Cardiovascular changes in pregnancy
Cardiovascular adaptations to pregnancy (Box 6.1) begin early, persist to an extent post partum, and appear to be enhanced by a subsequent pregnancy [1]. Cardiac output may decline during the third trimester [2]. This is partly because, in the supine position, the venous return to the heart is obstructed by the gravid uterus. This reduces cardiac output acutely by 20% or more [3], which may produce the supine hypotensive syndrome.
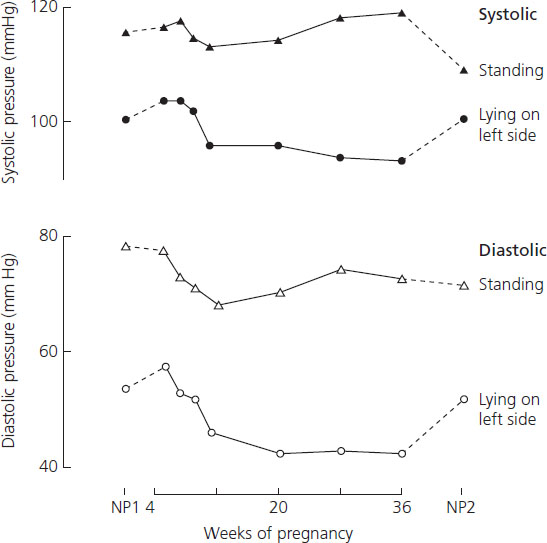
Arterial pressure falls during pregnancy (Figure 6.1), beginning in the first trimester, when the cardiac output is rising, and reaching a nadir in mid-pregnancy [4]. During the third trimester both systolic and diastolic readings slowly rise to about the pre-pregnant levels [4]. The reduced peripheral resistance of normal pregnancy appears to depend largely on increases in endothelial production of vasodilating factors.
Figure 6.1 The arterial pressures of 10 women were measured using a London School of Hygiene sphygmomanometer to reduce observer bias. Readings were taken in the right arm and phase IV of the Korotkoff sounds defined the diastolic pressures. Pressures taken before conception (NP1), during pregnancy, and 6 weeks after delivery (NP2) are shown, both standing and supine on the left side ({black dot}, {white dot}) (C.W.G. Redman, unpublished observations).
In nongravid women, blood pressures depend on age, sex, body build and other factors including the circumstances under which the measurement is taken and the time of day. Pregnant women are affected by many of these factors but with a narrower range of blood pressures than in the general population.
Box 6.1 Cardiovascular changes in pregnancy
- Increased cardiac output
- Increased plasma volume
- Increase heart rate
- Increased stroke volume
- Reduced arterial pressure (mainly in second trimester)
- Reduced peripheral resistance
Measurement of blood pressure
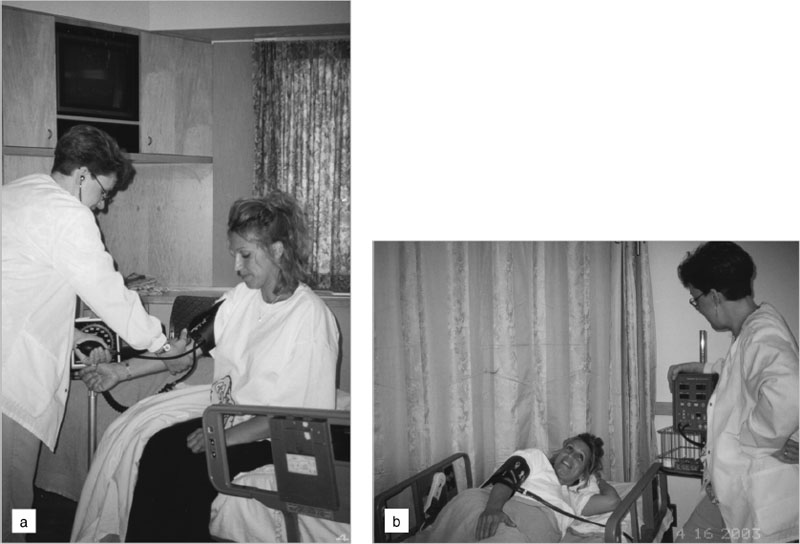
The indirect method of measuring blood pressure gives an estimate of the true intra-arterial pressure. The auscultatory technique with a trained observer and mercury sphygmomanometer remains the gold standard for clinical blood pressure measurement. The requirements for other instrumentation and standardized techniques are reviewed in Boxes 6.2 and Boxes 6.3. Figure 6.2 shows the preferred and the more commonly used techniques for blood pressure measurement.
Figure 6.2 (A) Blood pressure in pregnancy should be measured in the sitting position with the cuff at the level of the heart. The cuff should be appropriate for the arm circumference: with the increasing prevalence of obesity, this aspect of proper measurement is important. Ideally a mercury manometer should be used. If an aneroid sphygmomanometer is used it should be calibrated regularly against mercury. If an automated oscillometric technique is used, it should be a machine that has been validated for use in pregnancy. (B) The common practice of measuring blood pressure in the uppermost arm of women lying on their left sides is not recommended. The arm is elevated above the level of the heart, which leads to an underestimate of the true blood pressure.
Box 6.2 Methods of measuring arterial blood pressure
- Intra-arterial catheter with pressure transducer: accurate, no observer error, but not applicable in general clinical practice.
- Mercury sphygmomanometry in upper arm: the gold standard of indirect measurement of blood pressure, although the use of mercury is being phased out.
- Aneroid sphygmomanometry: inaccurate and requires frequent calibration (often not done at many centers).
- Hybrid manometry: indirect measurement via arm cuff with electronic transducer; uncommon but better than aneroid manometry.
- Oscillometric automatic devices: variable accuracy; no observer bias; each marketed device type needs validation for use in pregnancy.
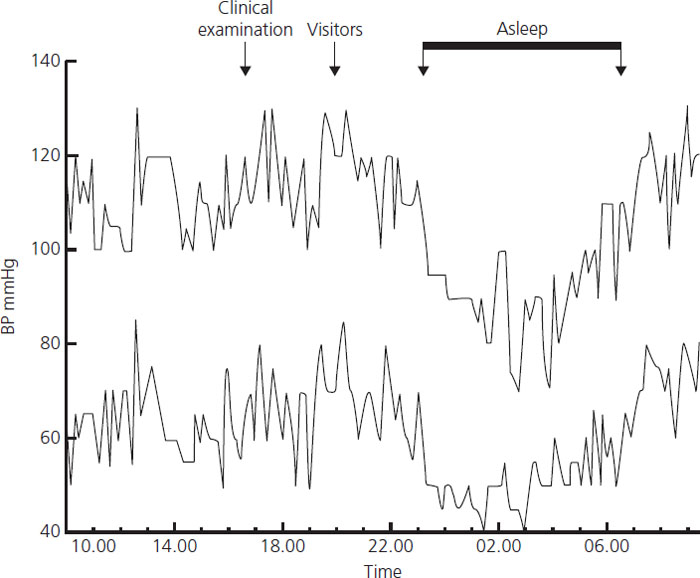
The time of day is an important determinant of blood pressure (Figure 6.3). As in nongravid individuals, the major change occurs in sleep. During waking hours, the patient’s blood pressure fluctuates minute by minute. Hence blood pressure measurements have large sampling errors that are distinct from the possible technical errors of measurement and can be reduced by averaging many readings. Ambulatory monitors can now be used for this purpose. A number of devices have been validated for use, and tested, in pregnancy and reference ranges have been published [5]. In general medical practice, ambulatory monitoring is the best way of diagnosing hypertension which predicts long-term cardiovascular risk and is useful for diagnosing white coat hypertension. However, it is expensive and cannot be recommended for routine use to screen pregnant women [6].
Figure 6.3 The circadian pattern of the arterial pressure of a pregnant woman at rest in bed during the third trimester is shown. The major change in the levels is the normal fall which occurs during sleep (C.W.G. Redman, unpublished observations).
Arterial pressure is distributed continuously in the general population. The dividing line between normotension and hypertension is an artificial concept, used for pregnant and nonpregnant individuals alike. An arbitrary threshold is imposed which divides the population quantitatively but not qualitatively – an important but frequently forgotten distinction. A high blood pressure may be unusual but not necessarily abnormal. This threshold is conventionally taken as ≥140 mmHg systolic or ≥90 mmHg diastolic.
The average blood pressure of more than 6000 women booking before 20 weeks for delivery in Oxford was 120/70. Two and three standard deviations above this mean were 144/87 and 156/95, respectively. The actual distribution of the readings is shown in Table 6.1. Later in pregnancy the blood pressure rises. A maximum antenatal reading (excluding those taken in labor) of 140/90 or more was found in 21.6% of all women and of 170/110 or more in 1.2%. By convention, the threshold for “diagnosing” hypertension in pregnancy is 140/90 mmHg. In the first half of pregnancy this identifies a small group (<2.0%) of hypertensive individuals. In the second half, a much greater proportion (21.6%) exceeds this limit at least once. So although 140/90 is an appropriate limit in the second half of pregnancy, it would be better to use a cut-off of 170/110 (maximum antenatal reading) to identify the extreme end of the distribution in populations that resemble the Oxford population. However, more usually, 160/110 or higher is considered to be “severe” hypertension [7].
Table 6.1 Blood pressures of 6790 women in pregnancy (cumulative frequency distribution)
| At booking before 20 weeks | Maximum during antenatal period | |
| (mmHg) | (%) | (%) |
| 170/110 | (0.0) | 1.2 |
| 160/100 | (0.1) | 3.7 |
| 150/95 | (0.4) | 8.7 |
| 140/90 | (2.0) | 21.5 |
| 130/85 | (5.0) | 38.7 |
| 120/80 | (21.0) | 76.7 |
Box 6.3 Technique of indirect blood pressure measurement
- The observer must be fully trained.
- Ideally, the patient should have rested for 10 minutes before measurement. Phase 1 (onset) and phase 5 (disappearance) of Korotkoff sounds should be used to record systolic and diastolic pressures respectively.
- The arm cuff should be level with the heart.
- The cuff size should be appropriate for arm size with a length that is 1.5 times the circumference of the upper arm or a bladder at least 80% of the arm. (Many cuffs are marked to show whether the size is appropriate.) Too small a cuff will overestimate blood pressure and too large a cuff will underestimate it. Practitioners caring for pregnant women should have ready access to small, medium and large cuffs.
- The patient’s position should be standardized as follows:
Outpatients: the woman should be seated
Inpatients: the woman may be either sitting or lying in the left lateral recumbent position; in either position, the arm cuff must be level with the heart. If it is above the heart the reading will be falsely low.
- The cuff pressure should be deflated slowly.
- Observer bias, especially digit preference, is a problem.
- Single readings can have large sampling errors.
- Clinic readings may be atypically high – white coat hypertension (see text).
- Readings in the third trimester, taken with the patient prone, may be atypically low because of the supine hypotension syndrome (see text).
Hypertension in pregnancy: introduction
Hypertension in pregnancy occurs in three contexts: chronic hypertension, pre-eclampsia-eclampsia and gestational hypertension. Chronic hypertension and pre-eclampsia are not mutually exclusive so that the two occur together as superimposed pre-eclampsia (Box 6.4). The incidence of all hypertensive disorders in pregnancy is about 6% [8]. The term hypertensive disease of pregnancy (HDP) is sometimes used to include all hypertension recorded during pregnancy regardless of its chronicity. This is imprecise and unhelpful since medical forms of hypertension are not diseases of pregnancy but of the affected woman regardless of pregnancy. Pregnancy-induced hypertension (PIH) is a term commonly used outside North America and means the same as gestational hypertension. Whether or not hypertension is truly gestational (or “pregnancy induced”) cannot, in fact, be known until nonpregnancy blood pressures have been defined in the remote puerperium.
Hypertension and blood pressure measurement would be of limited importance to obstetricians were it not for the syndrome of pre-eclampsia, which has also been called pre-eclamptic toxemia (PET) or gestosis. It is common, becomes detectable in the second half of pregnancy (although its origins may lie in the first half) and has been defined in terms of the development of new hypertension and proteinuria, which resolve after delivery. It is dangerous to both mother and baby, and of unknown cause. It is a difficult and elusive condition even for experienced clinicians. Toxemia is an obsolete expression, previously used to describe any hypertension or proteinuria in pregnancy, whether pregnancy induced or not. Pre-eclampsia is so called because it precedes eclampsia, which is characterised by grand mal convulsions, associated with signs of pre-eclampsia. In ensuing sections we shall see that the terminology is inexact. Eclampsia is not the only crisis of the condition, may occur without prodromal signs of pre-eclampsia, and is by no means the inevitable endstage of pre-eclampsia. Pre-eclampsia affects 2–5% of the pregnant population.
Etiology and pathogenesis of pre-eclampsia
Pre-eclampsia is a placental disease that evolves in two stages
Although the etiology of pre-eclampsia is not understood, the presence of a placenta is necessary and sufficient to cause the disorder [9,10]. A fetus is not required because pre-eclampsia can occur with hydatidiform mole. A uterus is probably not required because pre-eclampsia may develop with abdominal pregnancy. Central to management is delivery, which removes the causative organ, namely the placenta.
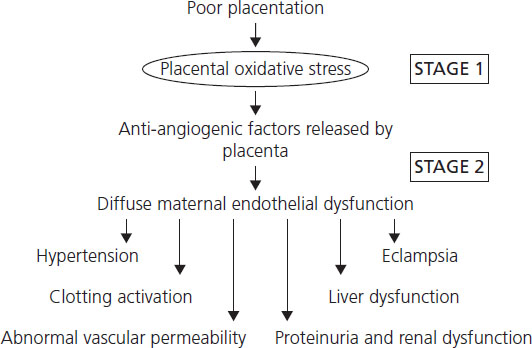
The disorder evolves in two stages [9] (Figure 6.4). The placental problem appears to be an inadequate maternal (uteroplacental) circulation leading to placental hypoxia, oxidative stress and infarction [11]. The uteroplacental circulation is unique because it lacks arterioles, capillaries or venules. Instead, about 40–50 spiral arteries deliver the 500 mL/min of blood required at the end of term pregnancy, directly into the intervillous space. In pre-eclampsia, two lesions may affect the spiral arteries. The first, called deficient placentation, comprises a relative lack of the structural remodeling and dilation that develop between weeks 8 and 18 [12] so that in normal pregnancy the arteries can transmit the expanded uteroplacental blood flow of the second half of gestation. At this time there is no clinical disease. The problem is not specific to pre-eclampsia; it is also found in some cases of intrauterine growth restriction (IUGR) without a maternal syndrome.
Figure 6.4 Pre-eclampsia evolves in two stages: poor placentation and then an excessive systemic inflammatory response of which maternal endothelial dysfunction in stage 2 explains the diversity of the pre-eclampsia syndrome.
Box 6.4 Classification of hypertension in pregnancy
- Hypertension: a blood pressure >140 mmHg systolic or >90 mmHg diastolic.
- Chronic hypertension: hypertension that is present before pregnancy or is diagnosed before the 20th week of gestation. The hypertension may be primary or secondary to medical conditions such as pheochromocytoma.
- Gestational hypertension (also called pregnancy-induced hypertension): new-onset hypertension after mid-pregnancy without proteinuria. This is a nonspecific term that includes women with incipient pre-eclampsia as well as those who do not develop pre-eclampsia.
- Pre-eclampsia-eclampsia: a pregnancy-specific syndrome that usually occurs after 20 weeks gestation and is characterized by new-onset hypertension and proteinuria, which regresses remotely after delivery.
- Pre-eclampsia superimposed on chronic hypertension may be more severe than simple pre-eclampsia.
The second lesion in the spiral arteries is “acute atherosis” – aggregates of fibrin, platelets and lipid-loaded macrophages (foam cells), which partially or completely block the arteries [12]. The time course of development is unknown, but it is likely that acute atherosis is a late pathologic feature. The cause is not known. The lesions are not the consequence of hypertensive injury. Acute atherosis and the associated thromboses are the cause of placental infarctions, which are more common in pre-eclampsia. The two spiral artery pathologies also can explain the reduced uteroplacental blood flow of pre-eclampsia. All these changes are consistent with an underlying placental ischemia to which the maternal signs of pre-eclampsia are secondary.
Impairment of the uteroplacental circulation affects the placental functions that sustain the fetus. Pre-eclampsia is conventionally considered to be a maternal disorder in which the fetus is an incidental participant. A more complete perception is that the placental problem causes both maternal and fetal syndromes [9]. The balance of the two syndromes varies: in some cases there is a major fetal problem and the maternal features are relatively trivial; in others the converse picture may be seen.
There are numerous animal models of pre-eclampsia, but differences in placentation between species limit their value.
The clinical features of pre-eclampsia are secondary to systemic endothelial dysfunction
The second stage of pre-eclampsia, the maternal syndrome, is surprisingly variable in the time of onset, speed of progression and the extent to which it involves different systems. It can cause hypertension, renal impairment and proteinuria, convulsions, hepatic dysfunction and necrosis, jaundice, abdominal pain, disseminated intravascular coagulation (DIC) or normotensive proteinuria (among others – see Box 6.5). Until relatively recently it was impossible to explain this astounding variability by a single underlying pathologic process; certainly hypertension could not account for all these features. But the concept that the maternal endothelium is the target organ for the pre-eclampsia process has resolved this difficulty [13]. In short, the maternal syndrome can be explained if it is seen not as a hypertensive problem, but as the sum of the consequences of systemic maternal endothelial dysfunction.
Box 6.5 Complications of pre-eclampsia
Central nervous system
- Eclamptic convulsions
- Cerebral hemorrhage
- Cerebral edema
- Cortical blindness
- Retinal edema
- Retinal detachment
Renal system
- Renal cortical necrosis
- Renal tubular necrosis
Respiratory system
- Laryngeal edema
- Pulmonary edema
Liver
- Jaundice
- Hepatic infarction
- HELLP syndrome (see text)
- Hepatic rupture
Coagulation system
- DIC
- Microangiopathic hemolysis
- HELLP syndrome
Placenta
- Placental infarction
- Retroplacental bleeding and abruptio placentae
Endothelial dysfunction appears to be caused by increased circulating levels of antiendothelial factors produced in excess by the oxidatively stressed placenta, namely the soluble vascular endothelial growth factor receptor 1 (also called sFlt-1) [14] and soluble endoglin [15]. There is also reduced availability of the angiogenic factor placental growth factor. Together, these factors synergize in their effects on the endothelium. These factors provide links between the pre-eclampsia placenta and the maternal disorder. A direct example of endothelial pathology of pre-eclampsia is the renal lesion of glomerular endotheliosis ([16] and see below).
In pre-eclampsia, endothelial dysfunction is one aspect of a maternal systemic inflammatory response
Systemic inflammation comprises multiple changes in the inflammatory network of the circulatory compartment. Apart from endothelial dysfunction, there is activation of circulating leukocytes (especially granulocytes and monocytes) and of the clotting and complement systems. All such changes have been demonstrated in pre-eclampsia [17]. Hence, it has been concluded that the endothelial dysfunction is one aspect of a more generalized process [17].
Pre-eclampsia and normal pregnancy are part of the same continuum
A maternal systemic inflammatory response is also detected in normal third-trimester pregnancy when it is not intrinsically different from that in pre-eclampsia except that it is milder. It has been suggested that pre-eclampsia develops when the systemic inflammatory process causes one or other maternal system to decompensate [17]. In other words, the disorder is not a separate condition but the extreme end of a range of maternal systemic inflammatory responses engendered by pregnancy. The problem is not the pre-eclampsia but pregnancy itself. This explains why, in clinical practice, it is often extraordinarily difficult to decide whether an atypical presentation is, or is not, pre-eclampsia.
Pre-eclampsia is heterogeneous and cannot be distinguished completely from normal pregnancy
This concept suggests that pre-eclampsia is the outcome of two opposing factors: a proinflammatory stimulus released by the placenta and a mother’s ability to respond and accommodate to the stimulus. Excessive inflammatory stimuli from the placenta could vary with either its physical size or a change in the intensity of (unknown) proinflammatory signals from the placenta. The larger the placenta, the larger the inflammatory burden – as, for example, with multiple pregnancy, a well-known predisposition to pre-eclampsia. In addition, advancing gestational age, which increases the physical bulk of the placenta, is itself a major risk factor for pre-eclampsia. Near term, normal pregnancy is characterized by many changes that are so well known that they are taken for granted, for example a rising diastolic pressure [4], which blur the distinction between normality and pre-eclampsia. In other words, during the last few weeks of normal pregnancy, more and more women appear to be driven towards the margin that distinguishes normal pregnancy from pre-eclamptic pregnancy.
Factors other than placental size must also be involved. With regard to the intensity of the proinflammatory stimulus of pregnancy, there are situations when, even from a small placenta, the inflammatory stimulus is excessive. It is suggested that, in this case, placental hypoxia, secondary to uteroplacental arterial insufficiency, amplifies release of inflammatory stimuli into the maternal circulation. These concepts imply that there cannot be a single cause of pre-eclampsia [17,18]. Different factors, particularly genetic factors that alter maternal inflammatory responses, will contribute. If the mother is genetically susceptible to inflammatory stimuli her constitution becomes the main determinant of the disorder (maternal pre-eclampsia). The other end of the spectrum is when the placenta is the main determinant (placental pre-eclampsia).
Heterogeneity of cause will lead to heterogeneity of clinical presentation. This is a main characteristic of the disorder that will be constantly emphasized in the ensuing sections. Furthermore, these considerations also lead to the conclusion that there can be no single preventive measure. Different prophylactic strategies will need to be targeted to the needs of different women.
The second stage of pre-eclampsia
Cardiovascular changes
The hypertension of pre-eclampsia is usually an early feature, not associated with a single hemodynamic pattern. Some investigators find increased cardiac output, others the converse. It is, however, agreed that peripheral resistance is increased once the condition is clinically apparent. The blood pressure is typically unstable at rest. Circadian variation is altered with, first, a loss of the normal fall in blood pressure at night and then, in the worst cases, a reversed pattern with the highest readings during sleep [19].
Pre-eclamptic hypertension is a form of secondary hypertension, arising from pathology in the placenta. Antihypertensive treatment is therefore not a cure; the definitive treatment is to remove the causative organ, the placenta, which means delivery. The hypertension is important because it is an accessible and early diagnostic sign of pre-eclampsia. In addition, if it is extreme, it may predispose to cerebral hemorrhage.
Complications of the hypertension
A sudden increase of blood pressure above a critical threshold causes acute arterial damage and loss of vascular autoregulation. The central nervous system appears to be particularly sensitive to hypertensive pathology, including cerebral hemorrhage, which antihypertensive treatment helps to prevent.
Pre-eclampsia may cause blood pressures which are well above the threshold (i.e. a mean arterial pressure of about 140 mmHg) at which organ damage would be expected. Cerebral hemorrhage remains a prominent cause of maternal death from pre-eclampsia and eclampsia in England and Wales [20], with a similar pathology to other hypertensive states. For this reason, adequate blood pressure control remains a priority. There is evidence that systolic rather than diastolic hypertension may be more important and the editors, but not the senior author, believe, but do not have published evidence, that the degree to which the blood pressure has increased acutely may also play a role independent of the absolute number.
Renal involvement (Box 6.6)
Glomerular filtration rate and renal plasma flow increase by 40–65% and 50–85%, respectively, during normal pregnancy [21]. Plasma creatinine and urea fall such that a plasma creatinine of more than 100 μmol/l (1.1 mg/dL) is always abnormal. Proteinuria is used as a defining sign of pre-eclampsia. Once present, it indicates a poorer prognosis for both mother and baby than when it is absent. It may be heavy (greater than 5.0 g/day). Overall, pre-eclampsia is the most common cause of nephrotic syndrome in pregnancy [22].
Proteinuria is one of several signs of involvement of the kidney in pre-eclampsia. Glomerular endotheliosis is a noninflammatory lesion that underlies this proteinuria [16]. However, renal biopsy is only indicated where the presentation strongly suggests an underlying glomerular pathology that could benefit from treatment. Otherwise, the investigation is reserved for those who continue to have significant proteinuric renal impairment at a remote time after delivery or very occasionally in early pregnancy where the specific diagnosis of renal disease will alter management in the short term (see Chapter 7).
Box 6.6 Renal system and pre-eclampsia
- Proteinuria
- Glomerular endotheliosis
- Reduced uric acid clearance
- Hypocalciuria
- Moderately reduced renal plasma flow
- Moderately reduced glomerular filtration
- Renal tubular necrosis
- Renal cortical necrosis
Renal function is also impaired. Often the changes are biphasic, involving first tubular dysfunction and later glomerular dysfunction. A common early feature of pre-eclampsia is a reduced uric acid clearance, reflecting altered tubular function and causing a reciprocal rise in plasma urate. Later, at about the time that proteinuria develops, glomerular filtration becomes impaired. At this point the plasma creatinine rises and clearance falls. A rising plasma urate may be an early sign of pre-eclampsia but it is not always demonstrated, reflecting the heterogeneity of the disease (see above). Another relatively early but inconsistent change in renal function is hypocalciuria [23], which is not a feature of pregnant women with other forms of hypertension. The endstage of renal involvement is acute renal failure, with tubular or partial cortical or total cortical necrosis. In relation to management, it is important to note that reduced renal function is rarely due to hypovolemia, but results from structural changes in the renal glomeruli [24].
Plasma volume, colloid osmotic pressure and edema
Maternal plasma volume increases during the second and third trimesters of normal pregnancy but is reduced in pre-eclampsia relative to normal pregnancy [25]. The vascular system in pre-eclampsia becomes “leaky”, with maldistribution of fluid: too much in the interstitial spaces (edema) and too little in the vascular compartment (hypovolemia). Hypoalbuminemia is also characteristic of the disorder, which causes a lower colloid osmotic pressure that may contribute to the problem.
Most women with proteinuric pre-eclampsia have edema. However, pre-eclampsia without edema – “dry pre-eclampsia” – has long been recognized as a particularly dangerous variant. Edema is difficult to quantify objectively. The best way is to chart weight gain. Those with excessive gain have an increased risk of pre-eclampsia [26]. Complications of the fluid retention include ascites, which is more common than generally recognized, affecting 13 of 99 women seen personally with severe pre-eclampsia. Pulmonary edema is a rare but life-threatening complication presenting before or after delivery [27]. Laryngeal edema may cause respiratory obstruction as well as difficulties at intubation if a general anesthetic is required.
Coagulation system (Box 6.7)
During normal pregnancy, the clotting system is activated such that pregnancy becomes a “hypercoagulable” state (see Chapters 3 and 4). This would be expected as part of the systemic inflammatory response that is intrinsic to late normal pregnancy (see above). The blood response to clotting stimuli is brisker and the natural “turnover” of the system is enhanced. Standard clinical tests do not detect these changes readily. In parallel, circulating platelets are also progressively activated.
Box 6.7 Coagulation in pre-eclampsia compared to normal pregnancy∗
Platelet activation
- Reduced platelet count
- Higher circulating beta thromboglobulin, platelet factor 4
- Younger, larger platelets
- Increased activation marker (CD63)
Plasma markers of activation of coagulation cascade
- Increased soluble fibrin
- Increased thrombin-antithrombin complexes
- Increased fibrinopeptide A (marker of thrombin activation)
- Increased circulating tissue factor (produced by activated endothelium)
Changes in fibrinolytic system
- Increased plasminogen activator type 1 antigen
- Increased d-dimer
- Reduced alpha-2 antiplasmin
Changes in clotting inhibitors
- Decreased antithrombin
- Decreased protein S
∗None of these changes is diagnostic of pre-eclampsia. Absence of any one change does not exclude the diagnosis.
Consistent with the continuum between normal pregnancy and pre-eclampsia, the activation of the clotting system is exaggerated in pre-eclampsia and may, in severe cases, decompensate as DIC. Activation causes changes in several tests of coagulation that are accessible in clinical laboratories, especially reductions of the platelet count. In the earlier stages, this is a chronic, fully compensated process which cannot be labeled as pathologic DIC, which is a late but inconstant feature of preictal pre-eclampsia and eclampsia. DIC is a condition in which intravascular activation of the clotting cascade leads to pathologic formation of fibrin within the circulation, with consumption and depletion of clotting factors. Complications can come both from bleeding and in situ microthrombi in the kidneys, brain, lungs and peripheral circulation. Microangiopathic hemolysis is a complication of DIC, associated with hemoglobinemia and a sudden fall in the hemoglobin concentration (see discussion of HELLP syndrome in the next section).
Liver dysfunction and the HELLP syndrome (see also Chapter 9)
Liver dysfunction is a feature of pre-eclampsia detected by elevations of circulating hepatic enzymes [28]. Epigastric pain and vomiting are the typical symptoms but are not always present. About two-thirds of women dying from eclampsia have specific lesions in the liver, which are periportal “lake” hemorrhages and various grades of ischemic damage, including complete infarction. Most of this pathology has been defined post mortem, although it has been confirmed in biopsy studies.
Liver damage is particularly associated with DIC in pre-eclampsia. If this occurs together with hemolysis, the acronym HELLP syndrome has been used to label the concurrence of Hemolysis, Elevated Liver enzymes and Low Platelet counts [29]. It is a dangerous complication that is often not associated with marked hypertension [28,29]. The severity of the presentation may be graded by the intensity of the laboratory changes. Hemolysis is detected by the presence of fragmented red cells (schistocytes) on a blood film, by the disappearance of haptoglobin (which binds and clears free hemoglobin from the circulation) or, less precisely, by acute increases of plasma lactate dehydrogenase (LDH), which is a nonspecific marker of cell necrosis.
Maternal mortality is of the order of 1% [28] and is commonly associated with cerebral hemorrhage. Recovery from the primary problem may take a week or longer if there is renal failure and be complicated by rebound hypercoagulability, which can cause fatal thrombosis. In rare cases, typically of multiparae rather than primiparae, there may be bleeding under the liver capsule. Subsequently this may rupture to cause massive hemoperitoneum, shock and (possibly) maternal death [30]. There may well be an overlap between the liver involvement in pre-eclampsia and acute fatty liver of pregnancy [31] as described in Chapter 9, which may depend on impaired fatty acid metabolism.
Eclampsia
Eclampsia is a form of hypertensive encephalopathy, an acute or subacute syndrome of diffuse cerebral dysfunction not ascribable to uremia or hypertension but commonly associated with both. Hypertensive encephalopathy is part of a wider syndrome – reversible posterior leukoencephalopathy (also known as PRES – posterior reversible encephalopathic syndrome) [32]. The latter describes the commonly seen changes on magnetic resonance imaging of the brain which may occur not only with hypertensive encephalopathy in nonpregnant individuals but in eclampsia, lupus or in those who require immunosuppressive treatment. The term is a misnomer, because the lesions may not be fully reversible nor confined to the posterior cortex or the white matter.
The symptoms include headaches, nausea, vomiting and cortical blindness. Convulsions commonly but not invariably occur. Blood pressure may be relatively low or normal [32]. This is not, therefore, a form of malignant hypertension which is characterized by gross papilledema and retinopathy, secondary to extreme hypertension, and are lesions which are rare in eclampsia.
The pathophysiology is thought to be vasogenic edema secondary to loss of autoregulation combined with endothelial dysfunction [33]. Autopsies on patients with hypertensive encephalopathy have demonstrated arteriolar fibrinoid necrosis with microinfarcts and failed to show brain edema; however, brain biopsy has shown edematous white matter with no evidence of vessel wall damage or infarction [34].
The role of hypertension in the genesis of pre-eclampsia associated encephalopathy is uncertain, since it may not be present in all cases. Protective vasospasm of cerebral vessels is sustained by myogenic tone and lost with pressure-forced dilation. Pregnant animals seem more susceptible to this type of damage and even in pre-eclampsia without convulsions, there is increased cerebral arterial flow [35]. Hypertension will certainly extend the problem even if it is not the primary cause.
Fetal and neonatal issues
Fetal and neonatal issues are discussed later in the chapter in a section entitled “The fetus in hypertensive pregnancies.”
Maternal mortality in pre-eclampsia
Pre-eclampsia and hypertensive diseases of pregnancy remain a prominent cause of maternal death worldwide. In relation to other causes, they are most important in Latin America and the Caribbean [36]. In undeveloped countries the main problems are access to medical services, the organization and range of available facilities, and the training of medical and paramedical staff [37]. In the UK pre-eclampsia is now a more important antecedent than eclampsia, in contrast to previous years. Cerebral hemorrhage continues to be the predominant cause of death in the UK [20]. In the USA higher mortality among black women is attributable not to a greater incidence of eclampsia but to higher case fatality rates [38].
Maternal risk factors (Box 6.8)
Risk factors may be specific to the mother or her pregnancy. Some, such as primigravidity or a past history of pre-eclampsia, are well known. The subject is systematically reviewed by Duckett & Harrington [39].
The predisposition to pre-eclampsia is, in part, inherited so that a positive family history is a risk factor. However, there is poor concordance between identical twin sisters so that maternal genes are not a dominant factor. Obese women are particularly susceptible but not to the variant of the HELLP syndrome. Obesity is associated with a constellation of other medical problems including type 2 diabetes and hypertension (syndrome X or metabolic syndrome). The separate parts of this constellation have all been associated with an increased tendency to pre-eclampsia [40]. The importance of renal disease increases with the degree of renal dysfunction and hypertension. The association with antiphospholipid antibodies is strong although the condition itself is rare. Antiphospholipid antibodies are an acquired cause of thrombophilia, which is a constitutional tendency to thromboembolism, some of which are genetically determined, for example possession of the Factor V Leiden gene or antithrombin (previously known as antithrombin III) deficiency. Thrombophilia is associated with more pregnancy complications and perinatal losses, including pre-eclampsia [41,42].
Box 6.8 Risk factors for pre-eclampsia
Maternal factors
- Primigravidity
- Primipaternity∗
- Short period of cohabitation†
- Increasing maternal age
- Previous pre-eclampsia
- Obesity (syndrome X, polycystic ovarian syndrome)
- Medical disorders
- Diabetes
- Chronic hypertension
- Chronic renal disease
- Antiphospholipid antibody syndromes
- Thrombophilia
- Migraines
- Asthma
- Diabetes
- Family history of pre-eclampsia
- Stressful job
Placental/fetal factors
- Advancing gestational age
- Poor placentation‡
- Multiple pregnancy
- Hydatidiform mole
- Triploidy
- Trisomy 13
- Trisomy 16 mosaic
- Placental hydrops
∗There may be partner specificity so it is not simply the first pregnancy that is an important risk factor but the first by the current partner.
†Stable cohabitation with a single partner seems to reduce the risk of pre-eclampsia in the first pregnancy by that partner.
‡See text for explanation of terminology.
Chronically hypertensive women are 3–7 times more likely to develop higher blood pressure combined with proteinuria or “superimposed pre-eclampsia” than are normotensive women. If hypertension is combined with renal disease, then the risk is particularly high. Since the first report [43], it has been repeatedly shown that cigarette smoking, for unknown reasons, is associated with a reduced incidence of pre-eclampsia, albeit with an increased perinatal mortality from other causes.
It is now known that nonpregnant individuals suffering obesity, syndrome X, type 2 diabetes or hypertension show evidence of endothelial dysfunction or systemic inflammation [44]. Thus, it is possible that these conditions all predispose to pre-eclampsia because they cause high susceptibility to processes that are important in pre-eclampsia.
There are a number of reports suggesting that stress at work may increase the risk of pre-eclampsia. This tends to become an issue after an episode of pre-eclampsia when women wish to review whether their lifestyle contributed to their problem.
Because pre-eclampsia is primarily a placental disease (see above), it is not surprising that placental/fetal factors may increase risk. Some are associated with larger than average placentas. One example is advancing gestational age, which is such a basic feature of the disease that it is rarely considered in this context. The other associations are listed in Box 6.8. If it is considered that poor placentation is a separate condition that may or may not be associated with pre-eclampsia, then it must be also considered to be a powerful predisposing factor [17].
It is not know whether all these risk factors can be formally combined to give clinically useful predictions of the onset of pre-eclampsia.
How can pre-eclampsia be defined? (Box 6.9)
The only consistent feature of pre-eclampsia is its inconsistency. There are many possible features, but no set pattern in the way that they occur. Definitions therefore are limited in their usefulness. For the clinician, they should point to situations that are dangerous, regardless of the fine detail found in various formal definitions. As already stated, pre-eclampsia is primarily a placental disorder. Hence, although it is defined by hypertension, it is not primarily a hypertensive disease. The raised blood pressure and other maternal signs by which it is recognized are secondary to intrauterine problems. Because the placenta is affected, the fetus may suffer its own morbidity or mortality. The mother suffers diffuse circulatory dysfunction secondary to endothelial involvement. Hence pre-eclampsia affects many maternal systems (see above) causing different presentations which are not included in the simple epidemiologic definition of pre-eclampsia. These problems have been recognized by proposing two definitions – a narrow one for research purposes and a broader one for clinical purposes. In clinical practice, pre-eclampsia is better sought using the broader definition (Box 6.9).
Box 6.9 Research and clinical definitions of pre-eclampsia
Strict research definition of pre-eclampsia (International Society for the Study of Hypertension in Pregnancy [7,45])
- New hypertension after 24 weeks: diastolic pressure ≥90 mmHg on two or more consecutive occasions >4 hours apart or >110 mmHg once AND
- New proteinuria after 24 weeks: 24-hour urine collection ≥300 mg protein or two mid-stream samples of urine collected more than 4 hours apart with >+ on stick test
Both features must be present.
Broad clinical definition of pre-eclampsia (adapted from Australasian Society for the Study of Hypertension in Pregnancy [46]
Hypertension arising after 20 weeks gestation and the new onset of one or more of:
- Proteinuria
- Renal insufficiency
- Hepatocellular dysfunction and/or severe epigastric/right upper quadrant pain
- Neurologic problems – convulsions (eclampsia); severe headaches; persistent scotomata
- Hematologic disturbances – thrombocytopenia; DIC; hemolysis
- Fetal growth restriction
The hypertension of pre-eclampsia will have returned to normal within 3 months post partum in both definitions.
The research definition is based on the presence of hypertension (see above) and new-onset proteinuria after 24 weeks gestation [7,45]. The idea that new hypertension is a mandatory part of the syndrome is historical, not logical. It is the focus of interest because this is what clinicians measure at the bedside. A syndrome requires at least two specific features before it can be recognized. Thus, new-onset hypertension (PIH) on its own (a common clinical presentation) is not pre-eclampsia; at least one more sign (by convention, pregnancy-induced proteinuria) is required. Some clinicians and investigators fail to appreciate this fact and use PIH and pre-eclampsia interchangeably, which confuses further an already confused subject. The clinical definition (Box 6.9) emphasizes the broader features of the disorder. It is to guide inexperienced clinicians and alert them to dangerous situations. With this approach, the term “nonproteinuric pre-eclampsia” in this chapter means new-onset or worsening hypertension in the second half of pregnancy combined with other features of pre-eclampsia, but without proteinuria.
Definitions are chosen for convenience, by consensus [46]. They describe outward appearances and embody no special truth about the underlying disease or diseases. When a syndrome such as pre-eclampsia is “defined,” rules are set that bring consistency to what is being discussed. The rules may be sensible or not, but their validity cannot be tested because there is no standard to which to refer. All definitions of pre-eclampsia have these limitations. Real progress is impossible until the mechanisms of the disease or diseases that contribute to the syndrome are fully understood.
Incidence of pre-eclampsia and eclampsia
The incidence of pre-eclampsia depends on how it is defined, how assiduously the signs are sought and how accurately the diagnoses are recorded on large databases. Some reports are of hospital-based incidences which may be biased by selective referral or incomplete ascertainment. The size of the problem can be estimated, but it is always approximate. A population with a higher proportion of primigravidae will have more pre-eclampsia. The current epidemic of obesity [47] would be expected to have the same effect. The incidence of pre-eclampsia is affected to an unknown extent by interventions to end a pregnancy, particularly at term for gestational hypertension (PIH) to prevent possible progression to pre-eclampsia. Just as the classification of hypertension has many observational problems, so does that of proteinuria, which is the second, defining feature of the syndrome. Urine testing is more often omitted than blood pressure measurements, particularly during labor or the early puerperium when significant pre-eclampsia may occur for the first time.
In Aberdeen the incidence in primigravidae has fluctuated between 3.0% and 7.7% since 1950. In the same study the incidence in multiparae was 0.8–2.6% [48]. The incidence was 5.3% in Norway in 1991–2003 [49] and 2.3% in the USA in 1979–86 [50]. Hence, the incidence of proteinuric pre-eclampsia is of the order of one in 20–40 maternities.
Eclampsia complicates one in 2000 pregnancies in the UK [51] and one in 1000 in the USA [8]. In about 10% of cases it is unheralded, without prodromal signs or symptoms [51]. In 10% of cases the only warning sign is proteinuria and in another 20% there is hypertension only [51]. Hence, extreme hypertension is not a necessary (or indeed sufficient) predisposing factor. This said, eclampsia occurs 7–8 times more commonly in the contexts of proteinuric than of nonproteinuric pre-eclampsia [52] and the average blood pressures of women with eclampsia are higher than those with severe pre-eclampsia.
In the UK (and probably other Western countries), the majority of cases occur during labor or after delivery. Such presentations are mostly at term and occur in hospital. Preterm eclampsia is more likely to be antepartum [51]. Eclampsia has been observed as early as 16 weeks of pregnancy [53]. Most postpartum fits occur within 24 hours of delivery but about one-fifth are defined as late post partum and occur more than 48 hours after delivery [54].
Screening for and prediction of pre-eclampsia
If the continuum theory of pre-eclampsia described above is accepted, then it is to be expected that there can be no reliable predictive test and indeed, this is the case to date, although numerous variables have been investigated. In brief, the maternal syndrome is too heterogeneous in its origins to be anticipated by a single test. Measurement of blood pressure and urine testing for proteinuria are the basic screening modes because they are relatively cheap, noninvasive and give an immediate result. Ways of improving their precision have been sought. Thus, 24-hour blood pressure monitoring (see above) eliminates many of the sampling errors of single measurements and provides a better estimate of the true blood pressure and may detect the changes of pre-eclampsia earlier. However, it is neither cheap nor simple to use and not all investigators agree that the information obtained is useful [6].
The different ways of assessing proteinuria have been summarized elsewhere [55] and are discussed in Chapter 7. A measurement of 24-hour protein excretion remains the gold standard but is too cumbersome and expensive for routine screening.
Dipsticks are the quickest and easiest but are not accurate for quantification because of natural variations in the degree of urinary dilution. This problem can be largely corrected by measuring urinary protein/creatinine ratios which can be obtained quickly from most laboratories from a small, randomly collected urine sample. Dipsticks for protein/creatinine ratio are also available that give convenient point-of-care estimates but may not be designed and calibrated to identify the mild level of proteinuria that is of concern in early pre-eclampsia. Any measurement based on single samples of urine will be affected to an unknown extent by variations in the rate of protein excretion. For the time being, standard dipsticks must remain the standard screening tool but positive results should be followed up by laboratory confirmation.
There are many possible circulating markers for pre-eclampsia reflecting endothelial dysfunction, activation of the clotting or inflammatory systems, renal or liver dysfunction. Their diversity reflects the huge range of systemic pathophysiology in pre-eclampsia. However, for prediction they have limited usefulness [56]. It is more logical to get as close to the primary pathology, the placenta, as possible. This can be done in two ways. The first is by ultrasound Doppler assessment for the presence of high-resistance blood flow patterns in the uterine arteries in mid-pregnancy which is gaining increasing acceptance as a moderately reliable technique [57]. A second way is to measure products of the placenta, secreted into maternal blood, that reflect the placental ischemia. These include factors that sustain or impair endothelial viability, including placental growth factor, soluble fms-like tyrosine kinase-1 (sFlt-1) and endoglin [14,58]. Further refinements of these techniques involve combining the Doppler measures and blood tests [57,59] or extending the screening from the second trimester to the end of the first trimester [60]. These techniques are under development and promise well for the future.
Diagnosis of pre-eclampsia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


