Background
Provoked vestibulodynia manifests as allodynia of the vulvar vestibular mucosa. The exact mechanisms that result in altered pain sensation are unknown. Recently, we demonstrated the presence of secondary lymphoid tissue, which is the vestibule-associated lymphoid tissue in the vestibular mucosa, and showed that this tissue becomes activated in provoked vestibulodynia.
Objective
The purpose of this study was to examine whether expression of intraepithelial nerve fibers and nerve growth factor are related to immune activation in provoked vestibulodynia.
Study Design
Vestibular mucosal specimens were obtained from 27 patients with severe provoked vestibulodynia that was treated by vestibulectomy and from 15 control subjects. We used antibodies against the protein gene product 9.5, the neuron specific neurofilament, and nerve growth factor for immunohistochemistry to detect intraepithelial nerve fibers and nerve growth factor expressing immune cells in the vestibular mucosa. For intraepithelial nerve fibers, we determined their linear density (fiber counts per millimeter of the outer epithelial surface, protein gene product 9.5) or presence (neuron specific neurofilament). Nerve growth factor was analyzed by counting the staining-positive immune cells. Antibodies against CD20 (B lymphocytes) and CD3 (T lymphocytes) were used to identify and locate mucosal areas with increased density of lymphocytes and the presence of germinal centers (ie, signs of immune activation). B-cell activation index was used to describe the overall intensity of B-cell infiltration.
Results
We found more protein gene product 9.5–positive intraepithelial fibers in vestibulodynia than in the control samples (6.3/mm [range, 0.0–15.8] vs 2.0/mm [range, 0.0–12.0]; P =.006). Neuron specific neurofilament –positive intraepithelial fibers were found in 17 of 27 vestibulodynia cases (63.0%) and in none of the control cases. Protein gene product 9.5–positive intraepithelial fibers were more common in samples with more pronounced immune activation. The density of these fibers was higher in samples with than without germinal centers (6.1/mm [range, 4.3–15.8] vs 3.0/mm [range, 0.0–13.4]; P =.020). A positive correlation between the fiber density and B-cell activation index score of the sample was found (Spearman’s Rho, 0.400; P =.004; R 2 =0.128). No significant difference, however, was found in the density or presence of nerve fibers between samples with high and low T-cell densities. We identified areas of minor and major vestibular glands in 16 of the patient samples and in 1 control sample. Protein gene product 9.5–positive nerve fibers were found more often in glandular epithelium surrounded by B-cell infiltration than in glands without B cells ( P =.013). Also, the presence of neuron specific neurofilament–positive fibers in glandular epithelium was associated with B-cell infiltrates ( P =.053). Nerve growth factor–positive immune cells were more common in mucosal areas with than without B-cell infiltration and intraepithelial nerve fibers.
Conclusion
Excessive epithelial nerve growth in provoked vestibulodynia is associated with increased B-cell infiltration and the presence of germinal centers. This supports the fundamental role of immune activation in provoked vestibulodynia.
Provoked vestibulodynia (PVD), which also is referred to as localized provoked vulvodynia, manifests as allodynia (severe pain by touch) of the vulvar vestibular mucosa in the absence of any other disease or identifiable cause.
Histopathologic investigation of PVD typically reveals increased lymphocytic infiltrates in the vestibular mucosa. Recently, we demonstrated the presence of secondary lymphoid tissue, which is the vestibule-associated lymphoid tissue (VALT) in the vestibular mucosa, and showed that VALT becomes activated in PVD. We showed higher numbers of B cells in PVD than in control samples but found no difference in the density of T cells between the groups. An exaggerated immunoinflammatory response and dysregulation of inflammation seem to be present in PVD. The close relation between immune and neuronal systems can activate neuroinflammatory processes and lead to sensitization of nerve fibers. Immune cells produce nerve growth factor (NGF), which may induce nerve sprouting and enhanced signaling of the nociceptive nerve endings. Thus, it is important to study the interrelation between immune activation and nerves in PVD. Previous studies have shown increased density of nerves in the vestibular mucosa in PVD and increased expression of transient receptor potential V1 (TRPV1) channels in these nerves, but no specific data on the density of intraepithelial nerve fibers (IENF) or expression of NGF exist.
We wanted to find out whether the density and localization of IENFs and the expression of NGF are related to immune activation in the vestibular mucosal tissue in PVD. In addition to the standard neural marker, the protein gene product 9.5 (PGP9.5), we used a specific marker for neurofilaments. To define the sites of immune activation, we used 2 standard markers, CD20 (B cells) and CD3 (T cells). We explored the differences in the expression of IENFs and NGF between PVD and control samples and in relation to different B-cell and T-cell densities.
Material and Methods
Study subjects
The study material consisted of 27 archival vestibulectomy specimens from posterior vestibulectomy operations. The patients were identified in the Helsinki University Hospital patient registry by matching the diagnosis (vulvar vestibulitis, vestibulodynia, and vulvodynia) and the surgical procedure (posterior vestibulectomy). Details of patient recruitment and data collection have been described previously. A good quality paraffin block of the tissue specimen was required. All the included patients had a long disease history (4.0 years; range, 2–18 years) of PVD. The diagnoses for 8 patients were classified as primary (symptoms already at the first vaginal entry); the diagnoses for 15 patients were classified as secondary (symptoms appearing later after an interval of painless intercourses), and the classification for 4 patients was unknown. All patients had been refractory to conservative treatments. The time from the last attempted medical management was >6 months. As control subjects, we recruited 15 healthy volunteers with no vulvar complaints who underwent benign gynecologic surgery. All participants were premenopausal. The median age of the patients with PVD was 27 years (range, 18–48 years); the median age of the control subjects was 30 years (range, 24–44 years; P =.017). A 4-mm punch biopsy specimen from the posterior vestibule at 5 o’clock position was obtained from the control subjects. Both patients and control subjects had provided informed consent. The local Ethical Committee approved the study.
Tissues
All vestibular tissues were embedded routinely in paraffin after a maximum of 24 hours fixation in 10% buffered formalin. Five-micrometer sections were first stained with hematoxylin-eosin to exclude dermatologic diseases and to confirm the quality of the samples. Immunohistochemistry for nerve fibers (10-μm sections) and B and T lymphocytes (5-μm sections) was performed at the Helsinki and Uusimaa Hospital District Laboratory Services tissue laboratory. Routine staining procedures according to the manufactures’ instructions were followed ( Table ). Immunostaining for NGF (5-μm sections) was performed at the Department of Clinical Chemistry, University of Helsinki (manual staining procedure ; Table ).
| Antibody | Clone; catalog number; manufacturer | Pretreatment buffer(pH); catalog number; manufacturer | Dilution; incubation time/°C | Detection system; catalog number; manufacturer | Staining instrument |
|---|---|---|---|---|---|
| Protein gene product 9.5 (PGP9.5) | Polyclonal | No pretreatment | 1:1000 | EnVision Detection SystemsPeroxidase/DAB | LabVision |
| RA95101 | 30 Min/room temperature | K5007 | |||
| Ultra Clone Ltd, Wellow, Isle of Wight, England | Agilent Technologies Inc, Santa Clara, CA | ||||
| Neuron specific neurofilament (NF2F11) | 2F11 | Tris-EDTA (pH 9,0) | 1:200 | EnVision Detection SystemsPeroxidase/DAB | LabVision |
| M0762 | S2367 | 30 Min/room temperature | K5007 | ||
| Agilent Technologies Inc | Agilent Technologies Inc | Agilent Technologies Inc | |||
| CD20 | L26 | CC1 (pH 8,0) | Ready to use | UltraView Universal DAB Detection Kit | Ventana XT |
| 760-2531 | 24 Min/room temperature | 760-500 | |||
| Roche Diagnostics Ltd, Rotkreuz, Switzerland | Roche Diagnostics Ltd | Roche Diagnostics Ltd | |||
| CD3 | 2GV6 | CC1 (pH 8,0) | Ready to use | UltraView Universal DAB Detection Kit | Ventana XT |
| 790-4341 | 950-124 | 32 Min/room temperature | 760-500 | ||
| Roche Diagnostics Ltd | Roche Diagnostics Ltd | Roche Diagnostics Ltd | |||
| Nerve growth factor (NGF) | Polyclonal | EnVision FLEX Target Retrieval Solution (pH 6,1) | 0.5 μg/mL | MACH 4 Universal AP Polymer Kit, BioCare Medical, Concord, CA | Manual |
| sc-548 | K8005 | Overnight/4°C | M4U536 | ||
| Santa Cruz Biotechnology Inc, Santa Cruz, CA | Agilent Technologies Inc | Biocare Medical Inc, Concord, CA |
Tissue analyses
Immunohistochemical scoring was performed under light microscopy (Nikon Eclipse E800; Nikon Instruments Inc, Melville, NY) at ×200 magnification. The scoring of each section was based on a consensus of 2 investigators (P.T., A.P., or S.M.) who were blinded to clinical data of the patients. The number of PGP9.5-positive IENFs was counted to calculate the linear density of IENFs (number of nerve fibers /millimeters of epithelial outer surface). For identification of individual fibers, we used the criteria that had been validated for the diagnostics of small fiber neuropathies. Briefly, the fibers were considered to be separate if there were clearly 2 individual parallel fibers and if the distance between 2 different perpendicular sections of a stained axon exceeded 5 times the diameter of an axon. Only fibers clearly penetrating into the epithelium through the basal membrane were counted as IENFs. For neuron specific neurofilament (NF2F11)–positive IENFs only the presence or absence was documented. The overall density of neural fasciculi in the neural plexus region in the subepithelial stroma up to the depth of 1.25 mm (diameter of the ×20 high-power field) was scored semiquantitatively for both neural markers. A single number score from 1–3 (1=low density, 2=moderate density, 3=high density) was given.
Evaluation of the vestibular glands was also limited to the depth of 1.25 mm. The glands were identified on the basis of typical morphologic condition. All comparisons were made between PVD samples and control samples. In PVD samples, densities of epithelial nerve fibers were also compared between areas with or without increased B-cell infiltration. The representative areas of B-cell infiltration in each sample were located with the use of CD20 staining. To reflect the overall level of B-cell infiltration of each sample, we used the B-cell activation index (BAI). BAI is the calculated sum (0–12) of 3 different parameters that were analyzed from each sample: (1) overall density of B cells in the epithelium (score, 0–4), (2) overall density of B cells in the stroma (score, 0–4), and (3) absence (score, 0) or presence (score, 4) of germinal centers. T-cell density was divided in the CD3-stained samples into 2 categories: “low density” (<50 cells/×20 high-power field) and “high density” (>50 cells/×20 high-power field). Germinal centers were visualized by CD20 and CD3 stainings.
For NGF quantification, 4 types of areas from the PVD samples were identified: (1) areas with increased B-cell infiltration without IENFs, (2) areas without increased B-cell infiltration with IENFs present, (3) areas with both increased B-cell infiltration and IENFs, and (4) areas lacking both B-cell infiltration and IENFs. The number of NGF-positive immune cells per visual field (×20 high-power field) in 3 of each type of areas in each sample was counted, and the mean number of positive cells was calculated. In the control samples, NGF-positive cells were evaluated only in the areas with IENFs present because no areas with increased B-cell infiltration were found.
The data were analyzed by Statistical Package for Social Sciences software (version 22; IBM Corporation, Armonk, NY). We report medians with minimum and maximum and interquartile range (IQR, 25–75%) when appropriate for continuous data. For comparisons, we used the Mann-Whitney U -test and Wilcoxon signed ranks test for continuous data and χ 2 analysis or Fisher’s exact test for categoric data. For correlations, the Spearman’s correlation test was used. A 2-tailed probability value of <.05 was considered significant.
Results
Intraepithelial nerve fibers
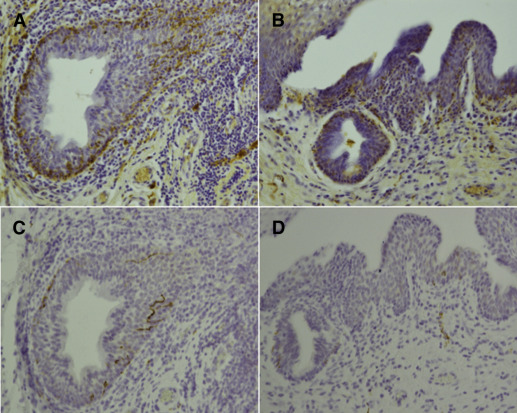
Because PVD may be related to increased pain sensitivity, we looked at individual nerve fibers in the vestibular epithelium. Both in PVD samples and control samples, PGP9.5-positive IENFs were expressed typically in clusters. Thus, such areas were studied for PGP9.5-positive IENFs. PGP9.5-positive IENFs were found in 24 of the 27 PVD samples (88.9%) and in 8 of the 15 control samples (56.2%). The density of PGP9.5-positive IENFs was significantly higher in PVD (6.3/mm [range, 0.0–15.8]; IQR, 4.4–9.2) than in control samples (2.0/mm [range, 0.0–12.0]; IQR, 0.0–4.3; P =.006; Figure 1 , A and B). No significant difference was found in the density of IENFs between 8 primary PVD cases (7.5/mm [range, 3.3–15.8]; IQR, 5.3–8.3) and 15 secondary PVD cases (5.0/mm [range, 0.0–12.4]; IQR, 2.5–9.2; P =.332). NF2F11-positive IENFs typically occurred as solitary fibers or in clusters of 3–8 fibers ( Figure 1 , C) and were found in 17 of the 27 PVD cases (63%) and in none of the control cases ( P <.001). NF2F11-positive IENFs were as common in primary and secondary PVD ( P =.627).
IENFs in relation to B-cell infiltrates
Our previous study showed increased density of B lymphocytes in the vestibular mucosa of patients with PVD. The density of PGP9.5-positive IENFs was greater in the areas with increased B-cell infiltration (5.3/mm; range, 0.0–23.3; IQR, 3.0–9.4) than in the areas with no B cells (4.0/mm [range, 0.0–11.3]; IQR, 1.0–8.0; P =.057; Figure 1 , D). In control samples, no areas with increased B-cell infiltration were found.
IENFs in relation to signs of immune activation
Germinal centers indicate immune activation and are the key component of the local lymphoid tissue, which we have termed as VALT. Infiltrating lymphocytes were primarily B cells that were stained by the CD20 marker. T cells were distributed more evenly across the samples and did not form clusters. The density of PGP9.5-positive IENFs was significantly higher in samples with germinal centers (6.1/mm [range, 4.3–15.8]; IQR, 5.0–9.4) than in samples without germinal centers (3.0/mm [range, 0.0–13.4]; IQR, 0.0–8.4; P =.020). A positive correlation between the density of PGP9.5-positive IENFs and the BAI score was found (Spearman’s Rho, 0.400; P =.004; R 2 =0.128). NF2F11-positive IENFs were not associated with the presence or absence of germinal centers. However, the BAI scores of the samples with NF2F11-positive IENFs were higher (5.0 [range, 1.0–9.0]; IQR, 4.0–6.0) than the BAI scores of the samples without fibers (2.0 [range, 1.0–9.0]; IQR, 1.0–3.0; P =.005). No differences were found in the densities of PGP9.5-positive fibers between the low-density and high-density T-cell groups (6.8/mm [range, 0.0–15.8] and 4.6/mm [range, 0.0–12.4], respectively; P =.112) or in the presence of NF2F11-positive fibers ( P =.080).
Glandular epithelium and nerve fibers in relation to B lymphocytes
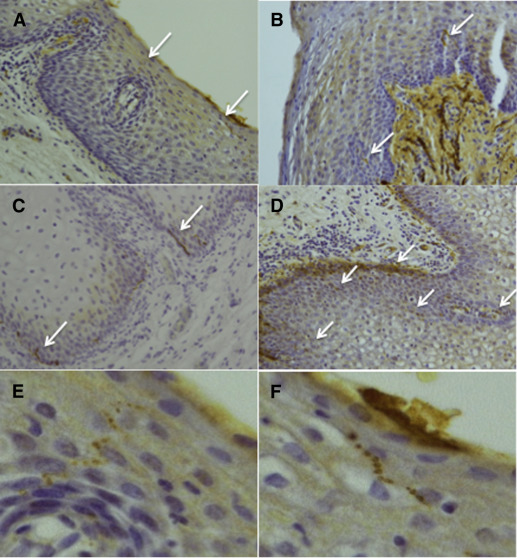
Vestibular glands typically occur in the subepithelial layer of the vulvar vestibular mucosa. In the PGP9.5-stained PVD samples, 14 regions with glandular epithelium were identified. Of these, 9 were in areas with increased lymphocytic infiltration that showed a glandular epithelial nerve fiber density of 25.0/mm (range, 3.1–48.0; IQR, 11.3–32.0; Figure 2 , A). Five regions were in areas without increased numbers of lymphocytes. Here the glands showed significantly lower epithelial nerve fiber density of 2.0/mm (range, 0.0–17.0; IQR, 0.0–16.5; P =.013). In the control samples, only 1 area of glandular epithelium was identified with an epithelial nerve fiber density of 2.0/mm, which was located in an area with no lymphocytic infiltration. In the NF2F11-stained PVD samples, 16 glandular regions were identified, 12 in areas with lymphocytes and 4 in areas without. Again, epithelial nerve fibers were present more commonly in glands that were surrounded by lymphocytic infiltrates (11/12) than in glands not surrounded by lymphocytic infiltrates (2/4; P =.053, Fisher’s exact test).