competent embryos appear to be ovulated first. When there are only a small number of oocytes remaining in the ovary, it is likely that these oocytes contain genetic abnormalities that reduce their potential to develop into competent embryos. Detection of women with a low number of ovarian oocytes is predictive of fertility and the effectiveness of fertility treatments. The best single method for detecting a low number of ovarian oocytes is measurement of serum follicle-stimulating hormone (FSH) during menstrual cycle day 2, 3, or 4 (the second, third or fourth day of menses). This is called the “day 3 FSH” test. During menses, FSH secretion is suppressed by the production of inhibin B from ovarian follicles containing competent oocytes. When the ovarian oocyte pool is small, inhibin B production is low and FSH secretion is increased resulting in serum FSH levels greater than 15 U/L on cycle day 3. Alternatively, serum inhibin B levels can be measured. Low inhibin B levels indicate few remaining ovarian oocytes. However, it is technically challenging to reliably detect low levels of inhibin B. High resolution pelvic sonography can also be used to count the number of follicles (which contain the oocyte) in the ovary. The greater the number of resident follicles detected by ultrasound scanning, the more likely that there is an adequate ovarian oocyte pool. The semen analysis is performed on an ejaculated semen specimen. In some cases, the semen can be obtained by suggesting the couple have sexual intercourse using a condom that contains no spermicide. The semen analysis includes the following measurements: semen volume, sperm concentration, sperm motility, and a microscopic evaluation of sperm morphology. The normal parameters for a semen specimen are listed in Table 42.1. In men with one abnormal or borderline semen analysis, it is prudent to repeat the test. If the semen analysis demonstrates a very low sperm concentration (<5 million/mL) a karyotype should be performed to look for defects in the Y chromosome and other problems such as balanced translocations. Obtaining a hysterosalpingogram (HSG) involves the injection of a contrast agent into the cervical os, so that the agent may travel through the uterus and fallopian tubes into the peritoneal cavity. The course of the contrast agent through the reproductive tract is monitored using fuoroscopy. The HSG is very sensitive for detecting tubal disease. It is especially specific for identifying distal tubal occlusion at the fimbriated ends of the tube. In some HSG tests, proximal tubal occlusion at the utero-tubal junction is detected, but follow-up tests such as selective catheterization of the fallopian tubes demonstrates the proximal tube to be patent. In some couples, laparoscopy of the female partner may be of benefit in the diagnosis and treatment of infertility. As noted above, about 7% of women with infertility have endometriosis, and the only test that can reliably diagnose endometriosis is laparoscopy. If endometriosis is detected at the time of laparoscopy, it can be surgically treated at the same time, which results in an improvement in fertility. Many clinicians reserve laparoscopy for women who have symptoms (severe dysmenorrhea) or physical findings (nodular uterosacral ligaments) suggestive of the disease; and for couples in whom all infertility testing was normal. Two commonly performed tests, the postcoital test and the endometrial biopsy, are of no value in evaluating most couples with infertility. The postcoital test is performed by getting the couple to have sexual intercourse just prior to ovulation. A speculum exam of the vagina and cervix is performed and a quantity of the cervical mucus is obtained with forceps for examination under the microscope. If motile sperm are present in sufficient quantities the test is normal. If no motile sperm are present, the test is deemed abnormal. In randomized trials of the clinical utility of the postcoital test it was found that it offered no incremental information that was not obtained with the basic three fertility tests; and that it did not influence treatment decisions or fertility outcomes. The test is of no value in evaluating the infertile couple. In the past, the endometrial biopsy was regarded as the “gold standard” for determining whether the endometrium was properly receptive for an incoming embryo. In a well-designed observational study, women with infertility and fertility had similar rates of abnormal, “out of phase” endometrial biopsy results. This indicated that the test cannot discriminate between fertile and infertile women. The endometrial biopsy is of no value in the evaluation of the infertile couple.
Definition
Causes
Diagnostic Tests
Tests of Ovulation
Testing of the Ovarian Oocyte Pool
Semen Analysis
Hysterosalpingogram
Laparoscopy
Other Tests
| Measurement | Normal range | Comments |
| Semen volume | 2–5 mL | A very low volume may be due to a poorly collected ejaculate or genital tract obstruction |
| Sperm concentration | >20 million sperm per mL | Sperm concentration is an important predictor of male fertility Values less than 10 million sperm per mL are especially indicative of male infertility |
| Sperm motility | >50% of sperm should be motile | When sperm concentration is low–normal, good motility may “make up” for the low number of sperm and be associated with good fertility prognosis |
| Sperm morphology | Evaluation of sperm shape, length, width, volume of acrosome body and assessment of head and tail defects Using strict criteria, normal morphology is diagnosed when =15% of sperm have normal form. Using lenient criteria, >30% should have normal form | Sperm morphology is an important predictor of male fertility |
Treatment
Whenever a clinician approaches the treatment of infertility, alternative choices, such as adoption or remaining childless should be explored with the couple. Treatment of infertility may be stressful for couples and disappointing if it is not successful. In many locales, some aspects of infertility treatment may not be covered by health insurance plans, making treatment an expensive part of a family budget.
Male Factor
Options for the treatment of male factor infertility include: semen donation from a well-screened anonymous donor; intrauterine insemination of the female with the male partner’s sperm; and assisted reproductive technology involving in vitro fertilization (IVF) combined with microinjection of a sperm into an oocyte in the laboratory.
Sperm Donation
Donor sperm is typically obtained from men in their 20s who have been thoroughly screened for genetic and sexually transmitted diseases. Donated specimens from these men are cryopreserved in aliquots, which are shipped to the point of clinical care. At the point of care, the sperm specimens are thawed and typically an intrauterine insemination (IUI) is performed to ensure that the maximal number of sperm are delivered high into the reproductive tract. In couples with male factor infertility and a baseline pregnancy rate of 2% per cycle, donor sperm may increase the per cycle pregnancy rate up to 15–25% per cycle. Success of sperm donation is highly dependent on the age of the female partner because, in older women, the ovarian oocyte pool is depleted.
Intrauterine Insemination
In couples where there is a mild male-factor infertility, intrauterine insemination may result in pregnancy. The IUI procedure consists of washing an ejaculated semen specimen to remove prostaglandins, concentrating the sperm in a small volume of culture medium with a high protein concentration which initiates the sperm acrosome reaction, and injecting the sperm suspension directly into the upper uterine cavity using a small catheter threaded through the cervix. IUI deposits a large number of motile sperm high in the female reproductive tract, increasing the number of sperm that reach the ovulated oocyte, and enhancing the pregnancy rate. After coitus, the sperm deposited in the vagina must traverse the cervical mucus, the entire uterus, and the fallopian tube. After IUI, the sperm only need to traverse the fallopian tube. The insemination is timed to take place just prior to ovulation, typically using home urine LH measurement. If the baseline pregnancy rate in an infertile couple is in the range of 2% per cycle, IUI improves the per cycle pregnancy rate to about 4–6% per cycle. A major advantage of the IUI is that it does not require either the male or female partner to take stimulatory hormones.
Intracytoplasmic Sperm Injection
Intracytoplasmic sperm injection (ICSI) is performed by injecting the female partner with FSH and stimulating the development of multiple follicles. The female partner undergoes ultrasound-guided aspiration of the oocytes from the ovary. Once the oocytes are mature, a single sperm is injected into each oocyte using a microscopic injection technique. Remarkably, ICSI can also be used in combination with testicular sperm extraction to produce paternity in men with absolute azoospermia (see Evidence Box 42.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


