Fig. 9.1
The Desormeaux’s endoscope (Courtesy of the Wellcome Library, London. Published under the Creative Commons Attribution)
Pantealoni performed the first investigation of uterine cavity in a 60-year-old woman with abnormal uterine bleeding by means of Desormeaux’s device in 1869. He could see an endometrial polyp and cauterized it with silver nitrate under endoscopic view. He was the first who used both diagnostic and treatment abilities of this approach, which can be considered as the beginning of the principle “see and treat” by direct visual control of uterine cavity.
It was obvious that visualization of the uterine cavity is an important tool for the diagnosis of endometrial pathology with enabling a new treatment choice. Therefore many researchers were interested in improvement of uterine cavity visualization. Achievements of the state of the art, including principles of optics, fiberoptics, Hopkins telescope, television, electricity, light, light sources, video and other technologies (Fig. 9.2) were step by step implemented to design modern hysteroscopic equipment (Fig. 9.3 and Table 9.1).
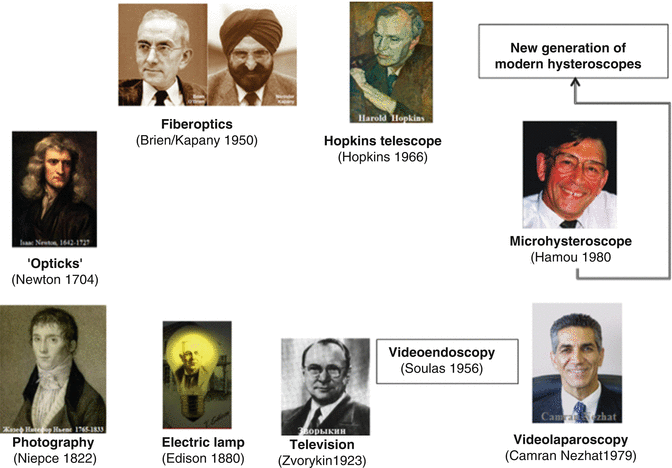


Fig. 9.2
Principles of basic sciences and technologies applied for a design of hysteroscopic tools

Fig. 9.3
Optical principles of modern hysteroscopic technique
Table 9.1
Principles of hysteroscopic equipment design
Purposes | Developed tools |
|---|---|
Delivery | Tubus or sheath |
Visualization | Optics/telescope, camera and screen |
Illumination | Light source and optical fiber (light guide) |
Distension | Distension medium (CO2 or liquid) and additional in/out flow channels |
Surgical | Manipulation channel, mechanical and electrical instruments, lasers |
Hamou’s hysteroscope was designed with an improved 4-mm rod lens system scope visual optics inserted into a diagnostic sheath to guide the distension media into the uterine cavity. Microcolpohysteroscope designed by Hamou revolutionized the hysteroscopy technique (Fig. 9.4), therefore according to his deserts in this field Hamou is considered as the father of modern hysteroscopy (Fig. 9.5).

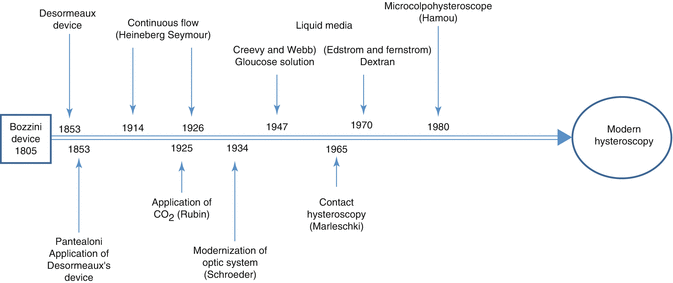

Fig. 9.4
Hamou’s microcolpohysteroscope

Fig. 9.5
History of the development of hysteroscopy
In turn, new advances in development of distension medium are based on principles of fluids diffusion and electrolytic conduction (Table 9.2). So, uterine cavity distention and illumination was also improved by irrigation with continuous flow (Heineberg 1914; Seymour 1926) and application of CO2 (Rubin 1925) and liquid media (Creevy and Webb 1947; Edstrom and Fernstrom 1970).
Table 9.2
Development steps of hysteroscopic equipment and distention media
Optics/telescope | Distension channel and media | Modern distention media |
|---|---|---|
Desormeaux’s endoscope (Desormeaux 1853) | Aspiration and irrigation channel (Heineberg 1914; Seymour 1926); | Isotonic ionic solutions; |
Photo endoscopy/cystoscopy (Nitze 1877) | CO2 – for distention (Rubin 1925); | Ringer’s Lactate; |
Modernization of optic system (Schroeder 1934) | Glucose solution – as a distention media (Creevy and Webb, 1947); | 5 % dextrose in water; |
Contact hysteroscopy (Marleschki 1965) | Dextran (Edstrom and Fernstrom 1970) | 1.5 % glycine, sorbitol, cytal; |
Microcolpohysteroscope (Hamou 1980) | Hyskon (32 % dextran 70) |
All developments of technologies have allowed to design modern hysteroscopic tool according to Valle and Sciarra [1], which consists of ‘a telescope 2–4 mm in diameter, with Foroblique vision; a metallic sheath for the telescope and accessory channels to deliver the distending medium and introduce operating instruments; a connecting bridge with special channels to introduce manipulating instruments; a cold light fiberoptic bundle to transmit the light; an external light source for illumination; and when electrocoagulation is to be used, an appropriate electrosurgical source´.
Most modern hysteroscopic procedures have developed in 1980s when new generation of miniaturized hysteroscopes was designed owing to Hopkins telescopic principles. Subsequently office hysteroscopies were mostly performed as the first diagnostic step to evaluate intrauterine pathologies and only easy procedures such as biopsy, polypectomy and adhesiolysis, were done by this approach at outpatient clinics. Further hysteroscopic surgical procedures have performed as the second step mostly by monopolar coagulation and mechanical instruments under anesthesia and dilatation of cervix at inpatient clinics in the operation theatre till 1997. Mechanical instruments were used mostly to remove polyps, adhesiolysis and for biopsy, whereas resectoscope was the main surgical tool for myomectomy and resection of uterine septum.
Introduction of bipolar system ‘Gynecare Versa Point’ by Ethicon Inc. in 1997 was the turning point of modern office hysteroscopy. This technology enabled to increase spectrum of intrauterine surgical procedures by means of thin miniaturized instruments without both anesthesia and dilatation of cervix at outpatient clinics.
Submucous Myoma
Submucous myoma (SMM) is a potentially dangerous pathology for women’s health with undesirable consequences. Therefore a hysteroscopic management of SMM represented a challenge in our practice since in the past hysterectomy was the only reliable surgical option of this pathology.
The first hysteroscopic myomectomy was performed by Robert Neuwirth, from Mount Sinai Hospital in NY in 1976, using an urologic resectoscope and he developed resectoscopic surgery of SMMs.
Since then many hysteroscopic procedures have replaced old, invasive techniques, such as dilatation, curetaje and even hysterectomy. Nowadays by means of advanced bipolar instruments and other alternatives such as laser and morsellation with miniaturized instruments we have modern office hysteroscopy, which has begun to completely replacing more invasive operating-room procedures.
In the last decades we have witness many developments in the surgical management of this SMM, with an increase in our efficacy to solve the problem by a minimally invasive way. Today we have better and thinner optical hysteroscopes, with more sophisticated system of lenses and prisms to give the operator a well-illuminated image, with excellent contrast and resolution. Although resectoscopy in theater is still the prevalent technique for myomectomy, in the last 20 years huge advances were made in the use of diagnostic hysteroscopes for treatment purposes with an outpatient on a “see and treat” principle.
The advantages of outpatient operative hysteroscopy include the possibility to diagnose and treat intrauterine lesions in a single session and the convenience and efficiency for both the physician and patient. An additional benefit of office-based operative hysteroscopy has been suggested by Lindheim et al. [2], who more than a decade ago, noted the cost saving per case of at least 50 % when compared with the hospital equivalent. The approach with vaginoscopy or “no touch” technique, without using speculum, tenaculum or even anesthesia is another well tolerated advantage of office hysteroscopy and patients reported just little discomfort [3].
Indications for Myomectomy
Any woman with symptomatic SMM is a candidate for hysteroscopic myomectomy. SMMs are represented only 5–10 % of all the myomas but in many cases are associated to symptoms such as heavy menstrual bleeding (HMB), infertility and recurrent pregnancy lost. [4, 5] Less frequent reported indications include dysmenorrhea [6], aspecific pelvic pain [7]. It is well known that HMB is related with SMM is not capable of being cured by conventional treatment ways [8]. A classic theory, first suggested by Sampson in 1912 (Surg Gynecol Obstet, 14:215–230), states that local dysregulation of the vascular structures in the uterus is responsible for this abnormal bleeding. Today we know that in addition to endometrial and uterine cavity local structure alterations due to growing SMM, several signaling pathways initiated by growth and angiogenic factors are involved in this process.
Infertile patients with fibroids that impinge upon the endometrial cavity have poorer reproductive outcomes than a population of infertile patients without fibroids and that removal of fibroids with a pathologic intracavitary component seems to be of benefit [9]. Therefore radical treatment choice by hysterectomy has considered as the main standard of surgical treatment for symptomatic submucous fibroids.
Advances of hysteroscopic technique and introduction of presurgical treatment to decrease size of fibroids have dramatically changed management strategy of submucous myomas.
Classifications of Submucous Myomas
A systematic classification of submucous myomas is necessary in order to standardize hysteroscopic diagnostic and treatment management as well as for a comparative analysis of results in research reports. Such parameters of myomas as their penetration into myometrium (sessile), localization in the uterine cavity and size are critical determinants of their clinical manifestation and for the treatment strategy. Expert teams of many international societies including European Society of Hysteroscopy (ESH), European Society for Gynaecological Endoscopy (ESGE) and American Association of Gynecologic Laparoscopists (AAGL) the International Federation of Gynecology and Obstetrics (FIGO) have developed classification systems to implement them with their guidelines concerning management of patients with myomas.
The most known and widely cited classification system of submucous myomas was proposed by Wamsteker et al. (1993) according to degree of intramural extension of sessile fibroids. Further this system named the Wamsteker classification has been adopted by many societies such as ESH in 1993, ESGE in 2003 and AAGL in 2012, FIGO in 2011 to a certain extent variations. This system is included three types of myomas. Pedunculated myomas entirely within endometrial cavity without myometrial extension are classified as type 0 fibroids. Sessile fibroids with intramural extension less than 50 % and with angle of myoma surface to uterine wall less than 90-degree are classified as type II fibroids, whereas intramural extension more than 50 % and angle of myoma surface to uterine wall more than 90-degree – as type II fibroids [10, 11].
FIGO working group on menstrual disorders introduced “Classification system including leiomyoma sub classification system” with modified the Wamsteker classification [12]. However this sophisticated classification system is difficult to apply for clinicians since the main purpose of this working group was the causes of abnormal uterine bleeding in nongravid women of reproductive age.
Further Lasmar et al. [13] introduced a classification system by incorporating four main parameters such as:
1.
The largest myoma diameter
2.
Extension of fibroid base to endometrial cavity surface
3.
Penetration of fibroid into myometrium
4.
Fibroid location along the uterine wall.
These four parameters were registered subsequently by their degree as 0, 1 and 2 points with an additional 1 point if fibroid located on the lateral uterine wall (Table 9.3). Depending on total score sum authors suggested their recommendations. In a cases of total score 0–4 it can be performed a low complexity hysteroscopic myomectomy, while for patients with 5–6 total score considered complex hysteroscopic myomectomy with presurgical GnRH analog treatment and/or two-stage surgery. In a case of 7–9 total score they recommend alternative nonhysteroscopic technique. Recently this classification system was revised and recommended by AAGL practice report as practice guidelines for the diagnosis and management of submucous leiomyomas [14].
Table 9.3
Lasmar classification of submucous myomas
Table classification Lasmar et al. (2005) | ||||||
|---|---|---|---|---|---|---|
Score | Size | Topography | Extension | Penetration | Lateral wall | Total |
0 | =2 cm | Lower | = 1/3 | 0 | ||
1 | >2a <5 cm | Medium | >1/3 a 2/3 | = 50 % | +1 | |
2 | >5 cm | Upper | >2/3 | = 50 % | ||
Total score | + | + | + | + | + | = |
Table showing the group and the suggested treatment according the highest score obtained | ||||||
Score | Group | Recommendations | ||||
0–4 | I | Low complexity hysteroscopic myomectomy | ||||
5–6 | II | Complex hysteroscopic myomectomy, consider preparing GnRH analog and/or two-stage surgery | ||||
7–9 | III | Recommend alternative nonhysteroscopic technique | ||||
Pre-surgical Preparation
GnRH Analogues
It has long been known that myomas are estrogen dependent, their size increases during pregnancy and shrink during menopause. GnRH analogues (GnRHa) induce hypogonadism through pituitary desensitization, down regulation of receptors and inhibition of gonadotrophins, this leads to decrease vascularity, resulting in the case of fibroids in a shrinkage of the mass. Because of these features, GnRHa has been found useful in the management of various hormone-dependant tumours, endometriosis and uterine fibroids.
The first successful report of the effect of GnRHa on uterine myomas was by Filicori et al. (1983). Since then many studies were published on this issue. We know that surgery is less technical demanding when myomas are smaller and a complete surgery can be achieve in a single session. The resection of large myomas is associated with increased bleeding during surgery, longer operative time, risk of fluid overload and sometimes with the necessity of repeated operations [10, 15]. GnRHa are commonly used pre-operatively before myomectomy so as to reduce the size of a fibroid in order to make surgery easier and safer [16, 17]. On the other hand GnRHa are expensive and have many unpleasant side effects such as menopausal symptoms due to estrogen deprivation.
Although the experience in the use of GnRHa for pre surgical preparation of fibroids, the medical evidence to support this practice has been weak. In one side we have observational studies such as Perino et al. [18] and Donnez et al. [19] that reported the use of GnRHa prior to hysteroscopic resection of myomas as useful, while in the other side we have Campo et al. [20] the found that this practice prolonged surgery time.
Recently was published a systematic review of the literature focused on the benefit of the preoperative use of GnRHa in women with submucous myomas, trying to determinate whether this treatment was more effective than placebo/no treatment prior to hysteroscopic resection, in terms of symptomatic relief, ability to complete surgery, operating time, complications and technical difficulties [21]. This publication shows with good quality evidence that there were no differences in terms of symptomatic relief, achieving a complete resection of the fibroid, operative difficulty and surgeon´s satisfaction. Cost analysis was not done in the included studies. There seems to be some benefit in terms of reduction in both operating time and fluid deficit. Kamath et al. conclude that there is inadequate evidence to support the routine use of preoperative GnRHa before hysteroscopic resection of submucous myomas.
Ulipristal Acetate
Since February 2012, ulipristal acetate (UPA) 5 mg/day, is approved in Europe for preoperative fibroid treatment [22].
UPA is a selective Progesterone receptor modulator (SPRM) that potently modulates P-receptor activity [23] with proapoptotic/ antiproliferative effects on fibroid cells [24] and with pharmacokinetic properties supporting once daily dosing [25]. Two short-term (3 months) randomized clinical trials showed that UPA effectively controls HMB and shrinks fibroids [26, 27]. After treatment cessation, menstruation usually returns within 4–5 weeks, but fibroid volume reduction can be sustained for up to 6 months. In addition, treatment with UPA reduced fibroid-associated pain, improved QoL, and revealed no safety concerns [26, 27].
When compared with a GnRHa like leuprolide acetate, both were effective in controlling bleeding with a difference the median times to amenorrhea were 7 days for patients receiving 5 mg of ulipristal acetate and 21 days for those receiving leuprolide acetate. In terms of myoma size reduction there were not significative differences between the treatments, but in the case of the patients treated with leuprolide, the size increased again by 84 % compared with the UPA group in which the sized remained stable during 6 month after treatment was finished [27]. But the big difference is found in the tolerance, moderate-to-severe hot flashes were reported only for 11 % of patients receiving 5 mg of ulipristal acetate and for 40 % of those receiving leuprolide acetate (P < 0.001).
Recently a new study on long-term safety of the use of UPA 10 mg/day in the treatment of symptomatic myomas was published. It seems that repeated 3-month courses of oral UPA 10 mg once daily effectively control bleeding and pain, reduce fibroid volume, and restore QoL over the long term in many women with symptomatic fibroids, providing an effective and well-tolerated long-term medical treatment for fibroids [28]. It looks like UPA could be an alternative medical treatment for symptomatic myomas avoiding in some cases necessity of surgery.
Clinical trials have also shown that UPA administration can lead to a pattern of benign, nonphysiological, nonproliferative, histological features of the endometrium termed P receptor modulator associated endometrial changes (PAEC). These changes spontaneously reverse over a few weeks to months after cessation of the 3-month UPA treatment. It is important to remember this fact prior to schedule hysteroscopic surgery, sometimes the endometrial thickness is above 16 mm and it will difficult the correct visualization of the endometrial cavity and even will cause the suspension of the procedure. The solution can be either wait until we can check the endometrium’s thickness with an ultrasound or to give a gestagene thinning the endometrium prior to the hysteroscopic procedure [29].
Misoprostol for Cervical Ripening
Misoprostol is a stable synthetic prostaglandin E1 analogue used for the prophylaxis and treatment of peptic ulcers resulting from long-term use of nonsteroidal anti-inflammatory drugs. It is inexpensive, can be kept at room temperature, and is associated with few adverse effects. The systemic bioavailability of misoprostol is three times higher after vaginal insertion than after oral administration. Misoprostol has a cervical ripening effect and induces uterine contractions Its ripening effect was shown by numerous clinical studies [30, 31], and is consistent with biological data.
The last systematic review and meta-analysis published by the Cochrane library [32] the conclusion was that misoprostol administration prior to hysteroscopy appears to have a beneficial role in premenopausal patients undergoing hysteroscopy in both the diagnostic and operative setting. In cases in which cervical dilatation is considered as easy, misoprostol may not be routinely administered as it may only increase patients’ discomfort without any substantial benefit.
It seems that the use of 400 mg of vaginal misoprostol 12–24 h before hysteroscopy may reduce the pain and the force needed to dilate the cervix, with only mild side effects.
Myomectomy Techniques
There are different factors to think about before we proceed to perform a hysteroscopic myomectomy.
1.
We need to know if the lesions are lying entirely or mostly in the uterine cavity or has a major intramural component. In this case, should the myometrial free margin still be considered a limiting factor? Some authors think that should be a limit [33] and a one-step hysteroscopic myomectomy may be performed to remove deeply infiltrating submucous myomas when myometrial thickness at the implantation site is as more than 5 mm. But, is this fact a real limitation? In another study [34] was evaluated the feasibility of the hysteroscopic resection of type II submucous fibroids regardless of the myometrial free margin separating them from the serosa. In this work the authors reported the dynamic changes the margin undergoes after the various phases of resection. During the hysteroscopic myomectomy ultrasound evaluation of myometrial free margin was measured before and alter each phase of the procedure. They found that myometrial free margin increases progressively with each step of the procedure probably leading to an increasing margin of safety. The importance of this margin is in the risk of perforation during the myomectomy, but it depends on the myome type and on the surgical technique, when the myoma is a type 2–5 traversing the myometrium and reaching the uterine serosa and the procedure is one step resectoscopy, in such circumstances it be neither feasible nor safe.
2.
The cleavage plane, it is the space between the fibroid and the adjacent myometrial tissue, also called “myoma pseudocapsule”. This space contains a proper vascular network, there isn´t any true vascular pedicle. The myoma is anchored to the pseudocapsule by connectival bridges. At this level there different elements such smooth muscle cells similar to the myometrium, neuropeptides and angiogenesis factors, this ultrastructural feature suggests that when removing fibroids their pseudocapsules should be preserved as much as possible, in order to preserve the myometrium [35, 36]. Depending of on the myoma type there are myomectomy techniques that respect this important cleavage plane, such as those that enucleate the myoma.
Today the tendency is to perform surgery in an outpatient setting, and this is also the tendency in hysteroscopy. New techniques and new instruments were developed during the last decade that allows us to perform office hysteroscopic myomectomy. We are going to review all the most used surgical option for submucous myomas, but only few are performed in an office setting.
Resectoscopy
This is the most commonly employed approach. The resectoscope is the instrument that resects under direct and constant visual control. The sheath has an outer diameter of 7–9 mm, and includes both inflow and outflow ports for distending media. The resectoscope is equipped with continuous flow and provides excellent irrigation for operative procedures. If surgical debris or the so-called ‘chips’ block the operative field, the resectoscope can be removed while the sheath is left in place. This allows for removal of large tissue while maintaining cervical dilatation. It has a straight-forward or a slightly fore-oblique telescope 12/30° [37].
Because of the diameter of the endoscope, most of the times it requires cervical dilatation. It is performed with an inpatient in surgery room under anesthesia (see Fig. 9.6).
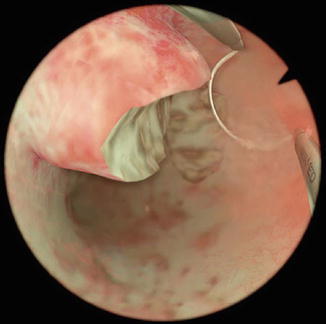

Fig. 9.6
Resectoscopy of a myoma
The distending solution depends on the electrosurgical system, if it is a monopolar system it will require a non conducting distending solution such as sorbitol 5 % or glycine 1.5 %, but if the system is bipolar the use of normal saline solution is allowed, avoiding the risk for the patient of excessive fluid absorption and the potential electrolyte imbalance [38, 39]. Fluid input and output should still also be monitored. If excessive intravasation occurs, the isotonic fluid overload is generally readily treatable with diuretics (e.g. furosemide 20 mg intravenously). Therefore, a higher amount of intravasation during surgery can be accepted. Generally, most protocols and guidelines mention about 2,500 ml as the upper limit of saline intravasation.
This endoscope uses a radiofrequency electrosurgical generator. The electrosurgical system can be monopolar or bipolar: in the monopolar one, from the extremity of the resectoscope (active electrode) the flow of current, in order to close the circuit, must reach the plate (passive electrode). The use of a bipolar set of instruments, in which both electrodes are introduced into the thermal loop, would be much safer. In this way the current will only have to pass through the tissue with which the thermal loop comes into contact, thus minimizing the danger deriving from the random passage through the corporeal structures. The passage of the electrical energy from the thermal loop to the tissues determines the cutting or coagulation action of the resectoscope. There are various types of thermal loops with different shapes and sizes. The diameters of thermal loops for bipolar resectoscopes are usually smaller than loops for a monopolar instrument with the same outer diameter, thus increasing the time required for resection (Indman 2006). The bipolar loop operates in a similar way to a monopolar electrode; however, as tissue contact is not necessary for activation, the electrodes do not ‘stick’ in the tissue while cutting (Stamatellos and Bontis 2007). Although bipolar techniques are less hazardous because of intravasation and electrolyte blood-imbalances, more gas-bubbles may hamper visualisation, and gas-embolimight even cause spasms in the lung capillaries, potentially disturbing gas diffusion in the lungs.
The removal of type-0 and most type-1 lesions is generally straightforward. It consists of repeated and progressive passages of the cutting loop beyond the myoma, with cutting only taking place during the backward or return movement of the loop. Excision usually begins from the top of the fibroid, progressing in an uniform way towards the base, it is also the technique for pedunculated fibroids. The surgery is considered finished when the fasciculate structure of the myometrium is visualized. In some cases this technique is associated to a lack of clear vision due to the accumulated fragments of fibroid in the cavity, the classical removal of tissue under visual control with the resectoscope may consume a lot of time, a resectoscope with automatic chip aspiration could be of help, or consider the use of a morcellator.
G1 and G2 fibroids should not exceed 5–6 and 4–5 cm respectively in order to succeed to remove them hysteroscopically. Although in some cases they can be removed by one-step procedure, most surgeons prefer to remove this kind of myomas through a two-steps procedure (Loffer 1990).
Myoma growth within the uterine wall produces dislocation, compression, and stretching without rupture of adjacent muscle fibers. When the intramural portion is resected by classic slicing technique, direct cut with the loop and thermal damage may cause injury to adjacent healthy myocytes.
For deep type 1 and type 2 there is a high risk of damage of the healthy myometrium during electrosurgery, both by cutting and by thermal damage. Other risks are bleeding, intravasation and the risk of perforation. In case of lesions that extend close to the serosa, within a few millimeters, sometimes there may be a role for the concomitant performance of laparoscopy, not necessarily because it reduces the chance of perforation, but because it allows for the creation of a safe buffer of gas around the uterus should perforation occur. Alternatively, intraoperative transabdominal ultrasonography can be performed when performing dissection of leiomyomas that are believed to be close to the uterine serosa [40].
In some cases it is possible to use a cold loop, those are structurally more robust than others as they are used in a mechanical way without electrical energy to carry out enucleation of the intramural component of the myoma.
Morcellator
Currently we can find in the market three different hysteroscopic morcellators, the Truclear (Smith and Nephew, Andover MA, USA), the Myosure by Hologic (Bedford MA, USA) and another company that recently introduced an alternative device is Storz (Tuttlingen, Germany).
The TRUCLEAR system approved by the FDA in 2005, is based on an instrument that consists of a set of two metal hollow rigid tubes that fit into each other [35]. The inner tube rotates within the outer tube, is driven mechanically by an electrically powered control unit, and is controlled by a foot pedal that activates the rotation and regulates the direction of rotation of the inner tube. The control unit is connected to a handheld motor drive unit in which the morcellator is inserted.
Both tubes have a window-opening at the end with cutting edges. By means of a vacuum source connected to the inner tube, the tissue is sucked into the window-opening, cut and ‘shaved’ as the inner tube is rotated [41]. The system uses no electrocoagulation, and there is no lateral thermal or electrical energy spread. Haemostasis occurs by spontaneous myometrial contraction. The removed tissue is discharged through the device, is collected in a tissue-trap, and is available for pathology analysis. As only one introduction is needed, the number of perforations is extremely low. The 4.0-mm morcellator is introduced in the uterine cavity through a straight-forward working-channel of a continuous flow 8–9 mm rigid hysteroscope. After dilatation of the internal orifice of the uterine cervix, atraumatic insertion is accomplished with the use of an obturator in the outer sheath of the hysteroscope.
Saline solution is used for distension and irrigation.
van Dongen et al. [42], conducted a randomised-controlled trial (RCT) to compare conventional resectoscopy and hysteroscopic morcellation among residents in training. The mean operating time for resectosocpy and morcellation was 17.0 (95 % confidence interval [95 % CI] 14.1–17.9, standard deviation (SD) 8.4) and 10.6 (95 % CI 7.3–14.0, SD 9.5) min, respectively (P < 0.008). Subjective surgeon and trainer scores for convenience of technique on a visual analogue scale were in favour of the morcellator.
A new development in hysteroscopic morcellation is the recent availability of a smaller outer diameter TRUCLEAR system, with a 2.9-mm cutting-blade and a 5.0-mm hysteroscope for office or ambulatory use with no or local anaesthesia. Polyps, small myomas, and retained products of pregnancy can be removed in that way.
MyoSure® Tissue Removal System (Hologic, Bedford, MA), was approved by the FDA in 2009; this morcellator consists of an electrical control box, a foot pedal, and a morcellator hand piece that features a rotating/reciprocating 2 mm OD cutter blade encased in a 3 mm OD outer tube. The cutter is connected to a vacuum source that aspirates resected tissue through a side-facing cutting window in the morcellator’s outer tube. Resected tissue is captured in a standard vacuum canister tissue trap and is available for pathological examination [43].
Regardless of the methodology used to resect intrauterine pathology, it is important to remember that resected tissue must be thought of in terms of three-dimensional rather than two-dimensional measurements. Thus, increasing pathology diameter yields a exponential rather than linear increase in volume following the equation ν = πd 3/6. This mathematical consideration becomes important as one plans a surgical approach for submucous myomas in which the resection rate and procedure time will be a function of the volume, density, and type of myoma tissue. With loop resectoscopy, the amount of tissue removed per minute will depend on:
1.
How quickly the surgeon deploys each pass of the loop
2.
How much tissue each bite with the loop resects
3.
How quickly the tissue chips can be removed from the uterine cavity.
On the other hand, with hysteroscopic morcellation, the amount of tissue removed per minute will only be a function of [1] how much contact the cutting window maintains with the myoma and [2] how quickly the device can cut tissue and aspirate it out. Because the devices’ cutting speeds are relatively fixed by their design characteristics, minimizing procedure time mostly depends on maintaining tissue contact between the cutting window and the pathology. Learning the correct resection technique, although not difficult, is of prime importance with hysteroscopic morcellation.
Although in vivo accurate measurements of tissue resection speed are challenging to conclusively determine due to surgeon and pathology variations, in vitro measurements have been performed to assess the tissue resection characteristics of the different devices. As part of an IRB-approved FDA submission study in 2008, the author (JAG) compared a working MyoSure device prototype with a TRUCLEAR device to assess tissue resection speed. Fresh, discarded uterine leiomyoma tissue was placed in a saline-filled container and each device was placed directly on the tissue in alternating 5-min intervals for 30 min. The trial was repeated on three different myoma specimens. The study was designed to compare tissue cutting on identical tissue and to assess decline of cutting speed over time as a result of blade dulling [44]. As these data demonstrate, both devices are capable of resecting submucous myomas 3 cm in diameter (~15 cc3) in 15 min or less, although the MyoSure device was consistently faster at tissue removal at every time interval despite its smaller diameter.
In addition, the smaller diameter of the MyoSure hysteroscope (6.25 mm) compared with the TRUCLEAR hysteroscope (9.0 mm) makes the MyoSure device potentially more compatible with an oral sedation/cervical block anesthesia protocol and therefore amenable to office-based treatments of polyps and Type 0 or I submucosal fibroids [45].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


