Immunologic Disorders in Pregnancy
D. Ware Branch
Robert M. Silver
Kjersti Aagaard-Tillery
Reproductive Immunology
The physiologic mechanisms that protect the fetus and its placenta from attack by the maternal immune system are complex, and many remain poorly understood. Nevertheless, the practicing obstetrician should be familiar with the immunologic aspects of the maternal–fetal relationship as well as the profound impact that aberrations in immunologic function have on pregnancy outcome.
Fundamental Immunobiology
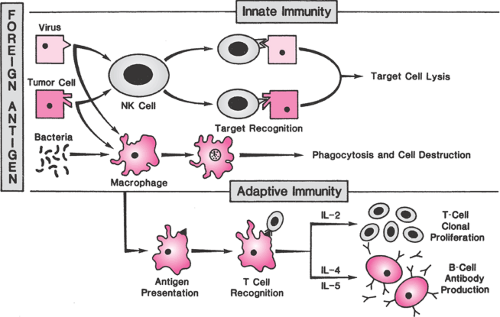
The primary function of the immune system is to protect against foreign pathogens, primarily by distinguishing biologic “self” from “nonself” (the clonal selection model of immunity). In the early stages of development, immune cell precursors are educated to recognize self. T cells that recognize self-antigens are killed or rendered inactive while T cells with specific receptor types against foreign pathogens continue to develop. The immune system has two primary effector mechanisms, which though overlapping and coordinated are classified for the sake of discussion as either innate or adaptive. The innate immune response is a continuous and relatively nonspecific process that recognizes foreign material as nonself, allowing it to operate effectively without prior exposure to a microorganism or its antigens. Components of the innate system include physical and biochemical barriers, primary effector cells such as mononuclear phagocytes, natural killer (NK) cells, and polymorphonuclear leukocytes and circulating biochemical factors such as the complement proteins. Recognition of foreign pathogens by membrane receptors on effector cells is followed by phagocytic destruction and induction of apoptosis by NK cells (Fig. 18.1). One family of pathogen recognition receptors, called Toll-like receptors (TLR), appears to play an important role in mediating the inflammatory responses in the reproductive tract. As membrane receptors on inflammatory cells, they initiate a nonspecific response to bacterial antigens and also may function to facilitate adhesion of immune cells on endothelium.
The hallmark of adaptive immunity is precise specificity of antigen recognition and memory so that repeated exposures to a specific antigen elicit an enhanced immune response. The adaptive response includes several stepwise processes that occur in the lymphatic system (Fig. 18.1). The primary adaptive effector cells are B cells, precursors of plasma cells that secrete specific antibodies that circulate through the bloodstream and destroy target cells in concert with complement or antibody-dependent cellular cytotoxic cells. Antibodies are heterogeneous proteins produced by gene rearrangement, a process that creates myriad possible immunoglobulin antigen-recognition sites. The first antibody to be produced in a primary response is immunoglobulin M (IgM), which is soon superseded by a predominantly immunoglobulin G (IgG) response.
As proliferating T cells mature, they differentiate into an array of subtypes that have diverse functions. CD4+ helper cells promote the proliferation of other immune cells and help B cells produce antibodies. CD4+ inducer cells control the subsequent development of other T cells. CD8+ cytotoxic cells lyse foreign or virus-infected cells, and CD8+ suppressor cells prevent an uncontrolled immune response. T-cell subsets destroy and remove foreign tissues and organisms by direct binding and secretion of cytokines that recruit and activate macrophages. Cytokines are a means of communication between immune cells. Interleukin (IL)-1 is produced by macrophages and monocytes
and promotes multiplication and activation of lymphocytes. IL-2, produced in response to lymphocyte activation, is the major T-cell growth factor for the proliferation of activated T cells.
and promotes multiplication and activation of lymphocytes. IL-2, produced in response to lymphocyte activation, is the major T-cell growth factor for the proliferation of activated T cells.
Cells of the innate immune system act as secondary effector cells in the adaptive response through T-cell recognition of human leukocyte antigens (HLAs), which are products of the major histocompatibility complex (MHC) located on the short arm of chromosome 6. Class I MHC antigens are expressed by nearly all nucleated cells and are identified by cytotoxic T cells. Class II MHC antigen expression is restricted to B cells, monocytes, macrophages, and activated T cells but is important for presenting antigen to helper T cells.
Embryologic Development of the Immune System
The development of the immune system begins at conception and continues throughout pregnancy and into the newborn period. During weeks 4.5 to 6.0 of gestation (menstrual weeks), pluripotential stem cells in the yolk sac and aortic–gonad–mesonephros form the precursors for all the blood cell series. Migration of these cells, probably in a series of migratory waves, to the liver, thymic bud, and eventually to the bone marrow and spleen establish the functioning immune system. The thymus develops in the human embryo at 6 weeks gestation, and lymphocyte differentiation proceeds in the absence of foreign antigens. Small lymphocytes appear in the peripheral blood at week 7 and around lymphocyte plexuses by week 8. As early as 13 weeks gestation, T cells that can respond to mitogens and recognize histoincompatible cells begin to appear. By 20 weeks gestation, the human fetus has the ability to respond to congenital infections by producing plasma cells and antibodies. Monocytes first appear in fetal blood at 18 to 20 weeks gestation and form approximately 5% of the circulating cells by 30 weeks. Polymorphonuclear cells (PMNs) are identifiable in the fetal liver and bone marrow in the mid-second trimester but are a relatively small component of circulating cells in utero and at birth. Circulating concentrations increase dramatically at birth, peaking at 12 to 24 hours and declining somewhat by 72 hours after birth in the term infant.
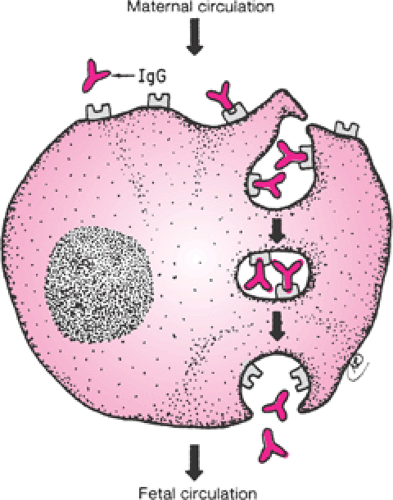
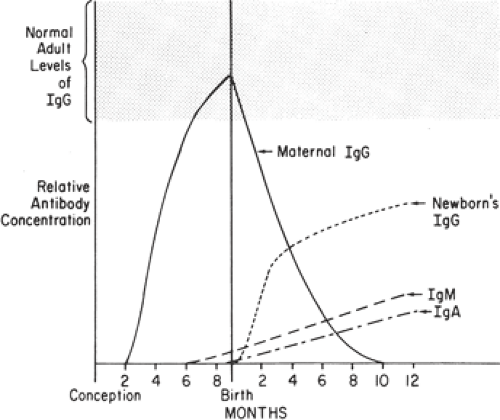
The presence of an intact trophoblastic cellular barrier prevents the movement of large numbers of immunocompetent cells into or out of the fetus during pregnancy. In contrast, maternal IgG, by virtue of its Fc fragment, is specifically selected for placental transfer (Fig. 18.2). Fetal IgG concentrations are about 10% of adult levels by the middle
of the first trimester (Fig. 18.3). Adequate humoral immunity in the neonatal period depends on the circulating immunoglobulins that have crossed the placenta, and fetal blood levels of IgG reflect maternal levels. The specific antibody protection depends on the mother’s own antigenic experience. The primary role of maternal antibodies is to protect the neonate from infections. However, several maternal autoimmune disorders are characterized by production of IgG antibodies and can be harmful to the infant.
of the first trimester (Fig. 18.3). Adequate humoral immunity in the neonatal period depends on the circulating immunoglobulins that have crossed the placenta, and fetal blood levels of IgG reflect maternal levels. The specific antibody protection depends on the mother’s own antigenic experience. The primary role of maternal antibodies is to protect the neonate from infections. However, several maternal autoimmune disorders are characterized by production of IgG antibodies and can be harmful to the infant.
Pathogenesis of Autoimmune Disease
The myriad specificities of the response of B cells and T cells to the wide array of antigenic material presented by nature results from various random combinations of the many genes coding for antigen binding sites on cellular receptors. It is estimated that this process can produce 109 different T-cell receptors. Not surprisingly, some of these millions of binding sites recognize, at least to some degree, antigenic structures present on the cells of normal tissues (autoantigens). The immune system is normally regulated so that cells capable of producing an immune response to self-antigens are suppressed; this process is often called immune tolerance.
Failure of immune tolerance may result in autoimmunity and autoimmune disease. A failure in B-cell tolerance would appear to result in the production of autoantibodies responsible for Graves disease, for example, because they bind to and stimulate the thyrotropin receptor. Autoantibodies also are the likely culprits in several other autoimmune disease, including pemphigus vulgaris, autoimmune hemolytic anemia, and autoimmune thrombocytopenia. A failure in T-cell immune tolerance likely is a primary cause of autoimmune thyroiditis, systemic lupus erythematosus, multiple sclerosis, and type I diabetes mellitus.
A complete discussion of the possible mechanisms of immune cell tolerance and the defects that may lead to a failure of B- or T-cell immune tolerance is beyond the scope of this chapter. Briefly, autoreactive B cells are normally deleted in the bone marrow or in the T-cell zones of the spleen or thymus, with the latter mechanism requiring the participation of the T-cell system. Autoreactive B cells also may be functionally inactivated in the periphery via the development of anergy or receptor editing.
Similarly, autoreactive T cells may be deleted in the thymus, where they encounter self-antigens presented in the context of MHC molecules. Those autoreactive cells with high affinity for the self-antigens undergo apoptosis—they are negatively selected by a process in thymus that requires self-antigen complexes, known as central tolerance. Autoreactive T cells in the periphery may be blocked from reacting with pertinent tissues via physiologic separation, for example, the blood–brain barrier, or by the binding of the autoreactive T cells to Fas ligand, which induces apoptosis and thus deletes the potentially damaging T-cell clone in periphery. Also, autoreactive T cells are regulated by “regulatory” T cells, likely composed of several cells types producing a variety of regulatory cytokines.
Autoimmune diseases result from a breakdown in normal regulatory mechanisms that allow the distinction between self and nonself. Autoimmunity is characterized by
persistent activation of immunologic mechanisms that affect the function and integrity of certain cells and organs. The process may be initiated in genetically susceptible individuals by environmental agents, such as infection, but is probably sustained by persistent T-cell activation that overrides normal tolerance of self-antigens.
persistent activation of immunologic mechanisms that affect the function and integrity of certain cells and organs. The process may be initiated in genetically susceptible individuals by environmental agents, such as infection, but is probably sustained by persistent T-cell activation that overrides normal tolerance of self-antigens.
Autoimmune diseases have a predilection for women of reproductive age and are often encountered during pregnancy. The effects of pregnancy on autoimmunity, and vice versa, depend to some degree on whether a condition is primarily cellular (T cell) or humoral antibody (B cell) in nature and to what degree the aberrant immune system might affect placentation or the fetus. Certain diseases with strong cellular pathophysiology, such as rheumatoid arthritis (RA) and multiple sclerosis, may be associated with remission during pregnancy and have little impact on the placenta or fetus, whereas autoantibody-mediated autoimmune diseases such as neonatal lupus may directly effect the fetus via the transplacental passage of autoantibodies.
Maternal–Fetal Immunology
Maternal immunologic reaction to the genetically dissimilar cells of the conceptus begins at fertilization and continues throughout pregnancy as differentiated fetal trophoblast cells interact with maternal uterine tissue and blood. The normal immunologic relationship between mother and fetus appears to be healthy and growth promoting rather than the usual allogeneic model of destruction.
Several mechanisms likely play a role in protection of the fetus from immunologic damage. Circulating blocking factors have been theorized to attenuate maternal immunologic reaction. One progesterone-induced blocking factor suppresses production of lymphocytes and proinflammatory cytokines. In addition, so-called blocking antibodies may prevent lymphocytic destruction by binding to receptors on fetoplacental tissues. Alternatively, blocking antibodies may be directed against antigen-specific combining sites (idiotypes) on maternally produced antibodies that prevent them from assisting lymphocytes in targeting cells on the conceptus. While the concept is appealing, the relevance of blocking antibodies and other alleged circulating pregnancy-maintaining factors remains unsettled. Not only do agammaglobulinemic women without antibody production have normal pregnancies, so-called blocking antibodies do not frequently appear until late in the first or second trimester of the first pregnancy.
Immunotolerance during pregnancy probably results from interactions at the local maternal–fetal interface rather than from generalized maternal immunosuppression. Before conception, endometrial stromal cells transform into decidual cells that contain T-cell subtypes with immunosuppressive activity. One T-helper cell subset secretes cytokines that are beneficial or neutral to the presence of the fetus. Another is thought to prevent colonization with microbial pathogens. Additional evidence suggests that some subsets of the decidual T cells promote growth of the placenta through the secretion of cytokines that suppress inflammation. Hormones, enzymes, growth factors, and endometrial proteins within maternal decidual tissue also have potent immunomodulatory properties that promote a favorable interaction between the conceptus and the mother.
The presence of large granular lymphocytes in luteal phase and early- to mid-pregnancy deciduas has generated considerable interest among reproductive immunologists. Under normal circumstances, these nonspecific innate immune effector cells, similar to NK cells, kill standard NK-cell targets with the notable exception of trophoblastic cells. Some experts find that alternations in uterine NK-cell numbers are associated with pregnancy loss.
The placenta also plays an active role in protection of the fetus from maternal immune responses. Villous cytotrophoblasts and syncytiotrophoblasts escape destruction because both express nonclassic MHC antigens that prevent trophoblast destruction through inhibition of lysis by activated NK cells, limitation of leukocyte cytotoxic activity, suppression of proinflammatory cytokine production, and induction of T-cell death. Nonclassic MHC antigens, specifically HLA-G, also promote trophoblast proliferation and invasion. Altered expression of nonclassic MHC antigens has been linked to recurrent miscarriage and preeclampsia.
Placental expression of a protein known as the Fas ligand also may play a role in pregnancy success through selective deletion of antifetal T-cell clones. In animal studies, binding to the Fas ligand causes death and removal of autoreactive T cells.
The placenta also may inhibit T-cell proliferation by sequestering nutrients. Placental indolamine 2,3-dioxidase (IDO) inactivates the amino acid tryptophan, which is essential for the proliferation of T cells. The role of IDO in recurrent pregnancy loss (RPL) in humans has not yet been widely investigated.
The inhibition of complement activation also may play a role in maternal–fetal tolerance. Mice lacking a protein that inhibits complement have a high rate of pregnancy loss characterized by placental inflammation. If complement is removed, the mice reproduce normally. Complement activation also has been shown to be important in the pathophysiology of pregnancy loss in women with antiphospholipid syndrome (APS).
Erythroblastosis Fetalis (Red Cell Alloimmunization)
Although the first description of erythroblastosis fetalis (hemolytic disease of the newborn) dates back to 1609, it was not until the early 1900s that the role of alloimmunization in the pathogenesis of erythroblastosis was
established. In this condition, the Rh-negative mother becomes alloimmunized by exposure to Rh-positive fetal erythrocytes during pregnancy or delivery. Maternally produced antierythrocyte antibodies, recognizing the Rh D antigen, pass across the placenta to the fetus where they react with the Rh-positive fetal erythrocytes, causing their destruction. In the past, Rh alloimmunization also has been referred to as Rh sensitization or Rh isoimmunization.
established. In this condition, the Rh-negative mother becomes alloimmunized by exposure to Rh-positive fetal erythrocytes during pregnancy or delivery. Maternally produced antierythrocyte antibodies, recognizing the Rh D antigen, pass across the placenta to the fetus where they react with the Rh-positive fetal erythrocytes, causing their destruction. In the past, Rh alloimmunization also has been referred to as Rh sensitization or Rh isoimmunization.
Many other erythrocyte antigens have been described since the discovery of the Rh D antigen, but only a few are clinically important causes of maternal alloimmunization. However, with the widespread use of Rh immune globulin prophylaxis and the decline in Rh D alloimmunization, the overall importance of the “minor antigens” in red cell alloimmunization has increased.
Genetics of the Rh Antigen
The Rh blood group was so named because rabbits immunized with rhesus monkey erythrocytes produced an antibody that agglutinated erythrocytes from 85% of whites. This antibody, initially known as the Rh factor, is directed against an erythrocyte surface antigen of the rhesus blood group system. The Rh blood group system has a high degree of polymorphism, with five major antigens and many variant minor antigens. While three systems of nomenclature have been suggested, the Fisher-Race system is probably best suited to understanding the inheritance of the Rh antigen and the clinical management of Rh alloimmunization. It assumes the presence of three genetic loci, each with two major alleles, lettered C, c, D, E, and e. No antiserum specific for a “d” antigen has been found.
The Rh gene complex is described by the three appropriate letters with eight possible combinations (listed in decreasing order of frequency among whites): CDe, cde, cDE, cDe, Cde, cdE, CDE, and CdE. Genotypes are indicated as pairs of gene complexes, such as CDe/cde. Certain genotypes, and thus certain phenotypes, are more prevalent than others. Although the alleles are always written in the order C(c), D, E(e), the actual order on chromosome 1 is of the genes coding for the antigens D, C(c), E(e). Anti-C, anti-c, anti-D, anti-E, and anti-e designate specific antibodies directed against the respective antigens. Because the majority of Rh D alloimmunization resulting in overt clinical disease results from incompatibility with respect to the D antigen, common convention holds that Rh positive indicates the presence of the D antigen and Rh negative indicates the absence of D antigen on erythrocytes.
Unique Rh antibodies have been used to identify more than 30 antigenic variants in the Rh blood group system, the most common of which is the Du antigen, now commonly referred to as weak D. This heterogeneous group of clinically important D-antigen variants most often is found in blacks. At least some weak D–positive patients are capable of producing anti-D, which could presumably result in a weak D–positive mother becoming sensitized to her D-positive fetus, but such an occurrence is exceedingly rare.
The Rh D antigen appears very early in embryonic life and has been demonstrated on the red blood cells of a 38-day-old fetus. The Rh erythrocyte glycoproteins are ancient in origin and occur widely in eukaryotic cells. They probably function in membrane ammonia homeostasis and also interact with cellular skeletal structure to help maintain the mechanical properties of the cell membrane. Rhnull individuals have a compensated hemolytic anemia with multiple red cell membrane abnormalities.
Pathophysiology of Rh Alloimmunization
The pathophysiology of Rh D alloimmunization is the best studied of the antierythrocyte alloimmunizations and thus may serve to illustrate the major principles of erythrocyte alloimmunization.
Rh D alloimmunization can occur only in the presence of three conditions: (a) the fetus must have Rh-positive erythrocytes, and the mother must have Rh-negative erythrocytes; (b) the mother must have the immunogenic capacity to produce antibody directed against the D antigen; and (c) a sufficient number of fetal erythrocytes must gain access to the maternal circulation.
Incidence of Rh D Incompatibility and Subsequent Alloimmunization
About 15% of whites, 5% to 8% of blacks, and 1% to 2% of Asians and Native Americans are Rh negative. In terms of risk, an Rh-negative woman has about an 85% chance of mating with an Rh-positive man, 60% of whom are heterozygous and 40% of whom are homozygous at the D locus. Assuming that one half of the conceptions of heterozygous men will be Rh D positive, the chance of an Rh-positive man producing an Rh-positive fetus is about 70%. An Rh-negative woman has about a 60% chance of bearing an Rh-positive fetus (0.85 × 0.70). About 10% of pregnancies are Rh incompatible (0.15 × 0.60). However, fewer than 20% of Rh-incompatible pregnancies result in alloimmunization because fetomaternal hemorrhage sufficient to trigger a maternal antibody response does not occur in every case. About 16% of untreated Rh-negative women become alloimmunized in their first Rh-incompatible (ABO-compatible) pregnancy. Half produce detectable anti-D antibody within 6 months of delivery, while the rest have undetectable amounts until early in the next incompatible pregnancy. Overall, even before the introduction of Rh immune globulin prophylaxis, only about 1% of pregnant women had anti-D antibody.
Maternal Immunologic Response
The probability and severity of Rh D alloimmunization varies depending on individual patient characteristics. As many as 30% of Rh-negative individuals appear to
be immunologic “nonresponders” who will not become sensitized. In addition, ABO incompatibility diminishes the risk of alloimmunization to about 1.5% to 2.0% after the delivery of an Rh-positive fetus. This is possibly due to rapid clearance of ABO-incompatible fetal cells from the maternal circulation or alteration or damage to the fetal Rh antigen so that it is no longer immunogenic. The effect is most pronounced if the mother is type O and the father is type A, B, or AB.
be immunologic “nonresponders” who will not become sensitized. In addition, ABO incompatibility diminishes the risk of alloimmunization to about 1.5% to 2.0% after the delivery of an Rh-positive fetus. This is possibly due to rapid clearance of ABO-incompatible fetal cells from the maternal circulation or alteration or damage to the fetal Rh antigen so that it is no longer immunogenic. The effect is most pronounced if the mother is type O and the father is type A, B, or AB.
Fetomaternal Hemorrhage
Fetal red cells may gain access to the maternal circulation during pregnancy, delivery, and the immediate postpartum period. Fetomaternal hemorrhage in a volume sufficient to cause alloimmunization is most common at delivery, occurring in 15% to 50% of births. The amount of fetal blood entering the maternal circulation is usually less than 0.1 mL but may be greater than 30 mL in 0.2% to 1.0% of cases. Risk factors for excessive postpartum fetomaternal hemorrhage include cesarean delivery, multiple gestations, bleeding placenta previa or abruption, manual removal of the placenta, and intrauterine manipulation. However, the majority of cases of excessive fetomaternal hemorrhage occur after uncomplicated vaginal delivery.
Antepartum events also can result in fetomaternal hemorrhage in sufficient volume to cause alloimmunization in 1% to 2% of cases, even without obvious disruption of the choriodecidual junction (Table 18.1). Fortunately, asymptomatic antepartum sensitization rarely occurs before the third trimester.
TABLE 18.1 Antepartum Events Associated with Fetomaternal Hemorrhage | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Rh D Immune Globulin and the Prevention of Rh D Alloimmunization
The prevention of alloimmunization to a specific antigen by the passive administration of antibody is termed antibody-mediated immune suppression. In the case of Rh D alloimmunization, a high degree of protection was first achieved by administering anti-D immune globulin (Rh D immune globulin) to Rh-negative male volunteers who had been infused with Rh-positive red cells. It was later established that the amount of Rh D immune globulin necessary to prevent alloimmunization varies according to the size of fetomaternal hemorrhage:
300 μg of Rh D immune globulin for exposure to 10 mL of fetal blood
20 μg of Rh D immune globulin for exposure to 1 mL of fetal erythrocytes
10 μg of Rh D immune globulin for 1 mL of whole fetal blood.
Postpartum Alloimmunization Prophylaxis
The early Rh D alloimmunization prevention trials found that administration of Rh D immune globulin within 72 hours of delivery reduced alloimmunization to less than 1.5% in Rh-negative women, a 7- to 10-fold decrease in alloimmunization compared with untreated controls. Although 300 μg of Rh D immune globulin was used (and continues to be the standard in the United States), it has subsequently been shown that a dose of 100 μg to 150 μg probably is adequate for routine use.
Rh D immune globulin should be given as soon as possible after exposure to Rh D–positive blood (delivery or other event associated with fetomaternal hemorrhage) and before the primary immune response is established. While 72 hours is the standard recommendation, it is an artifact from the early studies performed using inmates, during which prison officials would allow visits only at 3-day intervals. Prophylaxis beyond 3 days has never been extensively studied, but if for some reason Rh D immune globulin prophylaxis does not occur within 72 hours after exposure, susceptible Rh D–negative women should be treated up to 14 to 28 days. Further, if the neonatal Rh status is unknown 3 days after delivery, Rh D immune globulin should be given rather than waiting for the neonatal results.
Antepartum Alloimmunization Prophylaxis
During pregnancy, 1% to 2% of susceptible Rh D–negative women become sensitized despite postpartum Rh D immune prophylaxis. Most failures can be attributed to antepartum fetomaternal hemorrhage that often is not clinically apparent. Prophylactic administration of Rh D immune globulin at 28 weeks gestation reduces the incidence of alloimmunization from 1.8% to 0.1%. Initial concerns about potential adverse effects of antenatal Rh D immune globulin prophylaxis have been refuted by
decades of experience with Rh D immune globulin without reports of maternal or fetal complications. Further, routine antepartum prophylaxis is much less expensive than the neonatal intensive care required for severely anemic infants.
decades of experience with Rh D immune globulin without reports of maternal or fetal complications. Further, routine antepartum prophylaxis is much less expensive than the neonatal intensive care required for severely anemic infants.
Management of the Unsensitized Rh-Negative Pregnant Woman
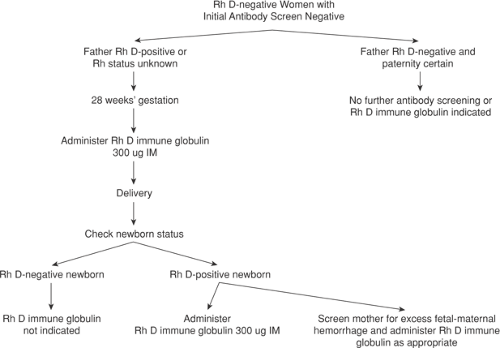
Prenatal care for Rh D–negative women without evidence of alloimmunization is straightforward (Fig. 18.4). Every woman should have her ABO blood group, Rh type, and antibody screen checked at the first prenatal visit of all pregnancies. If she is Rh-negative or weak D–negative and has no demonstrable antibody, she is a candidate for 300 μg Rh D immune globulin prophylaxis at around 28 weeks gestation and again immediately postpartum. The American Association of Blood Banks recommends obtaining another antibody screen before administration of Rh D immune globulin, including antepartum prophylaxis. The cost and inconvenience of this test must be weighed against documenting the rare case of Rh D sensitization in the first or second trimester in asymptomatic women.
After delivery, another antibody screen is routinely performed. If negative and the newborn is Rh D positive or weak D positive, women should be given 300 μg of Rh D immune globulin. In addition, because a small number of deliveries (0.1% to 1.0%) result in a fetomaternal hemorrhage greater than 30 mL (the largest volume of fetal blood adequately covered by a standard 300 μg dose of Rh D immune globulin), a screen for “excessive” fetomaternal hemorrhage should be performed routinely. Most laboratories use the erythrocyte rosette test, a simple and sensitive method for detecting fetomaternal bleeding. If the rosette test is positive, the volume of fetal red cells in the maternal circulation can be calculated by using the Kleihauer-Betke test. If the volume of fetomaternal hemorrhage is greater than 30 mL whole blood, an additional 10 μg of Rh D immune globulin should be administered for each additional milliliter of fetal blood.
A weak D–positive mother who delivers an Rh-positive infant is not at significant risk of Rh alloimmunization, probably because the weak D antigen actually is an incompletely expressed D antigen. Therefore, weak D–positive mothers usually do not require Rh D immune globulin. Occasionally, a woman previously typed as Rh negative is unexpectedly found to be weak D positive during pregnancy or after delivery. In this situation, the clinician should be suspicious that the patient’s “new” weak D–positive status actually is due to a large number of Rh D–positive fetal cells in the maternal circulation. Appropriate diagnostic studies should be performed, and if fetomaternal hemorrhage is
found, the mother should be treated with Rh D immune globulin.
found, the mother should be treated with Rh D immune globulin.
Several antepartum complications and procedures also may result in fetomaternal hemorrhage (Table 18.1). First-trimester complications, including spontaneous miscarriage, elective abortion, and ectopic abortion, may result in fetomaternal hemorrhage sufficient to result in alloimmunization.
Fetomaternal hemorrhage also has been demonstrated in up to one half of women with threatened first-trimester miscarriage but only occasionally is associated with alloimmunization.
An Rh-negative, unsensitized patient who has antepartum bleeding or suffers an unexplained second- or third-trimester fetal death should receive Rh D immune globulin prophylaxis and be evaluated for the possibility of massive fetomaternal hemorrhage. Several procedures also may result in fetomaternal hemorrhage sufficient to cause alloimmunization, including chorionic villus sampling (CVS), amniocentesis, and external cephalic version.
For first-trimester pregnancy complications and procedures, 50 μg of Rh D immune globulin is protective. Beyond 12 weeks, a full 300-μg dose is indicated, even in the absence of detectable hemorrhage. In addition, because excessive fetomaternal hemorrhage may occur with any complication or procedures performed in the second and third trimester, an assessment of the volume of fetal whole blood should be performed, and the appropriate amount of Rh D immune globulin should be given. Cases of chronic bleeding present a dilemma with regard to the optimal dose of Rh D immune globulin. An indirect antibody screen may be performed after the initial dose of Rh D immune globulin. A positive result likely indicates that enough antibody was administered (circulating unbound anti–Rh D). A negative result indicates antigen (fetal red blood cells) in excess of the antibody given and more Rh D immune globulin should be administered.
Failure to administer Rh D immune globulin when indicated is responsible for one fourth of new cases of alloimmunization. This oversight may be due to the following:
Failure to type the patient’s blood at the first prenatal visit or to order Rh D immune globulin when indicated
Error in transmitting the proper blood type to the mother’s chart and to the physician
Error in typing the mother, father, or baby’s blood
Failure to administer Rh D immune globulin when ordered
Unrecognized fetomaternal hemorrhage during pregnancy
Inadequate Rh D immune globulin for the volume of fetomaternal hemorrhage
Patient refusal.
Management of the Rh D–Alloimmunized Pregnancy
Obstetric History
A well-documented obstetric history is essential to guide the management of an alloimmunized pregnancy. Fetal hemolytic disease tends to become more severe in subsequent pregnancies. If hydrops occurred in a previous pregnancy, the next Rh-incompatible fetus has an 80% to 90% chance of becoming hydropic as well. With this in mind, patients are grouped into one of three categories:
mildly affected fetuses, which can be allowed to remain in utero until they have achieved pulmonary maturation
moderately to severely affected fetuses, which may need intrauterine treatment (transfusion) and very likely will require delivery prior to pulmonary maturation.
In general, hemolysis and hydrops develop at about the same time or somewhat earlier in subsequent pregnancies; this can be used as a rough guide for timing initial fetal studies and transfusions. For Rh D alloimmunization, fetal hydrops seldom develops in a first sensitized pregnancy.
Maternal Antibody Titers
Severe erythroblastosis or perinatal death does not occur if antibody levels remain below 1:16. Some centers use an anti-D titer of 1:8 as the critical threshold of concern because of variations in reliability and methods of titration. In general, women with anti-D titers of 1:8 or less and no history of a previously affected infant can be safely followed with anti-D titers every 2 to 4 weeks and serial fetal ultrasound assessment. Those with anti-D titers of 1:16 or greater should be referred for further evaluation regarding possible fetal anemia.
Once the critical anti-D antibody titer has been reached in a sensitized pregnancy, more antibody titers are not useful in the current pregnancy or subsequent pregnancies. Titers may remain stable in up to 80% of severely affected pregnancies. Variability between maternal antibody levels and severity of fetal disease is explained by the fact that antibody concentration is only one factor influencing the degree of anemia. Other factors include antibody subclass and degree of glycosylation, placental transfer of antibody, antigen expression on fetal erythrocytes, functional maturity of the fetal reticuloendothelial system, and the presence of HLA-related antibodies that inhibit fetal erythrocyte destruction.
Determination of the Fetal Antigen Status
The possibility that the fetus is Rh D negative (not at risk) should always be considered. Assuming the male partner is the father of the fetus, a reasonable first step in this process is to determine paternal Rh D–antigen status:
If the father is Rh D negative, the fetus must also be Rh D negative and no further testing is necessary.
If the father is Rh D positive but has previously fathered Rh D–negative children, he is heterozygous and the probability that this fetus is Rh D negative is 50%.
If the father is Rh D positive without other Rh D–negative children, zygosity can be established by using either DNA analysis or Rh antisera. If the father is homozygous, this fetus is Rh D positive and no other testing is necessary.
In the past, determination of fetal blood type required direct analysis of fetal blood obtained by umbilical cord blood sampling with its attendant risks of fetal loss and fetomaternal hemorrhage. The development of DNA tests that use polymerase chain reaction (PCR) has made it possible to determine fetal Rh status from uncultured amniocytes obtained from as little as 2 mL of amniotic fluid or 5 mg of chorionic villi. Though highly accurate, DNA testing for fetal Rh status is equivocal in about 1% of cases, probably because of the presence of gene rearrangements near the Rh D locus that can be missed by standard DNA primers used for PCR.
If the father of the fetus is heterozygous for the D antigen or if his D-antigen status is unknown, fetal antigen testing from amniocytes has become routine at most centers in the United States. Alloimmunized women having CVS or second-trimester amniocentesis for other unrelated conditions can have fetal antigen typing done in association with these procedures. If the DNA test indicates an Rh D–negative fetus, the small likelihood of misdiagnosis should be discussed and the patients offered standard antenatal surveillance.
Amniotic Fluid Optical Density Analysis
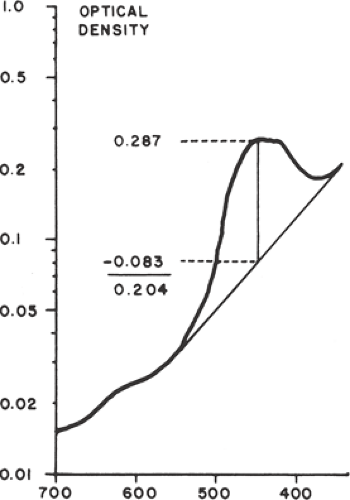
Assessment of amniotic fluid in Rh D alloimmunization is based on the original observations that spectrophotometric determinations of amniotic fluid bilirubin correlated with the severity of fetal hemolysis. Bilirubin, a by-product of fetal hemolysis, is excreted into the amniotic fluid through fetal pulmonary and tracheal secretions and by diffusion across the fetal membranes and the umbilical cord. Using a semilogarithmic plot, the curve of optical density of normal amniotic fluid is approximately linear between wavelengths of 525 and 375 nm. Bilirubin causes a shift in the spectrophotometric density, with a peak at a wavelength of 450 nm. The amount of shift in optical density from linearity at 450 nm (the ΔOD450) is used to estimate the degree of fetal red cell hemolysis (Fig. 18.5).
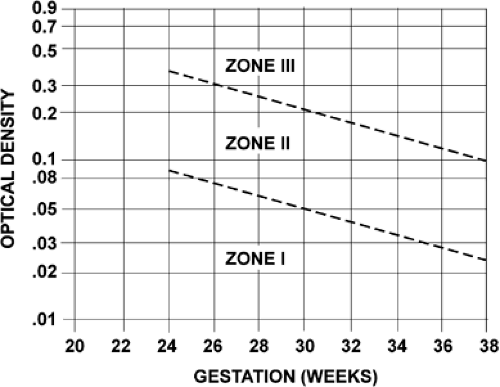
Amniotic fluid ΔOD450 values can be correlated with newborn outcome by dividing a semilogarithmic graph of gestational age versus ΔOD450 into three zones (Fig. 18.6), according to the work of Liley. Unaffected fetuses and those with mild anemia had ΔOD450 values in zone I (the lowest zone), while severely affected fetuses had ΔOD450 values in zone III (the highest zone). Fetuses with zone II values (the middle zone) had disease ranging from mild to severe, indicated primarily by the trend of the determinations of amniotic fluid bilirubin. Because there is a tendency for amniotic fluid bilirubin to decrease as pregnancy advances, the boundaries of the zones slope downward as gestational age increases.
Although the introduction of the method of fetal analysis substantially reduced perinatal mortality, the spectral
analysis of amniotic fluid is now primarily of historical interest. The current trend is management of alloimmunized pregnancy by primarily using fetal middle cerebral artery (MCA) Doppler imaging to assess for fetal anemia. However, amniotic fluid ΔOD450 measurement is sometimes performed in order to confirm or refute concern about fetal anemia in confusing clinical situations, such as an elevated MCA Doppler measurement after 34 to 35 weeks gestation.
analysis of amniotic fluid is now primarily of historical interest. The current trend is management of alloimmunized pregnancy by primarily using fetal middle cerebral artery (MCA) Doppler imaging to assess for fetal anemia. However, amniotic fluid ΔOD450 measurement is sometimes performed in order to confirm or refute concern about fetal anemia in confusing clinical situations, such as an elevated MCA Doppler measurement after 34 to 35 weeks gestation.
Ultrasound and Doppler Studies
Ultrasonographic examination of the fetus has become an extremely important adjunct in the management of the Rh D alloimmunized pregnancy, primarily as a guide for amniocentesis, cordocentesis, and intrauterine transfusion (IUT). Sonographic fetal findings also have been studied in an effort to identify those with severe anemia and reduce the need for invasive procedures. However, other than identifying frank hydrops, ultrasound is not sufficiently reliable in distinguishing mild from severe hemolytic disease.
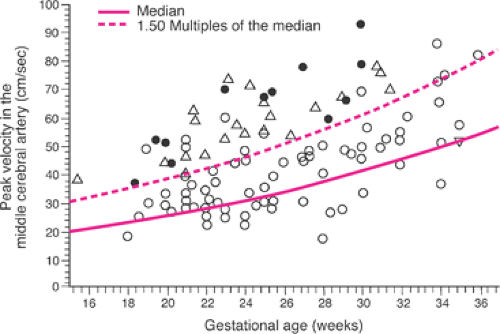
Doppler flow velocity waveforms of fetal cardiac output and red cell flow in numerous fetal blood vessels have been extensively investigated as noninvasive predictors of fetal anemia. Doppler flow waveforms of the fetal MCA have been established as the most useful in the management of Rh D alloimmunized pregnancies. Several investigators have reported that fetal MCA peak systolic velocity waveforms consistently identify fetuses with moderate or severe anemia when velocities are greater than 1.5 times the median of values derived from normal controls (Fig. 18.7), with a sensitivity of 100% and a false-positive rate of 12%. Though an element of controversy persists, fetal MCA peak systolic velocity also can be used to assess fetuses that have undergone IUT. The major limitation to this methodology is a higher false-positive rate after 34 to 35 weeks gestation—that is, a fetus with mild to moderate anemia is identified as more severely anemic by MCA peak systolic velocity assessment. As long as this caveat is taken into account, fetal MCA peak systolic velocity measurements may be used to monitor pregnancies complicated by Rh (and other erythrocyte) alloimmunzation.
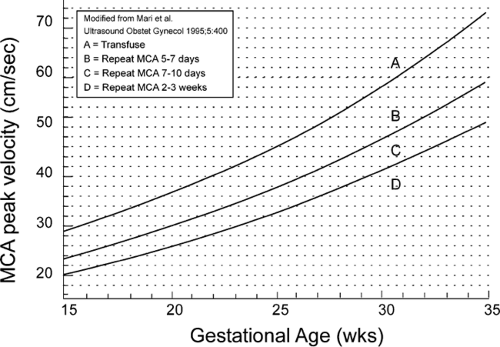
The graphic tool used in fetal MCA peak systolic velocity measurements at the University of Utah is shown in Figure 18.8




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree