Induction of Ovulation
 |
Although it seems commonplace today, indeed even routine, the ability to induce ovulation and attain pregnancy in anovulatory infertile women remains one of the greatest achievements of reproductive endocrinology. Once limited to clomiphene citrate, the therapeutic armamentarium for ovulation induction now includes a wide variety of agents.
Ovulatory disorders can be identified in 18-25% of infertile women.1 When anovulation is the only infertility factor, the prognosis for pregnancy generally is quite good because modern ovulation induction strategies are highly effective. When a specific cause for anovulation can be identified, treatment often restores normal cycle fecundity. Even when no specific cause can be found, as in most anovulatory women, empiric treatments with low costs and risks usually succeed. When those fail, other more complex forms of treatment are effective. One way or another, almost all anovulatory infertile women can be induced to ovulate. Unfortunately, many still do not conceive, often because there are other coexisting infertility factors.
Clinicians caring for infertile couples must have a thorough understanding of the methods for treatment of anovulatory infertility. This chapter reviews the principles that guide the choice of treatment, the results achieved with different therapies, and their associated risks.
Diagnosis of Anovulation
The diagnosis of anovulation generally is not difficult to establish. Women with irregular, unpredictable, or infrequent menses do not require specific diagnostic tests to prove what is already obvious. When anovulation is suspected but uncertain, a variety of methods can be used to evaluate ovulatory function, as discussed in Chapter 27 and summarized briefly here.
Ovulatory cycles typically are associated with a classic “biphasic” basal body temperature (BBT) pattern that is not difficult to recognize, when present.2 BBT recordings having no sustained interval of temperature elevation preceding the onset of menses strongly suggest anovulation. Biphasic recordings exhibiting a short luteal phase (onset of menses less than 12 days after the midcycle rise in BBT) suggest a subtle, but still important, form of ovulatory dysfunction. Although uncommon, BBT recordings are not clearly biphasic in some ovulatory women.
A serum progesterone measurement is the simplest, most common, objective and reliable test of ovulatory function, as long as it is appropriately timed. A progesterone concentration less than 3 ng/mL implies anovulation, except when drawn immediately after ovulation or just before the onset of menses, when lower levels naturally might be expected.3,4 A normal ovulatory cycle is 25-35 days in duration and exhibits a luteal phase lasting approximately 14 days. Ideally, the serum progesterone level should be drawn approximately one week before the expected onset of menses, when the concentration is at or near its peak. Contrary to popular belief and practice, cycle day 21 is not always the best time to measure the serum progesterone concentration and the threshold level indicating ovulation is not 10 ng/mL.
Cycle day 21 is a good choice for women with cycles lasting approximately 28 days, but a poor choice for women with 35 day cycles. A serum progesterone concentration greater than 10 ng/mL suggests normal luteal function, but not when the luteal phase is grossly short, and a level less than 10 ng/mL can be quite normal, because progesterone is secreted by the corpus luteum in distinct pulses, temporally linked to pulsatile luteinizing hormone (LH) secretion;5 random sampling can coincide with a transient nadir in serum levels.
Other more complicated or sophisticated tests of ovulation, such as monitoring urinary LH excretion and serial transvaginal ultrasonography, can be useful once ovulation has been achieved, but are unnecessary for the diagnosis of anovulation.
Classification of Ovulatory Disorders
After evaluation for the causes of anovulation is completed, virtually all women can be classified according to the criteria adopted by the World Health Organization (WHO).6 Hyperprolactinemic anovulation is considered as a fourth and specific category.
WHO Group I: Hypogonadotropic Hypogonadal Anovulation. The group accounts for approximately 5-10% of anovulatory women and includes those with low or low-normal serum follicle-stimulating hormone (FSH) concentrations, and low serum estradiol levels, due to absent or abnormal hypothalamic gonadotropin-releasing hormone (GnRH) secretion or pituitary insensitivity to GnRH. Examples include women with hypothalamic amenorrhea relating to physical, nutritional, or emotional stress, weight loss, excessive exercise, anorexia nervosa and its variants, Kallmann syndrome, and isolated gonadotropin deficiency. Women in the group may require hypothalamic-pituitary imaging to exclude a mass lesion.
WHO Group II: Eugonadotropic Euestrogenic Anovulation. This group is the largest, including 75-85% of anovulatory women, and is characterized by normal serum FSH and estradiol levels and normal or elevated LH concentrations.7 The most common examples are women with polycystic ovary syndrome (PCOS), some of whom ovulate at least occasionally. Women with PCOS should be screened for type 2 diabetes mellitus before treatment, due to the fetal risks associated with untreated diabetes.8 Weight loss generally is the best initial treatment for those who are obese because it can, by itself, restore ovulatory function.9,10 and 11
WHO Group III: Hypergonadotropic Anovulation. The group accounts for approximately 10-20% of anovulatory women and includes those with elevated serum FSH concentrations; most, but not all, have amenorrhea. The classic example is premature ovarian failure, due to follicular depletion, and few respond to treatment aimed at ovulation induction.
Hyperprolactinemic Anovulation. Approximately 5-10% of anovulatory women have hyperprolactinemia, which inhibits gonadotropin secretion. Consequently, serum FSH concentrations generally are low or low-normal and serum estradiol levels also tend to be relatively low. Most hyperprolactinemic women have oligomenorrhea or amenorrhea. When hyperprolactinemia cannot be attributed confidently to coexisting hypothyroidism or to medications, hypothalamic-pituitary imaging is indicated to exclude a mass lesion.
Pretreatment Evaluation and Treatment
The causes of anovulation are many and varied. Thyroid disease, hyperprolactinemia, adrenal disease, pituitary or ovarian tumors, eating disorders, extremes of weight loss or exercise, polycystic ovary syndrome (PCOS), and obesity all are commonly associated with ovulatory dysfunction. Treatment should be directed at the underlying cause, when that can be determined, because specific treatment is more likely to succeed and some conditions can have longer-term health consequences if not recognized and treated.
All anovulatory women deserve at least some preliminary evaluation, both to exclude important pathology that may require medical attention before ovulation induction begins and to identify the most likely successful form of treatment. Chapter 11 considers the causes and management of amenorrhea and galactorrhea. Chapters 12 and 13 discuss the pathophysiology and treatment of PCOS and hirsutism. Chapter 15 describes the evaluation of dysfunctional uterine bleeding. At a minimum, anovulatory women should be screened for thyroid disorders (serum TSH) and hyperprolactinemia (serum prolactin) because both require further evaluation and specific treatment.12,13 and 14 Depending on the menstrual history, endometrial sampling also merits consideration, because chronic anovulation is associated with increased risk for endometrial hyperplasia.
Screening for impaired glucose tolerance and diabetes is recommended for all obese anovulatory women with PCOS; up to 35% exhibit impaired glucose tolerance and 7-10% meet criteria for type 2 diabetes mellitus.15,16 Screening is best accomplished by measuring the glucose level 2 hours after a 75 gm oral glucose load; concentrations between 140 and 199 mg/dL indicate impaired glucose tolerance and levels of 200 mg/dL or greater indicate noninsulin-dependent diabetes.
Anovulation offers an obvious potential explanation for infertility, but often is not the only infertility factor. Before ovulation induction begins, a screening semen analysis is prudent because male factors are an important contributing cause in 20-40% of infertile couples.17 Early recognition of a significant co-existing male factor helps to avoid wasted time, effort, expense, and associated frustrations.
Additional preliminary evaluation with hysterosalpingography (HSG) or transvaginal ultrasonography merits serious consideration, particularly in women with a history of previous pelvic infection or surgery, ectopic pregnancy, inflammatory bowel disease, pelvic pain or other symptoms of endometriosis, or an abnormal physical examination. In the absence of such risk factors, the likelihood of tubal disease is low and HSG can be deferred safely in young women and those who do not require complicated and costly forms of ovulation
induction. In older women with a narrowing window of opportunity, it is generally wise to evaluate objectively all relevant infertility factors before reatment begins to ensure that time is used to best possible advantage. In women who require ovulation induction with exogenous gonadotropins, the associated costs, logistics, and risks also justify a thorough preliminary evaluation. Preliminary HSG and transvaginal ultrasonography are recommended when the medical history or physical examination raises suspicion for co- existing uterine or tubal infertility factors, for women over age 35, and when ovulation induction requires treatment with exogenous gonadotropins. Laparoscopy and hysteroscopy are unnecessary for most women, but certainly appropriate for those with an abnormal HSG or signs or symptoms of pelvic disease.
induction. In older women with a narrowing window of opportunity, it is generally wise to evaluate objectively all relevant infertility factors before reatment begins to ensure that time is used to best possible advantage. In women who require ovulation induction with exogenous gonadotropins, the associated costs, logistics, and risks also justify a thorough preliminary evaluation. Preliminary HSG and transvaginal ultrasonography are recommended when the medical history or physical examination raises suspicion for co- existing uterine or tubal infertility factors, for women over age 35, and when ovulation induction requires treatment with exogenous gonadotropins. Laparoscopy and hysteroscopy are unnecessary for most women, but certainly appropriate for those with an abnormal HSG or signs or symptoms of pelvic disease.
The best initial treatment for obese anovulatory women is weight loss, when it can be achieved. Even modest weight loss (5-10% of body weight) often restores ovulatory cycles in obese anovulatory women with PCOS.18,19,20,21,22,23,24 and 25 At a minimum, weight loss can increase sensitivity to ovulation-inducing drugs and decrease the complexity of treatment required. In one study, 60 of 67 obese anovulatory women (90%) who lost an average of 10 kg/m2 in a diet and exercise program resumed spontaneous ovulation and 52 (78%) ultimately achieved pregnancy, 18 (27%) without other interventions.26 A body mass index (BMI) less than 27 is a reasonable if also modest goal.
Clomiphene Citrate
Clomiphene citrate was first synthesized in 1956, introduced for clinical trials in 1960, and approved for clinical use in the United States in 1967.27,28 In early clinical trials, 80% of anovulatory women treated with clomiphene achieved ovulation and half of those who ovulated also conceived.27,28 The collected clinical experience gained in the years since remains consistent with those early observations.
Pharmacology and Mechanism of Action
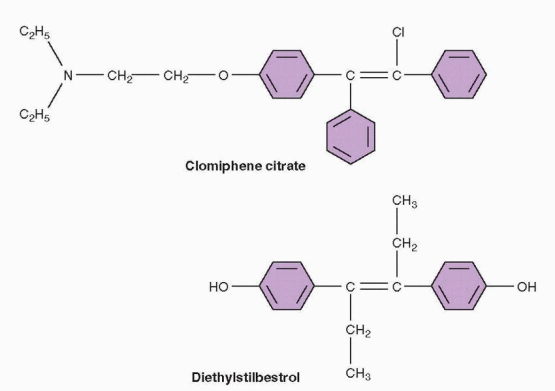
Clomiphene is a non-steroidal triphenylethylene derivative that acts as a selective estrogen receptor modulator (SERM), having both estrogen agonist and antagonist properties.29 However, in almost all circumstances, clomiphene acts purely as an antagonist or antiestrogen; its weak estrogenic actions are clinically apparent only when endogenous estrogen levels are very low. Clomiphene is cleared through the liver and excreted in the stool; approximately 85% is eliminated within a week, but traces can remain in the circulation for longer.30 Clomiphene is a racemic mixture of two different stereoisomers, enclomiphene (62%; originally known as cis-clomiphene) and zuclomiphene (38%; originally known as trans-clomiphene).29,31 Enclomiphene is the more potent isomer and the one responsible for its ovulation-inducing actions.29,32 The half-life of enclomiphene is relatively short, so serum concentrations rise and fall quickly during and after treatment.30,33 Zuclomiphene is cleared much more slowly; serum levels remain detectable for weeks after a single dose30 and may even accumulate gradually over a series of cycles, but there is no evidence that residual zuclomiphene has any important clinical effects or consequences.33
Structural similarity to estrogen allows clomiphene to compete with endogenous estrogen for nuclear estrogen receptors at sites throughout the reproductive system. However, unlike estrogen, clomiphene binds to nuclear estrogen receptors for an extended interval of time and thereby depletes receptor concentrations by interfering with receptor recycling.29 At the hypothalamic level, estrogen receptor depletion prevents accurate interpretation of
circulating estrogen levels; circulating estrogen levels are perceived as lower than they truly are. Reduced negative estrogen feedback triggers normal compensatory mechanisms that alter the pattern of gonadotropin-releasing hormone (GnRH) secretion and stimulate increased pituitary gonadotropin release which, in turn, drives ovarian follicular development. At the pituitary level, clomiphene also might increase the sensitivity of gonadotrophs to GnRH stimulation.34
circulating estrogen levels; circulating estrogen levels are perceived as lower than they truly are. Reduced negative estrogen feedback triggers normal compensatory mechanisms that alter the pattern of gonadotropin-releasing hormone (GnRH) secretion and stimulate increased pituitary gonadotropin release which, in turn, drives ovarian follicular development. At the pituitary level, clomiphene also might increase the sensitivity of gonadotrophs to GnRH stimulation.34
 |
When administred to already ovulatory women, clomiphene increases GnRH pulse frequency.35 In anovulatory women with polycystic ovary syndrome (PCOS) who already exhibit an increased GnRH pulse frequency, clomiphene increases only pulse amplitude.36 Serum levels of both FSH and LH rise during clomiphene treatment and fall again soon after the typical 5-day course of therapy is completed.37 In successful treatment cycles, one or more follicles emerge and grow to maturity. In parallel, serum estrogen levels rise progressively, ultimately triggering an LH surge and ovulation. In sum, clomiphene works primarily by stimulating the normal endocrine mechanisms that define the hypothalamic-pituitary-ovarian feedback axis. The importance of other effects it may have on insulinlike growth factors (decrease in IGF-I concentrations) and sex hormone-binding globulin (increase in serum levels) is uncertain.38,39
Peripheral Actions
In addition to its desirable central actions, clomiphene can exert less desirable anti- estrogenic effects at peripheral sites in the reproductive system, which some have suggested might explain the difference between ovulation and pregnancy rates achieved with clomiphene treatment. Adverse effects of clomiphene on the endocervix, the endometrium, the ovary, the ovum, and the embryo have been described, but there is no compelling evidence to indicate that such effects have important clinical consequences in most women.
On balance, the weight of evidence from controlled trials suggests that the quality and quantity of cervical mucus production can be decreased in clomiphene treatment cycles. Whereas some have observed no significant changes in mucus characteristics during treatment,40 others have found clomiphene has dose-dependent adverse effects.41,42 The conflicting results have several possible explanations. The effect may be more apparent when the interval between the end of treatment and ovulation is short.43 The effect might
often be negated by higher serum estradiol levels resulting from clomiphene-induced multifollicular development.44 It also is possible that some individuals may be more sensitive to the effect.45 Regardless, any adverse effect that clomiphene may have on cervical mucus now is largely moot (Chapter 27). In recent years, even the evaluation of cervical mucus has all but disappeared from clinical practice because controlled trials have demonstrated that postcoital testing (the traditional test of cervical factors) has little or no predictive value46,47 and because modern treatment regimens for persistent infertility now routinely incorporate intrauterine insemination (IUI), which bypasses the cervix altogether.48,49
often be negated by higher serum estradiol levels resulting from clomiphene-induced multifollicular development.44 It also is possible that some individuals may be more sensitive to the effect.45 Regardless, any adverse effect that clomiphene may have on cervical mucus now is largely moot (Chapter 27). In recent years, even the evaluation of cervical mucus has all but disappeared from clinical practice because controlled trials have demonstrated that postcoital testing (the traditional test of cervical factors) has little or no predictive value46,47 and because modern treatment regimens for persistent infertility now routinely incorporate intrauterine insemination (IUI), which bypasses the cervix altogether.48,49
Impaired endometrial growth also has been reported in clomiphene-treated women. However, preovulatory endometrial thickness in clomiphene-induced cycles remains well within the range normally observed in spontaneous ovulatory cycles in the large majority of women.50,51,52,53 and 54 Other subtle differences in endometrial morphology have been attributed to the effects of clomiphene, but their clinical relevance, if any, is uncertain.55,56 It is likely that clomiphene inhibits endometrial growth, at least in some women, for the same reasons that it may inhibit cervical mucus production, but the same caveats apply; the effect is inconsistent, may be offset by the higher estrogen levels in clomiphene-induced cycles, and probably has little clinical importance, except perhaps in those individuals exhibiting grossly poor endometrial growth (peak preovulatory thickness less than 5-6 mm).
Clomiphene does not appear to have any clinically relevant direct effects on the ovary or embryo. Although clomiphene can inhibit steroid hormone production by cultured avian,57 ovine,58 and human granulosa/luteal cells in vitro,59 serum estrogen and progesterone concentrations in clomiphene-induced cycles are typically higher, not lower, than in spontaneous ovulatory cycles. Adverse effects on mouse ovum fertilization and embryo development have been observed in vitro,60 but studies in women indicate that serum concentrations of enclomiphene and zuclomiphene never approach the levels required to induce such effects, even after several consecutive treatment cycles.33
Clinical Indications
Clomiphene citrate is the traditional drug of choice for ovulation induction in anovulatory infertile women with normal thyroid function, normal serum prolactin levels, and normal endogenous estrogen production, as determined by clinical observations (oligomenorrhea, estrogenic cervical mucus), a serum estradiol determination (greater than approximately 40 pg/mL), or a normal menstrual response to a progestin challenge (WHO Group II).61 Although the drug also frequently is used empirically to stimulate multi-follicular development in ovulatory women with unexplained infertility (usually in combination with IUI),48,62,63 and 64 the focus here is on ovulation induction in anovulatory women; empiric clomiphene and other treatments for unexplained infertility are discussed in detail in Chapter 27.
Given its mechanism of action, it is not surprising that clomiphene typically is ineffective in women with hypogonadotropic hypogonadism (WHO Group I). Together, low or lownormal FSH levels and low serum estrogen concentrations indicate that the hypothalamic-pituitary-ovarian axis is not functioning normally in women with hypothalamic amenorrhea; if it were, FSH levels would be high because estrogen concentrations are low. If low endogenous estrogen concentrations cannot stimulate increased FSH secretion, there is little reason to think that a clomiphene-induced decrease in the level of negative estrogen feedback will succeed, and it rarely does. Alternative treatments that directly stimulate the pituitary (pulsatile exogenous GnRH) or the ovary (exogenous gonadotropins) usually are required.
Because the corpus luteum derives from the ovulatory follicle, its functional capacity depends, in part, on the quality of preovulatory follicular development. Logically, inadequate follicular development can be expected to cause or predispose to poor luteal function,
if ovulation still occurs. Indeed, the most obvious example of poor luteal function, a short luteal phase, is associated with abnormally low follicular phase FSH levels.65,66 Consequently, clomiphene is both a logical and effective choice for treatment.67,68,69 and 70 Progesterone levels typically are higher in clomiphene-induced ovulatory cycles than in normal spontaneous cycles, likely because preovulatory follicular development is optimized and because treatment often results in more than one corpus luteum.71,72
if ovulation still occurs. Indeed, the most obvious example of poor luteal function, a short luteal phase, is associated with abnormally low follicular phase FSH levels.65,66 Consequently, clomiphene is both a logical and effective choice for treatment.67,68,69 and 70 Progesterone levels typically are higher in clomiphene-induced ovulatory cycles than in normal spontaneous cycles, likely because preovulatory follicular development is optimized and because treatment often results in more than one corpus luteum.71,72
Clomiphene citrate treatment is generally limited to women with demonstrated ovulatory dysfunction but may also be justified in normally ovulating women whose infertility remains unexplained, particularly when they are young and infertility is of short duration, and those unwilling or unable to pursue more aggressive treatments. The efficacy of clomiphene treatment in women with unexplained infertility can be attributed to optimizing follicular development or to the “superovulation” of more than a single ovum.62,63 Empiric clomiphene treatment is most effective when combined with intrauterine insemination (IUI), in an effort to increase the numbers of both ova and sperm (Chapter 27).48,73
Clomiphene Treatment Regimens
Clomiphene is administered orally, typically beginning on the third to fifth day after the onset of a spontaneous or progestin-induced menses. Ovulation and conception rates and pregnancy outcomes are similar when treatment starts anywhere between cycle days 2 and 5.74 In women with amenorrhea, treatment can begin immediately if pregnancy has been excluded. The dose of clomiphene required to induce ovulation correlates with body weight but cannot be predicted confidently for an individual woman.75,76 Although obese women often require higher doses of clomiphene treatment, the results achieved ultimately are similar to those observed in lean women.77,78 No clinical or laboratory parameter has proven utility for predicting the dose of clomiphene needed to induce ovulation.79
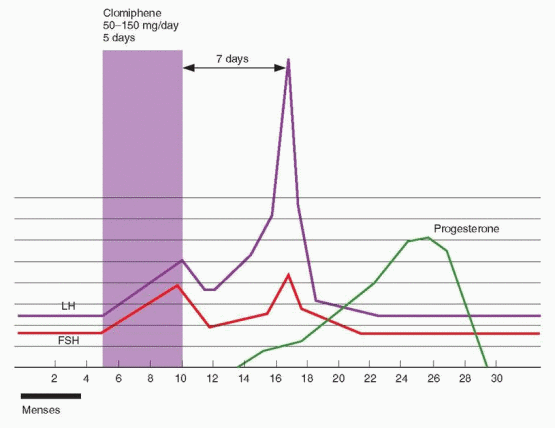 |
Treatment usually starts with a single 50 mg tablet daily for a 5-day interval and, if necessary, increases by 50 mg increments in subsequent cycles until ovulation is achieved. Most women who respond to clomiphene will respond to either 50 mg (52%) or 100 mg (22%).80,81 Lower doses (12.5-25 mg daily) deserve consideration for women who prove highly sensitive to the drug or develop large ovarian cysts that prevent continued treatment.82 Although not approved by the U.S. Food and Drug Administration, higher doses of clomiphene (150-250 mg daily) sometimes can succeed when lower doses fail (150 mg, 12%; 200 mg 7%; 250 mg 5%).80,81 We believe that treatment with doses up to 150 mg is reasonable before considering alternatives.83 Longer durations of clomiphene treatment (7-10 days) can succeed in some women when standard treatment does not and occasionally may be useful when there are no practical alternatives.84,85 and 86
The same methods used for diagnosis of anovulation can be used to evaluate the response to treatment. BBT recordings are simple and inexpensive, but can become tedious over time. A serum progesterone level greater than 3 ng/mL provides reliable evidence that ovulation has occurred,3,4 but must be timed appropriately for confident and correct interpretation. Measuring the serum progesterone concentration between cycle days 22 and 25 will minimize the risk of sampling immediately after ovulation (occurring as late as cycle day 19-20 in cycles lasting up to 35 days) or before menses, when levels less than 3 ng/mL might be observed and misinterpreted. More sophisticated tests of ovulation involving greater costs and logistical demands are unnecessary to determine only if ovulation occurred, but may be justified once successful ovulation induction has been achieved.
Commercial test kits can detect the midcycle urinary LH surge and help to determine not only whether ovulation occurred, but when, and to accurately define the length of the luteal phase.87 In clomiphene-induced ovulatory cycles in anovulatory women, the LH surge typically occurs 5-12 days after treatment ends, most often on cycle day 16 or 17 when clomiphene is administered on days 5-9.88 Ovulation generally occurs 14-26 hours after surge detection and almost always within 48 hours.87 However, in clinical practice, both false negative and false positive results are relatively common.89,90 An endometrial biopsy yielding secretory endometrium also implies recent ovulation,91 but the associated costs and discomfort cannot be justified for that purpose alone. Serial transvaginal ultrasonography can demonstrate the size and number of developing follicles, track endometrial growth, and provide presumptive evidence of ovulation,92,93 but is difficult to justify when less complicated and costly methods can provide the necessary information. Some advocate monitoring at least the first cycle of treatment to identify those who respond excessively.94 A study comparing fecundity in clomiphene-induced cycles monitored with BBT, urinary LH excretion, or serial transvaginal ultrasonography found no clear advantage for any one of the three methods.95
Traditionally, when the serum progesterone concentration reveals persistent anovulation after clomiphene treatment, a progestin is prescribed (e.g., medroxyprogesterone acetate, 5-10 mg daily for 5-7 days) to induce menses before the next cycle begins at a higher dosage. Although effective, the sequence takes time and several months may pass before a patient is proven unresponsive to clomiphene. A “stair-step” treatment protocol is an alternative that can shorten the time required to achieve ovulation and to identify those who require different treatment. The regimen involves treatment with clomiphene (50 mg) on cycle days 5-9 after a spontaneous or induced menses, ultrasonography on day 11-14, immediate treatment at the next higher dose level (100 mg) if no dominant follicle (≥15 mm) has emerged, repeated ultrasonography 1 week later, and if still no dominant follicle is observed, immediate treatment at the highest dose level (150 mg) and ultrasonography again 1 week later.96 In a case series involving 31 anovulatory infertile women with PCOS treated with a stair-step regimen, the time to ovulation was 23-35 days, cycle fecundability was 13%, and estimated costs were increased only modestly.96
In the past, monthly pelvic examinations to exclude residual ovarian enlargement were recommended before each new clomiphene treatment cycle began. It is still prudent to
postpone further treatment when symptoms lead to discovery of a large cyst or grossly enlarged ovaries, but clinical studies and experience indicate that routine baseline physical examination or ultrasonography is unnecessary.88 Nevertheless, regular contact should be maintained to review the progress of treatment and to ensure that any additional evaluation needed is accomplished efficiently.
postpone further treatment when symptoms lead to discovery of a large cyst or grossly enlarged ovaries, but clinical studies and experience indicate that routine baseline physical examination or ultrasonography is unnecessary.88 Nevertheless, regular contact should be maintained to review the progress of treatment and to ensure that any additional evaluation needed is accomplished efficiently.
Results of Clomiphene Treatment
Clomiphene will induce ovulation successfully in 70-80% of properly selected women.97,98 The likelihood of response decreases with increasing age and body mass index and with the extent of any associated hyperandrogenemia in anovulatory women. Interestingly, women with amenorrhea are more likely to conceive than those with oligomenorrhea,97 possibly because infertile women who menstruate also likely ovulate, albeit infrequently, and are more likely to have other co-existing infertility factors.
Among anovulatory infertile women who respond to clomiphene treatment, the overall cycle fecundability is approximately 15%. In women with no other infertility factors, cycle fecundability may reach as high as 22%, comparable to that observed in normal fertile couples after discontinuation of barrier contraception and those with male factor infertility receiving therapeutic donor inseminations.97 Cumulative pregnancy rates of 70-75% can be expected over six to nine cycles of treatment.99,100 Thereafter, cycle fecundability falls substantially. When pregnancy is not achieved within 3-6 clomiphene-induced ovulatory cycles, the infertility investigation should be expanded to exclude other infertility factors not yet evaluated, or to change the overall treatment strategy if evaluation is already complete. Prolonged treatment with clomiphene is inappropriate, particularly for women over age 35.
Side Effects
Clomiphene treatment generally is very well tolerated. Minor side effects are relatively common, but rarely are persistent or severe enough to require that treatment be discontinued.
Transient hot flashes, usually limited to the short interval of treatment, occur in 10-20% of women.83 Considering that clomiphene causes a central misperception that endogenous estrogen levels are low, vasomotor symptoms are not difficult to understand. Mood swings also are relatively common. Other mild and less common side effects include headache, breast tenderness, pelvic pressure or pain, and nausea. Visual disturbances (blurred or double vision, scotomata, light sensitivity) are uncommon (1-2%) and reversible, but reports of persistent “afterimages” (palinopsia) and light sensitivity (photopobia) make them nonetheless unnerving;101 when such symptoms appear, prudence dictates that treatment be abandoned in favor of alternative methods for ovulation induction.
Risks
Clomiphene treatment has risks, but serious complications are rare. Inevitably, questions arise concerning the risks for multiple pregnancy, congenital anomalies, and other potential adverse outcomes associated with clomiphene treatment.
The principal risk associated with clomiphene treatment is an increased risk for conceiving a conceiving a multiple pregnancy. The clomiphene-induced increase in FSH secretion is only transient, so normal selection mechanisms still operate to yield only a single mature follicle in most treatment cycles in anovulatory women. Nevertheless, multi-follicular development is relatively common and the overall risk of multiple pregnancy is increased to approximately 7-10%.102,103 and 104 The large majority of multiple pregnancies conceived in clomiphene-induced cycles are twins; the risk for triplets is 0.3-0.5%, for quadruplets 0.3%, and for quintuplets 0.1%.28 The higher risk of multi-fetal gestation is another reason to treat with the lowest effective dose of clomiphene; higher doses do not improve results and only increase the risk of superovulation and multiple pregnancy, with all of the attendant antenatal and neonatal complications.
There is no evidence that clomiphene treatment increases the overall risk of birth defects or of any one anomaly in particular.105 Several large series have examined the question and have drawn the same conclusion.102,103,104 and 105 In a series of 1,034 pregnancies resulting from clomiphene treatment, 14.2% ended in miscarriage, 0.5% in ectopic pregnancy, 0.1% in molar pregnancy, and 1.6% in stillbirth, and among 935 live born infants, malformations were detected in 21 (2.3%).105 Earlier suggestions that the risk of neural tube defects might be higher in pregnancies conceived after clomiphene treatment were not confirmed in later investigations.106,107 A small study of pregnancy outcomes in women exposed inadvertently to clomiphene during the first trimester of pregnancy found no evidence of teratogenicity.108 There also is no evidence that clomiphene treatment increases the risk of developmental delay or learning disability in children conceived during clomiphene treatment.109
Early studies suggested that the incidence of spontaneous miscarriage in pregnancies resulting from clomiphene treatment might be increased, but a number of others have observed miscarriage rates no different from those in pregnancies conceived without treatment.80,110
The incidence of ovarian hyperstimulation syndrome (OHSS) in clomiphene-induced cycles is difficult to determine confidently because definitions of the syndrome vary widely among studies. In general, mild symptoms of ovarian hyperstimulation (transient abdominal discomfort, mild nausea, vomiting, diarrhea, and abdominal distention) are not altogether uncommon but require only expectant management. When induction of ovulation proceeds in the recommended incremental fashion to establish the minimum effective dose, the risk of clinically significant OHSS (massive ovarian enlargement, progressive weight gain, severe abdominal pain, intractable nausea and vomiting, gross ascites, oliguria) is remote.
The incidence of ovarian cancer is decreased among parous women and those using hormonal contraception for prolonged intervals, suggesting that “incessant ovulation” (repeated epithelial disruption and repair) predisposes to development of ovarian cancer and that treatment with ovulation-inducing drugs might increase the risk.111 The results of case-control studies conducted in the 1990s lent credence to the notion and raised considerable concern,112,113 although their conclusions were challenged because of important methodologic flaws. One study compared infertile treated women to fertile women rather than to infertile untreated women, even though infertility and nulliparity were known risk factors for ovarian cancer.112 Another included cancers of all types and tumors of low malignant potential despite their differing pathophysiology.113 Since then, numerous studies have con-firmed that the incidence of ovarian cancer is increased in infertile women, but have failed to find any substantive evidence that ovulation-inducing drugs increase the risk.114,115,116,117,118,119,120 and 121 The results of studies of the risk for breast cancer have been conflicting, with some showing no association with ovulation-inducing drugs and others suggesting possible increases in risk in certain subgroups.120 No causal relationship between ovulation-inducing drugs and ovarian or breast cancer has been established, but prolonged treatment with clomiphene nonetheless should be avoided, primarily because it has little hope of success.
Adjuvant and Combination Treatments
Clomiphene failure describes women who do not ovulate in response to clomiphene treatment, not those who fail to conceive despite successful clomiphene-induced ovulation. In the latter group, additional evaluation is indicated to identify other potential co-existing infertility factors not already excluded. If or when that has been accomplished, persistent infertility is best regarded and treated as unexplained infertility (Chapter 27).
Although most properly selected women will ovulate in response to clomiphene treatment, many do not. Direct ovarian stimulation with exogenous gonadotropins, discussed later in detail, is an obvious alternative, but it is by no means the only option that merits consideration. Many clomiphene-resistant anovulatory infertile women will respond to supplemental or combination treatment regimens. Options include adjuvant treatment with glucocorticoids, exogenous human chorionic gonadotropin (hCG), or metformin, and preliminary suppressive therapy (hormonal contraceptives). It is helpful to be familiar with these less common ovulation induction strategies because many couples are understandably reluctant or unable to pursue the obvious alternative of gonadotropin treatment once fully advised of the associated costs, logistical demands, and risks.
Failure to respond to one or more of these less commonly employed treatment regimens is not a prerequisite for exogenous gonadotropin therapy. They are simply useful alternatives for those unwilling or unable to pursue gonadotropin therapy and, for some, may be the only options when clomiphene treatment has failed. A choice among them is not entirely arbitrary, but should consider specific elements of the patient’s history, the results of laboratory evaluation, and observations in previous unsuccessful clomiphene treatment cycles.
Clomiphene and Glucocorticoids
Numerous studies have examined the efficacy of adjuvant treatment with glucocorticoids in clomiphene-resistant anovulatory women and all have found that combined treatment with clomiphene and a glucocorticoid can successfully induce ovulation in many who fail to respond to clomiphene alone.86,122,123,124,125 and 126 Both continuous and more limited follicular phase treatment regimens (cycle days 5-14) have been described, using either prednisone (5 mg daily) or dexamethasone (0.5-2.0 mg daily). Whereas some studies have suggested that combined treatment with clomiphene and glucocorticoids is most effective in women having elevated serum dehydroepiandrosterone sulfate (DHEA-S) concentrations,122,123 others have found that treatment also can be effective in those with normal DHEA-S levels.124,126 and in unselected populations of clomiphene-resistant women.86,125
In the largest randomized trial involving more than 200 clomiphene-resistant anovulatory infertile women, over 80% of those receiving combined treatment with clomiphene (200 mg daily cycle days 5-9) and dexamethasone (2 mg daily, cycle days 5-14) ovulated, compared to 20% of controls treated with clomiphene and placebo; the cumulative pregnancy rate in women receiving dexamethasone (40%) was 10-fold higher than in those who received placebo (4%).126 The mechanism of glucocorticoid action remains unclear, but appears to involve more than simple androgen suppression. Other possibilities include direct effects on the developing oocyte and indirect effects on intrafollicular growth factors and cytokines, which may act synergistically with FSH.127 Regardless, adjuvant treatment with glucocorticoids may be justified for three to six cycles when it is successful, but should be promptly discontinued when it is not. There is no evidence that glucocorticoid treatment has any important side effects or risks when used in the doses and durations described.
Clomiphene and HCG
Although there are few if any data to demonstrate its value, exogenous hCG has been used commonly as a surrogate LH surge to trigger ovulation in clomiphene-induced cycles, particularly when IUI is performed, as in couples with unexplained infertility and those with a co-existing male factor. Adjuvant hCG treatment can be useful, but has limited indications, distinct disadvantages, and potential consequences.
In anovulatory women who fail to ovulate in response to clomiphene alone, adjuvant hCG treatment is based on the premise that clomiphene may be successful in stimulating the emergence of a preovulatory follicle but ultimately fail to trigger an endogenous LH surge and to induce ovulation. Physiologically, and in practice, the scenario is most unlikely. Moreover, serial transvaginal ultrasonography is required to demonstrate the phenomenon and to ensure that the ovulatory stimulus is delivered at the appropriate time. If administered blindly and prematurely, before the dominant follicle is mature enough to respond, hCG is more likely to induce atresia than ovulation. The question of when to administer hCG presents a dilemma. Although hCG commonly is administered when the lead follicle reaches 18-20 mm,128 clinical studies indicate that the peak preovulatory follicular diameter in successful clomiphene-induced ovulatory cycles ranges between 18 and 30 mm (mean 25 mm).88,129 Considering that the preovulatory follicle grows approximately 2 mm per day as it approaches maturity,92,93 the corresponding interval may thus span up to 6 days. Normally, the preovulatory follicle triggers its own ovulatory stimulus at the peak of maturity by generating and maintaining the estrogen levels that are required to induce the LH surge. The timing of the spontaneous LH surge is therefore always optimal, but that of hCG treatment can never be more than an educated guess.
When combined treatment with clomiphene and IUI is required, insemination usually is best performed on the day after detection of the spontaneous LH surge, using one of the now widely available commercial kits designed for the purpose, because ovulation generally occurs 14-26 hours after urinary LH surge detection.87,130 However, the lower limit of LH detection usually is between 20 and 40 IU/L and many ovulatory women exhibit peak LH levels below 40 IU/L or surges of brief duration that may escape detection;89 false negative results are therefore not uncommon, and frustrating. Exogenous hCG can be useful for the few women who require IUI but repeatedly fail to detect the LH surge despite other objective evidence of successful ovulation induction. In such circumstances, we believe that hCG is best postponed until the preovulatory follicle reaches or exceeds 20 mm in mean diameter. Ovulation occurs 34-46 hours after hCG injection,131 so IUI usually is performed approximately 36 hours later.
When the LH surge can be detected, adjuvant hCG treatment has no value and only adds unnecessary expense and inconvenience. Numerous studies have compared outcomes in clomiphene-induced cycles when IUI was performed after an endogenous LH surge or exogenous hCG injection; results are no better when hCG is administered and, in some cases, worse.64,89,130,132,133 and 134 The idea that hCG may still serve to ensure or improve the quality of luteal function even if is not required to trigger ovulation also is not supported by existing data. In spontaneous ovulatory cycles, hCG treatment superimposed on the endogenous LH surge has no effect on luteal phase duration or serum estrogen or progesterone concentrations;135 the same is true in clomiphene-induced ovulatory cycles.133 In sum, adjuvant hCG treatment is best limited to those few women who require IUI and ovulate but cannot reliably detect a midcycle LH surge.
Clomiphene and Metformin
Insulin resistance and hyperinsulinemia are common features of PCOS and an important contributing cause of the hyperandrogenism and chronic anovulation that characterize the disorder. Anovulatory infertile women with PCOS and hyperinsulinemia also are typically more resistant to clomiphene treatment.
Recognition of the pathophysiologic importance of insulin resistance in PCOS stimulated intense interest in the use of insulin-sensitizing agents for the treatment of the disorder. Metformin is a biguanide oral insulin-sensitizing agent that acts primarily by reducing hepatic gluconeogenesis, but also decreases intestinal absorption of glucose and increases peripheral glucose uptake and utilization. The effects of metformin on insulin levels and sensitivity, androgen concentrations and other metabolic and clinical measures are considered at length elsewhere in this text (Chapter 12); its adjunctive use an ovulation-inducing agent is the focus here.
Ttreatment with metformin alone or with other insulin-sensitizing drugs (thizolidinediones, D-chiro-inositol) can increase ovulation rates in some women with PCOS,136,137 and 138 but there is no practical method for predicting those who will respond. Fasting insulin concentrations and glucose-insuin ratios do not predict response to metformin,139 and overall, metformin appears most effective in patients who also respond to clomiphene.138,140 A meta-analysis of studies involving the use of metformin as an ovulation-inducing drug in women with PCOS concluded that its efficacy compared favorably with that of clomiphene,139 but subsequent randomized trials comparing the two drugs, alone and in combination, have found that clomiphene is superior to metformin and that combined treatment is no better than treatment with clomiphene alone.141,142 and 143 In the largest single trial, the live birth rate achieved with clomiphene treatment was significantly higher than that of metformin (22.5% vs. 7.2%) and the results of combined treatment were not significantly different (26.8%)142 Metformin treatment also did not decrease the dose of clomiphene required to induce ovulation.144
In a few small studies involving clomiphene-resistant anovulatory women with PCOS, combined treatment has increased ovulation and pregnancy rates over those achieved with clomiphene alone.145,146,147 and 148 A 2008 meta-analysis including 17 randomized trials concluded that combined treatment with metformin and clomiphene achieves higher ovulation and pregnancy rates than treatment with clomiphene alone.138 Although there is no convincing evidence that combined treatment with metformin and clomiphene can increase live birth rates over those achieved with clomiphene alone,149 the attempt seems justified for women having few alternatives besides ovarian drilling or treatment with exogenous gonadotropins. Limited evidence indicates that combined treatment with metformin and roziglitazone,150 or with clomiphene and rosiglitazone,151 is no more effective than metformin alone. Combined with the safety alert issued by the U.S. Food and Drug Administration concerning a possible increased risk of ischemic cardiovascular events in patients receiving treatment with thiazolidinediones,152 these data argue against their adjuvant use for ovulation induction. In summary, combined treatment with metformin and clomiphene deserves consideration in women who prove clomiphene resistant before proceeding to ovarian drilling or treatment with gonadotropins.
Metformin treatment is commonly associated with gastrointestinal side effects including nausea, vomiting, abdominal cramps, and diarrhea that can be severe enough to limit the dose administered or require discontinuation of treatment.139,153,154,155 and 156 Because side effects
tend to be dose-dependent and diminish with time, it is usually best to begin with a low daily dose (500 mg), increasing gradually at weekly intervals to a daily dose of 1500-2000 mg, as tolerance allows. Lactic acidosis can be a rare complication of metformin treatment, although recent systematic reviews have questioned whether there is a true causal relation-ship.157,158 Women at highest risk are those with chronic hypoxemic conditions related to cardiovascular, renal, hepatic and pulmonary disease and advanced age.
tend to be dose-dependent and diminish with time, it is usually best to begin with a low daily dose (500 mg), increasing gradually at weekly intervals to a daily dose of 1500-2000 mg, as tolerance allows. Lactic acidosis can be a rare complication of metformin treatment, although recent systematic reviews have questioned whether there is a true causal relation-ship.157,158 Women at highest risk are those with chronic hypoxemic conditions related to cardiovascular, renal, hepatic and pulmonary disease and advanced age.
Although there is no evidence that metformin treatment during pregnancy is associated with any increased risk for major fetal malformations,159 its safety during pregnancy is not yet established. Although some have advocated metformin treatment to reduce the increased risk for miscarriage in women with PCOS, which might relate to an underlying metabolic disorder,160,161 and 162 no differences in the miscarriage rates of women who did or did not receive metformin treatment have been observed in large randomized trials.141,142 and 143 Metformin treatment during pregnancy also has been advocated to reduce the risk for developing gestational diabetes and other pregnancy complications in women with PCOS.163 In diabetic women, treatment with metformin during pregnancy has been associated with an increased prevalence of pre-eclampsia and increased perinatal mortality in some studies,164 but not in others.165 Currently, routine metformin treatment during pregnancy is not recommended for women with PCOS.140
Preliminary Suppressive Therapy
Considering that anovulation reflects a dysfunctional hypothalamic-pituitary-ovarian axis, it is reasonable to think that an interval of preliminary suppressive therapy might help to restore harmony and ovulatory function, at least temporarily. The idea is consistent with clinical observations of a few normal menstrual cycles immediately following discontinuation of estrogen-progestin contraception in some women who previously exhibited abnormal menstrual patterns. Limited data suggest that a 2-month interval of continuous estro-gen-progestin contraception can effectively suppress serum LH and androgen levels and that ovulation rates up to 70% and cumulative pregnancy rates over 50% can be achieved with clomiphene treatment immediately thereafter in women who were previously clomi-phene-resistant.166,167
A long-acting gonadotropin-releasing hormone (GnRH) agonist, alone or in combination with an estrogen-progestin contraceptive, can be used for the same purpose. Combined suppressive therapy with a GnRH agonist and an estrogen-progestin contraceptive (3-6 months) achieves a greater and longer-lasting reduction in serum LH and androgen concentrations than treatment with estrogen-progestin contraception alone and also prevents the otherwise inevitable estrogen deficiency symptoms associated with use of a GnRH agonist. Spontaneous resumption of ovulatory cycles can follow,168,169 and 170 potentially eliminating even the need for clomiphene treatment.
Aromatase Inhibitors
Aromatase inhibitors are used primarily in the treatment of postmenopausal breast cancer but are emerging rapidly as a new class of ovulation-inducing agents. Their use for ovulation induction has been controversial, primarily because they are not approved for that purpose and because early preliminary data suggested they might have significant fetal toxicity, which prompted the manufacturer to issue a caution against the use of letrozole in premenopausal women. Although two subsequent studies have found no evidence to
indicate that birth defects are more common in children conceived after treatment with aromatase inhibitors than in those conceived naturally or after treatment with clomiphene citrate,171,172 concerns persist due to the risk of inadvertent exposure in early pregnancy and evidence of teratogenic potential from animal studies.173
indicate that birth defects are more common in children conceived after treatment with aromatase inhibitors than in those conceived naturally or after treatment with clomiphene citrate,171,172 concerns persist due to the risk of inadvertent exposure in early pregnancy and evidence of teratogenic potential from animal studies.173
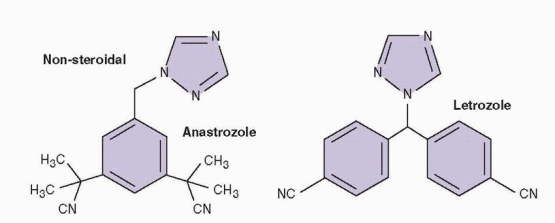
Anastrozole and letrozole are triazole (antifungal) derivatives that act as potent, competitive, nonsteroidal inhibitors of aromatase,174,175 the enzyme that catalyzes the rate-limiting step in estrogen production. They block estrogen production both in the periphery and in the brain, resulting in a compensatory increase in pituitary gonadotropin secretion that stimulates ovarian follicular development.176,177 and 178 In this regard, their mechanism of action is similar to, but also distinct from, that of clomiphene. Although both stimulate increased gonadotropin secretion by decreasing the negative feedback effects of estrogen during treatment, clomiphene does so via depletion of central estrogen receptors whereas aromatase inhibitors decrease estrogen production directly.
 |
At least in theory, the different actions of aromatase inhibitors and clomiphene may have functional, and clinical, importance. After treatment with an aromatase inhibitor ends, estrogen production in growing follicles increases promptly and rising serum concentrations exert negative feedback on gonadotropin secretion, thereby restoring the mechanism that normally serves to select and promote development of a single dominant follicle. After treatment with clomiphene ends, rising estrogen levels cannot immediately exert negative feedback due to the depletion of central estrogen receptors, resulting in a more sustained increase in gonadotropin levels that is more likely to support multifollicular development. The transient accumulation of androgen substrate during treatment with aromatase inhibitors also may increase FSH receptor expression179,180 and production of insulin-like growth factor-1 (IGF-1),181,182 and 183 amplifying the actions of FSH. Moreover, because aromatase inhibitors do not interfere with the actions of estrogen in the periphery, they may be less likely than clomiphene to inhibit estrogen-stimulated cervical mucus production and endometrial proliferation.
Aromatase Inhibitor Treatment Regimens
In almost all studies conducted to date, letrozole (2.5-7.5 mg daily) and anastrazole (1 mg daily) have been administered for a 5-day interval in a manner very similar to that typical for clomiphene treatment (e.g., cycle day 3-7). One trial comparing outcomes achieved with 5 or 10 days of letrozole treatment (2.5 mg daily, beginning on cycle day 1) in clomiphene-resistant women (100 mg daily) observed significantly more preovulatory follicles (3.0 vs. 1.8) and higher pregnancy rates (17.4% vs. 12.4%) in women receiving the longer course of treatment.184 A single-dose treatment regimen of letrozole (20 mg on cycle day 3) also has been described, with preliminary data suggesting it can achieve results similar to those observed with a lower multi-dose treatment protocol.185
The optimal dosage of letrozole and anastrazole has not been established firmly. In most trials involving anovulatory women, 2.5 mg of letrozole or 1 mg of anastrozole has been administered. In a trial comparing outcomes achieved with a 2.5 mg or 5 mg dosage of letrozole in ovulatory women with unexplained infertility, the 5 mg dose yielded significantly greater numbers of follicles and a higher pregnancy rate (26.3% vs. 5.9%).186 In another trial comparing outcomes in women with unexplained infertility randomly assigned to receive treatment with letrozole (7.5 mg daily) or clomiphene (100 mg daily), followed by intrauterine insemination, the two treatments yielded similar numbers of preovulatory follicles (2.1 vs. 1.7) and pregnancy rates (11.5% vs. 8.9%); results also suggested that such higher doses of letrozole may result in greater and longer suppression of estrogen production that could limit endometrial proliferation.187
Altogether, the available data suggest that the optimal dose of letrozole probably ranges between 2.5 mg and 5 mg daily; outcomes achieved with doses of anastrazole greater than 1 mg daily remain to be evaluated.
Results of Treatment with Aromatase Inhibitors
Evidence for the efficacy of aromatase inhibitors for ovulation induction is accumulating rapidly. Early studies exploring the use of aromatase inhibitors focused on anovulatory women considered resistant to clomiphene, because they failed to ovulate or exhibited poor endometrial proliferation during treatment. More recent studies have sought to compare the effectiveness of aromatase inhibitors to that of clomiphene in unselected populations of anovulatory infertile women.
In an early proof of concept trial involving anovulatory clomiphene-resistant women, 9/12 patients (75%) ovulated after treatment with letrozole (2.5 mg daily) and hCG (lead follicle follicle > 20 mm), three conceived (resulting in two singleton births), and normal endometrial proliferation was observed in all.188 In a subsequent trial involving clomiphene-resistant (150 mg daily) anovulatory women with polycystic ovary syndrome (PCOS), 22/44 patients (50%) ovulated after treatment with letrozole (2.5 mg daily) and hCG (lead follicle > 18 mm) and six conceived; response did not relate to age, BMI, or menstrual pattern (amenorrhea vs. oligomenorrhea) and mean endometrial thickness was 10.2 mm.189 In a third involving 64 women with clomiphene-resistant anovulation (100 mg daily), patients were randomized to receive treatment with letrozole (7.5 mg daily) or clomiphene (150 mg daily), followed by hCG (lead follicle ≥ 18 mm); women receiving letrozole had higher ovulation (62.5% vs. 37.5%) and pregnancy rates (40.1% vs. 18.8%) but the differences were not significant.190
Two randomized trials have compared the effectiveness of letrozole (2.5 mg daily) and anastrazole (1 mg daily) for ovulation induction in clomiphene-resistant anovulatory women with PCOS also receiving hCG (lead follicle follicle ≥ 18 mm). One involved 40 clomiphene-resistant patients (200 mg daily, or endometrial thickness ≤ 5 mm) and observed significantly greater endometrial thickness (8.2 vs. 6.5 mm), ovulation rates (84% vs. 60%), and pregnancy rates (19% vs. 10%) in women receiving letrozole.191 In the larger trial involving 220 clomiphene-resistant patients (100 mg daily, or endometrial thickness < 5mm), ovulation rates (62% vs. 63%), pregnancy rates (12 % vs. 15%), and endometrial thickness (9.1 vs. 10.2 mm) in the two groups were similar.192 Taken together, these observations suggest strongly that aromatase inhibitors can be effective in anovulatory women who fail to ovulate in response to clomiphene treatment. Aromatase inhibitors also might be considered for women who respond to clomiphene but exhibit grossly poor endometrial proliferation. Virtually all studies have included monitoring with ultrasonography and adjuvant treatment with hCG, so it remains unclear whether these additions
to the treatment regimen are necessary to achieve success. If not, the substantially lower complexity, risks, and costs of treatment, compared to the alternative of gonadotropin therapy, make it easy to justify a trial of treatment with an aromatase inhibitor for clomiphene-resistant anovulatory women.
to the treatment regimen are necessary to achieve success. If not, the substantially lower complexity, risks, and costs of treatment, compared to the alternative of gonadotropin therapy, make it easy to justify a trial of treatment with an aromatase inhibitor for clomiphene-resistant anovulatory women.
The results achieved with aromatase inhibitors in clomiphene-resistant anovulatory women suggested that aromatase inhibitors might be considered a first-line treatment for ovulation induction. Three trials have compared letrozole and clomiphene in treatment-naïve anovulatory women. The first involved 106 patients who were randomized to receive letrozole (2.5 mg daily) or clomiphene (100 mg daily), followed by hCG (lead follicle ≥ 18 mm); a significantly greater endometrial thickness (8.4 vs. 5.2 mm), ovulation rate (82% vs. 64%), and pregnancy rate (22% vs. 9%) was observed in women receiving letrozole.193 In a second trial with the same design, ovulation occurred in 65/99 letrozole-induced cycles (66%) and in 71/95 (75%) clomiphene-induced cycles, but endometrial thickness (8 mm vs. 8 mm) and pregnancy rates (9% vs. 7%) were not different.194 The largest of the three randomized trials involved a total of 438 women who received treatment with letrozole (5 mg daily) or clomiphene (100 mg daily) and hCG (lead follicle follicle ≥ 18 mm); ovulation rates (365/540, 68% vs. 371/523, 71%) and pregnancy rates (15% vs. 18%) were not different and endometrial thickness was significantly greater in women receiving clomiphene (9.2 vs. 8.1 mm).195 In the only trial involving anastrazole, 115 patients received treatment with anastrazole (1 mg daily, cycle days 3-7, 243 cycles) and hCG (lead follicle follicle ≥ 18 mm) and outcomes were compared to those in matched historical controls treated with clomiphene (100 mg daily, 226 cycles); endometrial thickness was significantly greater in anastrazole cycles (10.1 vs. 8.2 mm), but ovulation rates (68% vs. 69%) and pregnancy rates (10.2% vs. 7.9%) were similar in the two groups.196
A few studies have examined the outcomes of pregnancies conceived after treatment with aromatase inhibitors. A cohort study found that pregnancies conceived in letrozole-induced cycles are significantly more likely to be singletons than those conceived in cycles involving treatment with clomiphene or gonadotropins.197 One case series comparing the incidence of congenital malformations in 911 newborns of women who conceived after treatment with letrozole (14/514, 2.4%) or clomiphene (19/397, 3.0%) found no difference.171 Another comparing the incidence of birth defects in children born to mothers treated with letrozole or clomiphene to that in pregnancies conceived without treatment also observed no differences.172
In sum, the available data suggest that aromatase inhibitors may be as effective, but not more effective, than clomiphene as a first-line treatment for ovulation induction. Early trials all have used surrogate endpoints, which do not correlate consistently with live birth rates. Uniformly, they also have included adjuvant treatment with hCG, which likely is unnecessary (as in clomiphene-induced cycles) and certainly is not desirable because it requires serial ultrasonography, with all of the added costs and inconvenience. Aromatase inhibitors have great promise and appear to be associated with a lower risk for conceiving a multiple pregnancy. Their use seems certain to expand, but larger randomized trials are required to better define their efficacy and rightful place in the treatment of anovulatory infertility.
Laparoscopic Ovarian Drilling
Surgical treatments aimed at restoring ovulatory function in anovulatory infertile women date back to the classic bilateral ovarian wedge resection described originally by Stein and Leventhal in 1935.198 The procedure understandably fell out of favor after the introduction
of clomiphene citrate and gonadotropins for ovulation induction. Advances in laparoscopic surgery sparked renewed interest in the procedure, with ovarian “drilling” now representing the modern equivalent of the classical wedge resection and another treatment option for clomiphene-resistant, hyperandrogenic, anovulatory women.
of clomiphene citrate and gonadotropins for ovulation induction. Advances in laparoscopic surgery sparked renewed interest in the procedure, with ovarian “drilling” now representing the modern equivalent of the classical wedge resection and another treatment option for clomiphene-resistant, hyperandrogenic, anovulatory women.
Several methods for ovarian drilling have been described, including electrocautery or laser vaporization (approximately four to six sites per ovary, avoiding surfaces near the tubo-ovarian interface to minimize the risk for adhesions that might adversely affect ovum capture) and multiple biopsy.199,200 and 201 All are intended to cause focal destruction of the ovarian stroma in efforts to decrease both intraovarian and systemic androgen concentrations. There is no evidence for the superiority of any one method, but the most common technique involves electrocautery using a unipolar needle electrode insulated above the distal 1-2 cm.
Postoperative serum concentations of androstenedione and testosterone decrease, at least for a time,202,203 and 204 and inhibin concentrations also decline.205,206 Both changes likely contribute to an associated increase in FSH levels. As with the classical surgical procedure, the principal risk associated with laparoscopic ovarian drilling is postoperative adnexal adhesion formation that may decrease overall fertility, although the risk and severity of adhesions are lower;207,208,209 and 210 second-look laparoscopy and adhesiolysis do not appear necessary or useful.211,212 There is one report of unilateral ovarian atrophy after ovarian drilling with electrocautery.213 Whether ovarian drilling might adversely affect ovarian reserve and predispose to early menopause has not been investigated specifically, but other data indicate that reductive ovarian surgery, including ovarian wedge resection, increases risk for early menopause.214
In numerous uncontrolled observational studies, 40-90% of women have ovulated after laparoscopic ovarian drilling and approximately half of those have conceived.199,200 and 201,215,216 In truly clomiphene-resistant women, ovarian drilling can improve clomiphene sensitivity or response to exogenous gonadotropins when it does not restore spontaneous ovulatory cycles.217 When considering laparoscopic ovarian drilling as a treatment option in clomiphene-resistant anovulatory infertile women, the most relevant data derive from randomized controlled trials comparing surgical treatment with ovulation induction using exogenous gonadotropins.211,218,219 A 2007 systematic review and meta-analysis including nine trials found no evidence of a difference in live birth (OR=1.04, CI=0.59-1.85) or clinical pregnancy rate (OR=1.08, CI=0.69-1.71).220 After 12 months, the ovulation rate achieved with drilling (52%) was similar to that with gonadotropin therapy (62%). However, as might be expected, laparoscopic ovarian drilling yields far fewer multiple pregnancies than gonadotropin treatment (1% vs. 16%; OR=0.13, CI=0.03-0.52); the miscarriage rates associated with surgical and medical treatment are comparable.220
The ideal candidate for ovarian drilling is not obese and has no other infertility factors. The procedure often is unsuccessful in obese women (BMI >30 kg/m2),221,222 and whereas over 80% of women can be expected to conceive after surgery when clomiphene-resistant anovulation is the sole cause of infertility,222,223 only 15-30% achieve pregnancy when there is a coexisting tubal, male, or other infertility factor.203,223
Laparoscopic ovarian drilling can be an effective therapeutic option for clomipheneresistant anovulatory infertile women, but the temporary effects of treatment, the risk of postoperative adhesions, and the theoretical risk of adverse effects on ovarian reserve deserve careful consideration and discussion. The procedure is perhaps best reserved for women who are unable or unwilling to accept the costs and risks associated with gonadotropin therapy.
Exogenous Gonadotropins
Exogenous gonadotropins have been used to induce ovulation in gonadotropin-deficient women and those who fail to respond to other, less complicated forms of treatment for nearly 50 years. They are highly effective, but also very costly and associated with substantial risks including multiple pregnancy and ovarian hyperstimulation syndrome. Consequently, exogenous gonadotropins should be used only by clinicians having the training and experience necessary to provide safe and effective treatment.
Gonadotropin Preparations
Gonadotropin preparations have evolved gradually over the years, from relatively crude urinary extracts, to more highly purified urinary extracts, to the recombinant preparations in common use today.224,225
For almost 30 years, the only exogenous gonadotropins available were human menopausal gonadotropins (hMG, menotropins), an extract prepared from the urine of postmenopausal women containing equivalent amounts (75 IU) of FSH and LH per ampule or vial and requiring intramuscular injection. Originally, the urinary source was a single convent in Italy, but later collections were expanded to a number of centers in other countries.226 Urinary menotropins also contain small but measurable and varying amounts of hCG, most of it added intentionally during the manufacturing process to provide the appropriate amount of LH activity and some derived from other sources.227 Clinical use of hMG began in 1950 but the clinical trials did not begin until after 1960.228,229 Relatively crude gonadotropin extracts like traditional hMG also contained significant amounts of uncharacterized urinary protein that may be antigenic.230 Contemporary hMG preparations are more highly purified than in the past and can be administered subcutaneously.231
Beginning about 25 years ago, more purified urinary FSH preparations (urofollitropin) were developed by removing LH from urinary extracts using immunoaffinity columns containing polyclonal anti-hCG antibodies.232 Early preparations of purified urinary FSH (75 IU) contained less than 1 IU LH but a considerable amount of other urinary protein and still required intramuscular administration. Further purification using monoclonal antibodies specific for FSH yielded a preparation containing less than 0.1 IU LH and less than 5% unidentified protein. The even more highly purified products now in use contain less than 0.001 IU LH, very low levels of urinary protein, and can be administered subcutaneously.
Just over 15 years ago, the in vitro production of recombinant human FSH was achieved through genetic engineering. Briefly summarized, the process involves introduction of the genes encoding the a- and b-FSH subunits into the genome of a Chinese hamster ovary cell line, which then synthesizes and secretes a glycosylated bioactive dimeric FSH that is finally purified by immunochromatography using a specific anti-FSH monoclonal antibody. Recombinant FSH preparations contain less acidic FSH isoforms that have a shorter half-life than those derived from human urine but stimulate estrogen secretion as or even more efficiently.233 The advantages of recombinant FSH preparations include the absence of urinary protein, more consistent supply, and less batch-to-batch variation in biologic activity. The two recombinant FSH preparations currently available are marketed
as follictropin alpha and follitropin beta. They are both structurally identical to native FSH and contain 1 alpha and 1 beta glycoprotein chain, but the post-translational glycosylation process and purification procedures for the two are different.234 Despite the subtle differences in structure, they are functionally the same. The biological activity of all FSH preparations, including recombinant formulations, is ultimately confirmed using the classic Steelman-Pohley ovarian bioassay.235
as follictropin alpha and follitropin beta. They are both structurally identical to native FSH and contain 1 alpha and 1 beta glycoprotein chain, but the post-translational glycosylation process and purification procedures for the two are different.234 Despite the subtle differences in structure, they are functionally the same. The biological activity of all FSH preparations, including recombinant formulations, is ultimately confirmed using the classic Steelman-Pohley ovarian bioassay.235
Most recently, recombinant technology has been used to create a new chimeric gene containing the coding sequences of the FSH β-subunit and the C-terminal peptide of the hCG β-subunit (containing additional glycosylation sites). Co-expression of the α-subunit and the chimeric FSH β-subunit produces a new molecule called corifollitropin alpha, which has a prolonged half-life and enhanced in vivo bioactivity compared with wild-type FSH. Early studies in women suppressed by treatment with a long-acting GnRH agonist have confirmed the extended half-life of the compound and clinical trials have demonstrated that corifollitropin alpha can induce and sustain multifollicular growth for a week in women receiving ovarian stimulation for IVF. Corifollitropin alpha provides the means for simpler and more convenient treatment compared with conventional treatment regimens involving daily injections with FSH and GnRH agonist. Although the new recombinant gonadotropin is likely to find applications in assisted reproductive technologies where the goal is to induce multi-follicular development, it is not well suited for ovulation induction where unifollicular development is the objective.236,237
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


