Hypothyroidism in the Infant
Delbert A. Fisher
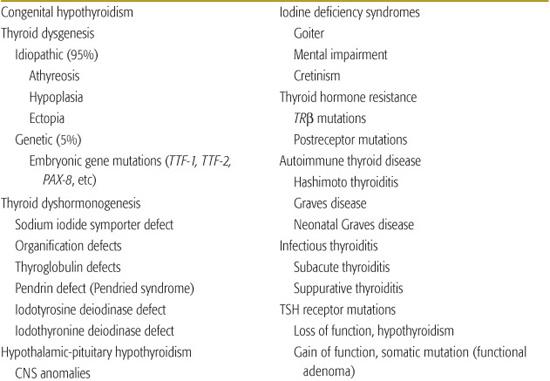
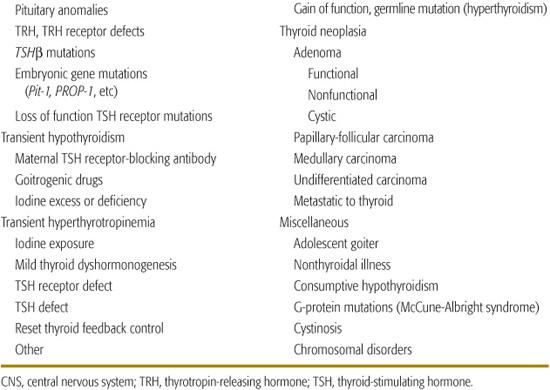
Causes of hypothyroidism are listed in Table 527-1. Transient neonatal hypothyroidism occurs in premature infants, and may be caused by drugs or maternal antibodies. The most common cause of nonendemic congenital hypothyroidism is defective thyroid embryogenesis. Inborn defects in thyroid hormone synthesis or action are the second most common cause of congenital hypothyroidism.1-13 Other causes include intrauterine exposure to goitrogenic agents and hypothalamic-pituitary disorders. These usually occur in the setting of panhypopituitarism, but isolated defects in thyrotropin-releasing hormone (TRH) or thyroid-stimulating hormone (TSH) do occur.5-7
Table 527-1. Thyroid Disorders in Infancy and Childhood
THYROID DYSFUNCTION IN THE PREMATURE INFANT
Premature infants are defined by gestational age (GA) and weight. By weight, premature infants are classified as low birth weight (LBW, 2500–1500 g), very low birth weight (VLBW, 1500–1000 g), and extremely low birth weight (ELBW, < 1000 g); they range from 34 to 35 weeks’ to 23 to 24 weeks’ GA (the current threshold of viability).3 Most premature infants have some degree of hypothyroxinemia (T4 < 6.5 μg/dL, 84 nmol/L). The prevalence of T4values < 6.5 μg/dL approximates 50% in VLBW infants. These infants have a relatively immature hypothalamic-pituitary-thyroid axis, immature metabolic systems, and high prevalence of neonatal morbidities, including respiratory distress, hypoxia, undernutrition, gastrointestinal and cardiac dysfunction, sepsis, and cerebral pathology. As result, they are predisposed to development of transient primary hypothyroidism and the syndrome of transient hypothyroxinemia of prematurity (THOP), which probably represents transient hypothalamic-pituitary hypothyroidism and/or nonthyroidal illness (NTI; the low T3 syndrome).
 TRANSIENT PRIMARY HYPOTHYROIDISM
TRANSIENT PRIMARY HYPOTHYROIDISM
Transient primary hypothyroidism is characterized by low serum T4 and high thyroid-stimulating hormone (TSH) concentrations.3 The prevalence of transient hypothyroidism among premature neonates depends on the extent of iodine deficiency in the environment. Term infants can have transient neonatal hypothyroidism in areas of low iodine intake (endemic goiter). Transient primary hypothyroidism develops during the first 1 to 2 weeks of extrauterine life and is superimposed on the usual state of transient hypothyroxinemia characteristic of prematurity. Urinary iodine excretion and thyroid iodine content are reduced, suggesting that the acquired primary hypothyroidism is the result of relative iodine deficiency. Iodine supplementation of neonates in areas of iodine deficiency prevents transient hypothyroidism.
Administration of iodine-containing drugs to the mother or amniotic injection of radiographic contrast agents for amniofetography can induce hypothyroidism. Premature infants are also particularly susceptible to transient, iodine-induced hypothyroidism.  The hypothyroidism is transient but may persist for several weeks; thus, thyroxine (6–8 μg/kg/d) treatment is recommended.
The hypothyroidism is transient but may persist for several weeks; thus, thyroxine (6–8 μg/kg/d) treatment is recommended.
TRANSIENT HYPOTHYROXINEMIA OF PREMATURITY
Relative to term infants at birth, serum thyroid-binding globulin (TBG) and total T4 concentrations in premature infants are lower, the neonatal thyroid-stimulating hormone (TSH) surge is obtunded, tissue iodothyronine monodeiodinase 1 (MDI-1) activity and serum T3levels are lower, postnatal TSH and free T4concentrations are lower, bioinactive thyroid hormone analog levels are higher, brown adipose tissue thermogenic mechanisms are immature, and tissue thyroid hormone response systems are variably immature.3 The extent of these immaturities is related inversely to gestational age. In extremely low birth weight (ELBW) and very low birth weight (VLBW) infants, the TSH surge and the early thyroidal response are limited and followed by a progressive decrease in serum total T4 with nadir values at 7 to 10 days of postnatal life. 
These infants also have a low serum T3 concentration secondary to decreased T4 to T3 conversion, and decreased T4 concentrations. TBG levels tend to be low, and there may be an inhibitor of T4 binding to TBG as seen with nonthyroidal illness (low T3 syndrome). Free T4levels are variable especially in VLBW infants, where levels below 10 and 12.5 pmol/L (0.8–1.0 ng/dL) are seen in 15% to 20%. Serum TSH concentrations are normal or low. 
It is difficult to differentiate the contribution of nonthyroidal illness versus hypothalamic-pituitary immaturity in the pathogenesis of transient hypothyroidism of prematurity, and there is no consensus regarding treatment. Thyroxine supplementation is not used in most neonatal intensive care units (NICUs) but in one preliminary study, thyroxine was administered at a dose of 8 μg/kg/d given parenterally over a 6-week period, with some potential developmental benefits.4
TRANSIENT CONGENITAL HYPOTHYROIDISM
Ingestion of goitrogenic substances by a mother can cause fetal goiter and neonatal hypothyroidism.5-7 The most frequently ingested drug is iodide, usually prescribed in expectorants or as therapy for maternal thyrotoxicosis. Common goitrogenic agents are listed in Table 527-2. The fetus is unusually sensitive to iodide-induced hypothyroidism, probably because of the immature mechanisms for decreasing thyroid iodide uptake to compensate for high plasma iodide levels. Other goitrogens that have caused neonatal goiter include the thioureas, sulfonamides, and hematinic preparations containing cobalt. Neonatal goiters caused by propylthiouracil are uncommon unless large doses (more than 150 mg/d near term) are given to the mother. Transient hypothyroidism is also caused by transplacentally acquired maternal antithyroid antibodies. These can be measured as thyroid-stimulating hormone (TSH)–binding inhibiting immunoglobulin (TBII) or as cAMP (TSH)-blocking antibodies (TBA). These mothers usually have atrophic thyroiditis. The antibody half-life in the neonate approximates 2 to 3 weeks, and hypothyroidism can persist for 2 to 4 months.
DEFECTIVE THYROID EMBRYOGENESIS (THYROID DYSGENESIS)
The term thyroid dysgenesis describes ectopia or hypoplasia of the thyroid gland (or both) or total thyroid agenesis.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Thyroid dysgenesis is the cause of decreased thyroid function among most infants with permanent congenital hypothyroidism detected by neonatal screening programs. The prevalence approximates 1 in 4000 newborns.5-7 Thyroid dysgenesis is more prevalent among girls than among boys (ratio approximates 2:1), less prevalent among black infants (1 in 11,000 births), and more prevalent among Hispanics relative to white infants. The prevalence is also high among infants with Down syndrome. 
 PATHOPHYSIOLOGY AND GENETICS
PATHOPHYSIOLOGY AND GENETICS
Although the disorder is usually sporadic, familial examples have been described. These are presumably caused by mutations of the homeobox genes, TTF-1 (NKF2.1), TTF-2 (FOXE1), and PAX-8, which are involved in thyroid gland embryogenesis and are associated with developmental defects in other tissues.2 More recently, mutations in GL153, another transcription factor, have been described as the cause of a rare syndrome of neonatal diabetes, congenital hypothyroidism, and congenital glaucoma.8 First-degree relatives of children with thyroid dysgenesis have an increased prevalence of thyroglossal duct cysts, pyramidal thyroid lobe, thyroid hemiagenesis, and ectopic thyroid.
In rare instances, thyroid dysgenesis has occurred in association with maternal autoimmune thyroiditis. However, this appears to be coincidence; there is no correlation between thyroid dysgenesis and the presence of maternal autoimmune thyroiditis or circulating thyroid antimicrosomal or antithyroglobulin autoantibodies.
 CLINICAL FEATURES
CLINICAL FEATURES
Some thyroid tissue is present among 40% to 60% of these infants and represents a spectrum of severity of thyroid deficiency. Thyroid isotope scanning or ultrasonography may not be sensitive enough to detect small amounts of residual functioning thyroid tissue. In such infants, normal or near-normal circulating levels of T3 in the face of a low T4 value suggest residual thyroid tissue, as do measurable levels of serum thyroglobulin. An increased prevalence (8–10%) of nonthyroidal anomalies, including cardiac anomalies, nervous, musculoskeletal, digestive and urologic systems, cleft palate, and eye, are reported in infants with congenital hypothyroidism.9
Most infants with thyroid dysgenesis have no symptoms; perhaps 15% to 20% have suggestive signs of hypothyroidism during the first 2 to 3 weeks of life.5-7 Most affected infants have low serum T4 and high thyroid-stimulating hormone (TSH) concentrations in cord blood or in filter paper blood spots collected in newborn screening programs. An additional group of infants has T4 levels in the low-normal or normal range and increased TSH values. These infants usually have ectopic thyroid tissue on scans and may constitute 10% to 15% of infants with congenital thyroid dysgenesis. One percent to 5% of infants with congenital hypothyroidism have a delayed increase in serum TSH. They manifest a low serum TSH level at birth with an increase to primary hypothyroid TSH levels during the first 2 to 3 months of life. These infants may be missed by the newborn screening programs. Treatment is discussed as follows.
Table 527-2. Drugs That Impair Thyroid Function
Anions |
Iodine (in large amounts) |
Radiographic contrast agents |
|
|
Perchlorate |
Thiocyanate |
Cations |
Cobalt (in certain hematinic preparations) |
Arsenic salts |
Lithium salts |
Drugs |
Propylthiouracil |
Methimazole |
Aminosalicylic acid |
Aminoglutethimide |
Phenylbutazone |
Amiodarone |
Cholestyramine |
Phenytoin |
Propranolol |
Dexamethasone |
Ferrous sulfate |
Phenobarbital |
Carbamazepine |
Rifampin |
Naturally Occurring Substances |
Goitrin (present in cabbage and other members of the genius Brassica) |
Soybeans (not soybean milk as currently prepared) |
Linamarin (a glycoside in cassava) |



