Infection
George T. Drugas
Steven Ognibene
Walter Pegoli Jr.
Department of Surgery, Division of Pediatric Surgery, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642-8410.
Department of Surgery, Division of Pediatric Surgery, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642-8410.
Department of Surgery, Division of Pediatric Surgery, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642-8410.
Prior to the acceptance and application of Joseph Lister’s principles of antisepsis in the late 1860s, surgical interventions were often associated with “irritative fever,” followed by purulent drainage from their wounds, overwhelming infections, and even death. Lister’s contributions changed the practice of surgery from an activity inextricably linked to infection and death to a discipline that could eliminate suffering and prolong life.
Currently, in the United States, more than 28 million surgical procedures are performed yearly (1). Surgical site infections (SSIs), referred to as surgical wound infections prior to 1992, rank third among those reported to the Centers for Disease Control and Prevention’s National Nosocomial Infections Surveillance System (NNIS) and account for 15% of all nosocomial infections among hospitalized patients (2). Two-thirds of SSIs were confined to the wound, and one-third involved organs or body spaces accessed during the procedure. When surgical patients with nosocomial SSI died, 77% of the deaths were infection related, and the majority (93%) involved organs or spaces accessed during the operation (3).
SSIs are defined as either incisional or organ/space infections that occur within 30 days after operation or within 1 year if an implant is left in place and the infection appears to be related to the operation. Incisional SSIs are further subdivided into those that involve skin and subcutaneum (superficial incisional SSI) or those involving the deeper soft tissues such as muscle and fascia (deep incisional SSI). Organ/space SSIs involve any body part, other than the incised wall layers, that was violated during an operation.
DETERMINANTS OF INFECTION
The risk of any infection can be expressed by the following equation:
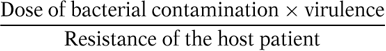
The dose of bacterial contamination is a key determinant of infection. Quantitatively, if a surgical site is contaminated with more than 105 microorganisms per gram of tissue, then the risk of SSI is markedly increased (4). The dose of microorganisms required to produce infection may be considerably lower in the presence of foreign material (i.e., 100 staphylococci per gram of tissue introduced on silk suture) (5). The number of organisms required to produce clinical infection predictably decreases in states of diminished host resistance.
The virulence of a microorganism refers to its ability to invade, damage, or survive in host tissue. Some gram-negative bacteria elaborate endotoxin that causes no local injury, but stimulates cytokine production. Cytokines trigger the systemic inflammatory response syndrome (SIRS), which may lead to organ failure. Other bacteria possess cell surface polysaccharide capsules that inhibit phagocytosis, an early host defense to microbial contamination. Certain strains of Clostridia and Streptococci elaborate exotoxins that disrupt host cell membranes or alter cellular metabolism. Coagulase-negative Staphylococci produce glycocalyx, a “slime” that physically shields the bacteria from phagocytes or inhibits antibiotic uptake (6). Virulence is also impacted by the interactions between different species of microorganisms. Most notable is the species synergism in mixed anaerobic and aerobic infections associated with intraabdominal abscess. Anaerobic bacteria flourish in the presence of aerobic species capable of reducing the oxygen content of local tissues. In turn, the aerobes benefit from the increased soft tissue invasion imparted by the anaerobes.
Host resistance to infection occurs on multiple fronts, ranging from simple mechanical barriers to complex interactions mediated by lymphocytes, complement, and antibodies. These defense mechanisms work to protect the body from virulent pathogens. The initial defense is a physical barrier that prevents microorganisms from
penetrating inner compartments. Intact skin has a pH between 5 and 6, and the fatty acids secreted from sebaceous glands inhibit proliferation of bacteria on the skin. The mucociliary transport mechanism lining the respiratory tract traps and propels inhaled pathogens to the oropharynx. The harsh acidic gastric milieu destroys organisms ingested orally. The periodic flushing of the urinary tract reduces the number of adherent microorganisms. Likewise, the washing action and immunoglobulin and lysozyme components of tears protect the corneal surface of the eye (7). Alterations of these anatomic barriers diminish the host’s resistance to infection. Surgical incisions, trauma, and burns allow access to deeper soft tissues. Smoke inhalation disrupts the mucociliary ladder of the respiratory tree.
penetrating inner compartments. Intact skin has a pH between 5 and 6, and the fatty acids secreted from sebaceous glands inhibit proliferation of bacteria on the skin. The mucociliary transport mechanism lining the respiratory tract traps and propels inhaled pathogens to the oropharynx. The harsh acidic gastric milieu destroys organisms ingested orally. The periodic flushing of the urinary tract reduces the number of adherent microorganisms. Likewise, the washing action and immunoglobulin and lysozyme components of tears protect the corneal surface of the eye (7). Alterations of these anatomic barriers diminish the host’s resistance to infection. Surgical incisions, trauma, and burns allow access to deeper soft tissues. Smoke inhalation disrupts the mucociliary ladder of the respiratory tree.
The second line of host defense is provided by the immune response, both humoral and cell mediated. Host defenses in infection are discussed in detail elsewhere.
PREVENTION OF SURGICAL SITE INFECTIONS
Without an infecting agent, there is no infection. Endogenous pathogens on the host’s skin, on the mucous membranes, or within a hollow viscous are the most important sources for SSI. In clean-contaminated, contaminated, and dirty-infected operations, the source and size of the pathogenic inoculum are functions of the patient’s disease and site of procedure. The risk of an SSI is contingent upon host factors and operator-dependent variables.
Host Factors
Immunocompetence requires a period of maturation. Efficient T-cell and B-cell responses require previous antigen exposure. Passive fetal immunity is imparted by maternal transfer of IgG during the third trimester (8). The level of IgG in premature infants is proportional to gestational age. Postnatally, IgG levels decline during the first 4 months. Prematurity is effectively a hypogammaglobulinemic state. These infants manifest suboptimal protection against gram-negative organisms and exhibit impaired opsonization, increasing the risk of infections from Streptococcus B, Escherichia coli, and Serratia. Serum concentrations of complement component peptides are decreased in the newborn period, but rise rapidly following birth. Low levels of complement adversely affect opsonization (8).
The fetus begins to produce IgM at 10 weeks gestation. However, at birth the infant only has 10% the normal adult level. The infant’s response to antigen is an elevation of IgM. IgA is not detected until near term and does not achieve normal levels until puberty. Diseases whose defenses depend primarily on secretory IgA, such as respiratory syncytial virus, remain prevalent throughout infancy.
Host susceptibility to infection can be predicted by the following variables: extremes of age, severity of disease, classification of physical status, presence of infection at other sites, prolonged preoperative hospitalization, malnutrition, morbid obesity, and immunosuppressive therapy, including exposure to transfusions. The Study on the Efficacy of Nosocomial Infection Control demonstrated that patients most clearly at risk for SSI are those with three or more concomitant diagnoses; those undergoing a clean-contaminated, contaminated, or dirty-infected procedure; or any procedure expected to last longer than 2 hours (3).
The Surgeon’s Influence
Many of the factors that predispose a surgical site to infection are under the control of the surgeon. Traditionally, disinfection of the patient’s skin, sterilization of surgical equipment, control of the operating room environment, use of prophylactic antibiotics, and expeditious operation have been used to prevent SSI. Preoperative shaving with a razor carries an SSI rate as high as 5.6% compared with 0.6% for those not shaved (3). Hair should not be removed unless it interferes with the operation. If hair removal is necessary, it should be done with electric clippers immediately before the procedure. An antiseptic should be used for skin preparation. Chlorhexidine gluconate achieves greater reduction in skin microflora and has longer residual activity than povidone-iodine (3). Alcohol is effective, fast acting, and inexpensive, but it is also flammable. Drying and evaporative heat loss can be problematic for small children. There is minimal evidence to support the view that antiseptic impregnated adhesive drapes or scrubbing all wounds affects the incidence of SSI.
Cleansing the hands of members of the operative team is imperative. Solutions containing chlorhexidine gluconate or an iodophor are most effective. These agents have the fewest problems with stability, contamination, and toxicity (3). Evidence suggests that a 2-minute scrub is sufficient, provided a brush is used to remove resident bacteria about the nail folds. Alcohols applied to skin are among the safest antiseptics, producing the greatest and most rapid reduction in bacterial counts on the skin. A 1-minute scrub with alcohol has been shown to be the most effective method for hand antisepsis.
Adherence to basic Halstedian principles decreases the risk for SSI. Hemostasis, sharp dissection, fine sutures, gentle tissue handling, and anatomic dissection can mitigate the development of tissue necrosis, seromas, or hematomas. Logarithmically, fewer bacteria are required to produce infection in the presence of a foreign body (i.e., suture, drain, catheter, graft, mesh) or necrotic tissue (poor hemostasis or thermal injury from electrocautery). Use of electrocautery to provide pinpoint coagulation or divide tissue under tension produces minimal tissue
destruction, no charring, and does not change the wound infection rate (9).
destruction, no charring, and does not change the wound infection rate (9).
The surgeon and the operation are both capable of influencing the immunologic efficacy of the host. Contamination increases with time, and operations expected to last longer than 2 hours are at greater risk. Extremes of age, basal nutritional state, transfusion, and use of steroids or other immunosuppressive drugs, including chemotherapy, are associated with an increased risk for SSI (3).
Antimicrobial Prophylaxis
Surgical antimicrobial prophylaxis refers to an agent administered prior to an operation whose function is to reduce the burden of contamination to a level manageable by host defenses. Antimicrobial prophylaxis anticipates the surgical wound class (Table 13-1). Prophylactic agents should be given 30 minutes prior to surgery to establish a bactericidal concentration of the drug in the tissues at the time of skin incision. A second dose is warranted if the operation lasts longer than 3 hours, exceeds twice the half-life of the antibiotic, or massive hemorrhage has occurred. No benefit has been demonstrated in continuing prophylaxis beyond the day of operation (3).
Prophylaxis is not indicated for clean operations, or if the operation does not involve placement of prosthetic materials. Cardiac operations, vascular procedures involving graft or stent placement, and neurosurgical operations and orthopedic procedures using hardware are notable exceptions. Clean cases that are at risk are in patients with more than three concomitant diagnoses, those whose operation will exceed 2 hours, and those whose operations are abdominal (3).
TABLE 13-1 Surgical Wound Classification. | ||
|---|---|---|
|
Antimicrobial prophylaxis is indicated for all clean-contaminated operations. Prophylaxis is appropriate for biliary procedures, including cholecystectomy (open or laparoscopic), common bile duct exploration/reconstruction, choledochal cyst excision, portoenterostomy, and pancreatic procedures. Gastroduodenal procedures warrant prophylaxis in patients with low gastric acidity, active hemorrhage, cancer, gastric ulcer, and obstruction (3). Patients undergoing procedures of the oral cavity, pharynx, and esophagus should receive preoperative antibiotics. In principle, urologic procedures in patients with sterile urine do not require antibiotics. Patients with documented urinary tract infections should be treated with culture-specific antibiotics. Gynecologic procedures are usually adequately covered with cephalosporins, tetracyclines, aqueous penicillin G, or metronidazole. In vaginal procedures, there is no added benefit to the use of a preoperative vaginal antiseptic over saline irrigation alone.
Certain clean-contaminated procedures involving the colon or rectum benefit from preoperative “bowel preparation” to reduce fecal mass and number of microorganisms. Most protocols employ mechanical cleansing, coupled with preoperative oral antibiotics or perioperative parenteral antibiotics. Mechanical cleansing can be performed by whole gut lavage using an electrolyte solution, polyethylene glycol or dietary restriction, cathartics, and enemas for several days before operation. In the pediatric population, bowel preparation may result in dehydration and electrolyte abnormalities. Infants and toddlers may require admission for intravenous hydration and monitoring.
Antibiotics are used to suppress both aerobes and anaerobes. There is no consensus on which agents to use or their route of administration. Oral regimens need to exhibit (1) rapid, highly bactericidal activity against gastrointestinal pathogens; (2), low local and systemic toxicity; and (3) limited gut absorption. Most oral regimens use neomycin and erythromycin base, or neomycin-metronidazole or kanamycin-metronidazole, given in three doses the day prior to operation (9). Parenteral antibiotics shown to be effective for elective colon resection include cefoxitin, cefotetan, and metronidazole, alone or in combination with an aminoglycoside.
Antimicrobial prophylaxis in contaminated or dirty cases is not indicated. Patients are frequently receiving therapeutic antibiotics for established infections. In colorectal procedures, a second-generation cephalosporin with activity against aerobes and anaerobes is recommended. For uncomplicated appendicitis, a single dose of cefoxitin or cefotetan, or in β-lactam allergic patients,
metronidazole, is effective. The combination of an aminoglycoside with clindamycin or metronidazole is effective for perforated appendicitis. Prophylaxis for an emergency laparotomy is best managed with an agent active against aerobes and anaerobes. When laparotomy is performed for nonpenetrating trauma, coverage for both aerobes and anaerobes is indicated and 24 hours or less of therapy is adequate. For penetrating abdominal injuries, treatment with cefoxitin, cefotetan, or a combination of agents active against both aerobes and anaerobes is recommended. Duration of use should again be limited to 24 hours. Open fractures with mild to moderate tissue damage should be managed with a first-generation cephalosporin. Combination therapy is appropriate for open fractures with gross contamination, major tissue damage or soft tissue loss. Major soft tissue injury is usually treated with parenteral cefazolin. Infected or dirty cases require a therapeutic course of antibiotics. Wounds with extensive contamination or tissue destruction should be left open for later inspection.
metronidazole, is effective. The combination of an aminoglycoside with clindamycin or metronidazole is effective for perforated appendicitis. Prophylaxis for an emergency laparotomy is best managed with an agent active against aerobes and anaerobes. When laparotomy is performed for nonpenetrating trauma, coverage for both aerobes and anaerobes is indicated and 24 hours or less of therapy is adequate. For penetrating abdominal injuries, treatment with cefoxitin, cefotetan, or a combination of agents active against both aerobes and anaerobes is recommended. Duration of use should again be limited to 24 hours. Open fractures with mild to moderate tissue damage should be managed with a first-generation cephalosporin. Combination therapy is appropriate for open fractures with gross contamination, major tissue damage or soft tissue loss. Major soft tissue injury is usually treated with parenteral cefazolin. Infected or dirty cases require a therapeutic course of antibiotics. Wounds with extensive contamination or tissue destruction should be left open for later inspection.
Prophylaxis of Endocarditis
Patients with cardiac or vascular conditions and with intravascular prosthetic material should receive prophylactic antibiotics (9). In oral and dental procedures, viridans streptococci pose the greatest risk. The genitourinary or gastrointestinal tracts predispose to enterococcal infection (Table 13-2). Oral amoxicillin has replaced penicillin V and ampicillin because of its superior absorption and improved serum levels. In penicillin-allergic patients, clindamycin is recommended but clarithromycin, azithromycin, or cephalexin are acceptable alternatives. Exposure to enteric flora warrants parenteral ampicillin plus gentamicin. In penicillin-allergic patients, vancomycin is recommended, with gentamicin added in high-risk patients. For patients with a history of rheumatic fever, erythromycin rather than amoxocillin should be used to protect against endocarditis (9).
TABLE 13-2 Likely Pathogens and Prophylactic Antibiotic of Choice According to Operative Site. | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Surgical Drains
Appropriate surgical drain use and details of drain placement vary widely. Some general points should be emphasized. To minimize the risk of SSI, most authorities suggest placing drains through separate incisions distant from the primary wound. Closed suction drains are favored over open drains because they pose less potential for contamination and infection. Timing of drain removal is important because bacterial colonization of initially sterile drain tracts is a function of the duration of time a drain is kept in place (9).
POSTOPERATIVE INFECTIONS
Nosocomial infections (NIs) are major contributors to hospital-associated morbidity and mortality. Critically ill pediatric patients are at high risk for acquiring NIs (10). The exact incidence of NI is not known. In pediatric general surgical patients, the incidence of NI is as low as 6%. The rate of NI in pediatric intensive care unit patients is as high as 20% (10). In neonatal intensive care unit (NICU) patients, the incidence has been reported as high as 50% (11). Infection site and pathogen vary with patient age, comorbidities, use of mechanical ventilation, and intravascular access devices. Parenteral nutrition and the use of antibiotics have been associated with the greatest probability of developing a NI in children (10).
Lower Respiratory Tract Infections
Pneumonia is the most frequently encountered hospital-acquired infection, accounting for approximately one-third of all NI (10). The frequency of nosocomial pneumonia in pediatric patients ranges from 1% to 5% (12). In the NICU population, this rises to nearly 12%, primarily in low birth weight (less than 1,000 g) neonates (13). Specific risk factors for the development of nosocomial pneumonia include intubation and mechanical ventilation, admission to an intensive care unit (ICU), burns, immunosuppression, and central nervous system injuries.
Nosocomial respiratory tract infection is often diagnosed by clinical suspicion (i.e., new onset of purulent sputum) supported by microbiological studies (i.e., blood and sputum cultures). Chest radiography is a useful adjunct to diagnosis, especially in the absence of positive cultures. Alternative methods of diagnosis, including bronchoscopy and bronchoalveolar lavage, have not proven effective in the pediatric population (12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


