Human Papillomavirus and the Management of the Abnormal Pap Test
Christine H. Holschneider
Since the advent of widespread Papanicolaou (Pap) smear screening in the United States during the 1950s, the incidence of invasive cervical cancer and mortality from this disease has fallen more than 70%. The Pap test collects exfoliated cells from the surface of the cervix. Exfoliation occurs from most normal, precancerous, and cancerous cervical epithelium. It is the detection of precancerous cells, which predate invasive disease by years, that allows for the prevention of cancer.
The management of the abnormal Pap test has undergone numerous updates, which are reflected in the consensus guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP). The current guidelines were the result of a widely inclusive consensus conference convened in 2001 and reflect the new terminology for Pap test classification, known as the Bethesda system, which also underwent consensus review in 2001. In the fall of 2006, a follow-up consensus conference was convened in Bethesda. The 2006 revised guidelines are available at http://www.asccp.org. An abnormal Pap test triggers follow-up evaluation, typically colposcopy with biopsies. Treatment should be based on such biopsy diagnosis. Screening and subsequent treatment are aimed at the detection and elimination of preinvasive cervical disease, thus eliminating subsequent invasive disease.
The majority of cervical lesions, approximately 70% of squamous cell carcinomas, and more than 80% of adenocarcinomas are attributable to human papillomavirus (HPV) types 16 and 18. Two vaccines, a Food and Drug Administration (FDA)-approved quadrivalent vaccine and a bivalent vaccine in phase 3 trials, protect against these HPV types. Once widely implemented, these vaccines should reduce the incidence of preinvasive cervical disease. Currently, the quadrivalent vaccine is indicated for use in girls and women 9 to 26 years of age. The Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) recommends that all girls 11 to 12 years of age receive the quadrivalent vaccine as well as girls and women age 13 to 26 who have not yet been vaccinated and girls as young as 9 years, if indicated. These recommendations are not altered if a girl or woman in the qualifying age group is found to have an abnormal Pap or a positive HPV test. By their mid 20s, approximately 25% of women test positive for one of the four HPV types in the quadrivalent vaccine (HPV 16, 18, 6, or 11), but only 1% test positive for HPV 16 and 18, and only 0.1% test positive for all four HPV types. Thus, the quadrivalent vaccine should offer benefit to almost all women in the indicated age range. It is critical that women, whether vaccinated or not, follow current cervical cancer screening guidelines.
Evolution of Screening Cervical Cytology and the Bethesda System
The evolution of the Pap test is instructive in many aspects. First devised as a simple method to determine the reproductive cycle of laboratory animals by George Papanicolaou, it has evolved into a source of cellular material
for sophisticated molecular and diagnostic techniques. Although apparently ever changing, the one consistent aspect of its past and future is the success that Pap screening has had in the prevention of invasive cervical cancer.
for sophisticated molecular and diagnostic techniques. Although apparently ever changing, the one consistent aspect of its past and future is the success that Pap screening has had in the prevention of invasive cervical cancer.
Papanicolaou performed collection of cervical cells from the posterior vaginal fornix as a method of finding early invasive cervical cancer. Ayers introduced direct sampling of the cervix with the spatula that still bears his name. Such direct sampling significantly increased the cellular yield.
The next major advance was the support that the beginnings of the American Cancer Society (ACS) provided for increasing the visibility of the Pap test. Such exposure led to increasing adoption of the technique, and this coincided with the realization that Pap smears could detect preinvasive disease as well. Richart and colleagues identified the preinvasive component to squamous cell cancer of the cervix. This was achieved, in part, by use of the colposcope in defining the cervical transformation zone.
Terminology was developed for squamous preinvasive disease and reflected its origin in the cervical epithelium. The term cervical intraepithelial neoplasia (CIN) was developed and began to replace the term cervical dysplasia. Increasing involvement in the epithelial layer was reflected by CIN grades. CIN 1 is used when only the lower third of cells of the squamous cervical epithelium are abnormal. CIN 2 represents approximately two thirds involvement, and CIN 3 represents involvement into the outer third. Full-thickness involvement is also referred to as carcinoma in situ. These terms still are used today to describe the histology of squamous cervical lesions (Fig. 59.1).
Technology also has led to changes in the way that cervical cytologic specimens are obtained. The first advance was the Ayers spatula, as noted previously. Next, cotton swabs, often moistened with saline, were used to obtain cells directly from the cervical transformation zone. Although an improvement, a major advance in obtaining endocervical cells occurred when the endocervical brush was introduced in the 1980s. In the late 1980s, increased attention was paid to the potential false-negative result rate of the Pap smear, widely quoted to be as high as 50%. A false-negative Pap smear result does not reflect the current condition of the cervical epithelium and can arise from errors in screening, interpretation, or sampling. Several studies have shown that the inability of a Pap smear to render the true cervical diagnosis is more likely due to the smeared cellular sample not representing the state of the epithelium rather than the technical interpretation being misleading. Given these data, several efforts were made to reduce the false-negative rate and resulted in technology that allows for the cervical cellular sample to be rinsed into a preservative solution. Data have shown that up to 80% of the cells collected are thrown away after a conventional smear is made. Less than 10% are left on the collection device when the device is rinsed rather than smeared onto glass. From the concept of improving the cellular sample came the basis for liquid-based Pap tests, thus attempting to decrease the false-negative rate.
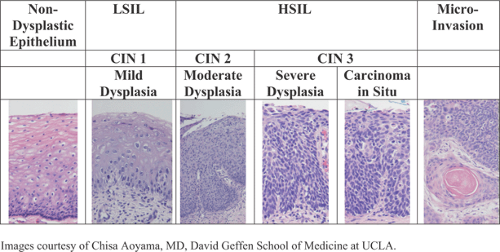 Figure 59.1 Correlating cytologic and histologic terminologies for neoplastic squamous epithelial changes in the cervix. (LSIL, lowgrade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CIN, cervical intraepithelial neoplasia.) (Images courtesy of Chisa Aoyama, M.D., David Geffen School of Medicine at UCLA.) (See Color Plate) |
Since then, the FDA has approved several liquid-based cervical cytology systems. These allow for the cellular material to be deposited in a liquid preservative that rinses the collection device and fixes the cells. Liquid-based collection and processing provide more representative cervical sampling than conventional smearing of the specimen on a glass slide. In multiple studies during the last decade, liquid-based cytology for cervical cancer screening has been shown to increase the detection rate for preneoplastic squamous intraepithelial lesions compared with that found with the conventional Pap smear method.
Given the significant concern regarding the quality of Pap tests, the U.S. Government developed the Clinical Laboratories Improvement Amendment released in 1988. This guideline addressed the number of Pap tests that could be screened by cytotechnicians and the need for quality assurance review. Additionally, this amendment directed the National Institutes of Health to establish guidelines on
Pap test terminology in order for the cytology results to be more consistent. Until then, frequently some iteration of the histologic CIN categories was also used to describe cervical cytology results. As a result of this direction, the first Bethesda conference was held in 1990, leading to the development of the Bethesda system for Pap cytology reports. The most recent update of the Bethesda system terminology for reporting results of cervical cytology was in 2001 at the Third Consensus Conference.
Pap test terminology in order for the cytology results to be more consistent. Until then, frequently some iteration of the histologic CIN categories was also used to describe cervical cytology results. As a result of this direction, the first Bethesda conference was held in 1990, leading to the development of the Bethesda system for Pap cytology reports. The most recent update of the Bethesda system terminology for reporting results of cervical cytology was in 2001 at the Third Consensus Conference.
The 2001 Bethesda system is summarized in Table 59.1. It requires evaluation of specimen adequacy, which is one of the key quality assurance elements of the Bethesda system. Although earlier thinking was that squamous metaplastic cells or endocervical cells were important in determining the adequacy of cervical screening, this concept has not been supported by the data. It has since become evident that a certain amount of squamous cellularity is more reflective of adequate sampling. Minimum squamous cellularity requirements are 8,000 squamous cells for a conventional preparation and 5,000 squamous cells for one that is liquid based. A notation is made regarding the presence or absence of an endocervical or transformation zone component.
TABLE 59.1 The 2001 Bethesda System | ||
|---|---|---|
|
The report then gives a general categorization of the findings followed by the interpretation of the results. One of the main changes for reporting epithelial cell abnormalities in 2001 was to the category of atypical squamous cells of undetermined significance (ASCUS). ASCUS describes cellular abnormalities that are more marked than those attributable to reactive changes but that fall short of meeting the diagnosis of squamous intraepithelial lesion. The intent in 2001 was to remove from the ASCUS category those Pap tests that contained cells suspicious but not diagnostic of high-grade squamous intraepithelial lesion (HSIL). Therefore, the category of atypical squamous cells suspicious for high-grade squamous intraepithelial lesion (ASC-H) was developed. The remaining atypical Pap test results would be designated atypical squamous cells of undetermined significance (ASC-US). Low-grade squamous intraepithelial lesion (LSIL) is being used for cells with findings consistent with either HPV effects or consistent with CIN 1–type changes since there is little ability to discern cytologically koilocytic effect from CIN 1 changes. HSIL denotes cells with findings consistent with CIN 2, 3, or carcinoma in situ. The ability to discern among these categories is limited, but they are cytologically distinct from CIN 1–HPV effect. The 2001 Bethesda system designated a specific cytology category for adenocarcinoma in situ (AIS). Atypical glandular cells (AGC) now also have a “not otherwise specified” (AGC-NOS) and a suggested neoplasia category. Within the glandular neoplasia section, the cytopathologist is asked to designate further whether the cells are from the endocervix or endometrium, if possible.
The 2001 Bethesda system requests reporting of benign endometrial cells, when seen in the cytology specimen, for women age 40 and older. As a women nears and goes beyond the menopausal years, the presence of benign endometrial cells can be indicative of more significant endometrial cavity pathology, such as polyps, hyperplasia (both simple and atypical), and endometrial cancer.
Educational notes are recommended at the end of the Bethesda system report and now should reflect the recommendations of the ASCCP consensus conference guidelines. Many of the recommendations made at the 2001 and 2006 Bethesda conferences are based among other studies on data from the ASCUS/LSIL triage study (ALTS trial). The ALTS trial is a large, multicenter, randomized clinical trial
of the management of women with ASCUS and LSIL screening cytology sponsored by the U.S. National Cancer Institute. The study was primarily designed to compare the sensitivity and specificity for the detection of CIN 3 of immediate colposcopy, HPV testing, or serial accelerated cytology. Data from the initial publications have been strengthened over the past 5 years by additional clinical studies from other groups and by additional analyses of the ALTS data. A number of ALTS publications are suggested at the end of this chapter for the interested reader.
of the management of women with ASCUS and LSIL screening cytology sponsored by the U.S. National Cancer Institute. The study was primarily designed to compare the sensitivity and specificity for the detection of CIN 3 of immediate colposcopy, HPV testing, or serial accelerated cytology. Data from the initial publications have been strengthened over the past 5 years by additional clinical studies from other groups and by additional analyses of the ALTS data. A number of ALTS publications are suggested at the end of this chapter for the interested reader.
Epidemiology, Molecular Biology, and Natural History of Human Papillomavirus Infection
Since the early 1990s, HPV has been accepted as a necessary but not sufficient cause in the development of invasive cervical cancer, both squamous cell cancer and adenocarcinoma. HPV is accepted as a necessary cause because 99.7% of all cervical cancers test positive for HPV based on data by the International Agency for Research on Cancer (IARC). It is the natural history of an HPV infection that has given rise to the not-sufficient portion. Excellent data support the concept that the majority of HPV infections, particularly the initial exposure in a woman’s teens and early 20s, regress spontaneously.
Although HPV-related diseases have been noted in the medical literature since the Roman-Hellenic era, it was not until researchers using electron microscopy identified mature viral particles in condylomata during the 1950s that a viral cause for these diseases was entertained strongly. The next breakthrough came with the isolation of HPV type 6 DNA from condylomata by zur Hausen and colleagues during the early 1980s. Since that time, more than 100 HPV types have been identified. A new type is designated when there are sufficient differences in the DNA sequences but still enough homology such that it is consistent with the overall family of papovaviruses.
Interestingly, HPV types have specific preferences for the type of epithelium that they infect. Our interest is in those more than 40 HPV types that infect the lower genital tract epithelium. These HPV types have been categorized further according to their ability to cause cervical neoplasia. HPV types that rarely, if ever, are found in preinvasive or invasive cervical cancer are put in the category of low-risk human papillomavirus (LR HPV). During an active infection with LR HPV, expression of the viral proteins may lead to a proliferative epithelial response and the formation of condylomata or, in some cases, low-grade CIN. The prototypes of LR HPV are HPV 6 and 11. Conversely, those types found at least occasionally in high-grade CIN or cancer are categorized as high-risk human papillomavirus (HR HPV) (Table 59.2). Prototypes of HR HPV are HPV 16 and 18. Combined, HPV 16 and 18 are present in close to 90% of CIN 2/3 and over 70% of invasive cervical cancers. Epidemiologic evidence also indicates a significant role for HPV in cancers of the vagina, vulva, and anus. Between 64% and 91% of vaginal cancers are HPV positive. In anal cancers, HPV is detected in 88% to 94%. Of the basaloid or warty types of vulvar cancers, typically occurring in young women who frequently smoke, 60% to 90% are HPV positive. HPV has also been strongly linked to nonanogenital tract cancers, such as cancers of the oral cavity, pharynx, and larynx.
TABLE 59.2 Epidemiologic Classification of Human Papillomavirus Types | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
When infection occurs, replication of the viral particle requires mature squamous keratinocytes. An active HPV infection is initiated when the infectious particles reach the basal layer of the epithelium, where they bind to and enter into cells. It has been suggested that for the maintenance of infection, the virus has to infect an epithelial stem cell. The HPV DNA sequence consists of early (E) and late (L) open reading frames. The critical molecules in the process of viral replication and cellular transformation are the E6 and E7. The E6 and E7 protein products bind tumor suppressor genes p53 and pRB, respectively. Cell transformation with oncogenic HPV subtypes, such as HPV 16 or 18, may be accompanied by the fact that HPV is no longer in an episomal state but rather is integrated into the host genome. Opening of the virus for genomic integration usually occurs in the E1/E2 region. Disruption of E2, which acts as a repressor of E6/E7, may result in unregulated expression of the transforming E6/E7 proteins and inactivation of p53 and pRB. Variations in the oncogenicity of different HPV subtypes may be due to differences in the binding efficacy of E6 and E7 to p53 and pRB or differences in their ability to inactivate these tumor suppressor genes. Low-risk types do not give rise to such changes.
As a consequence of disruption of these tumor suppressor genes, the dependence on cell cycle control is abolished and normal keratinocyte differentiation is retarded. With HPV integration into the human genome, there is constant activity of viral proteins E6 and E7, leading to increasing genomic instability, accumulation of oncogenic mutations, future loss of cell cycle control, and ultimately cancer. The viral capsule is quite uniform among the HPV types and is formed from the L1 and L2 reading frames, encoding the structural proteins. These capsule proteins are utilized in prophylactic vaccine therapy, whereas various manipulations of either the protein or HPV DNA from E6 and E7 are
used in therapeutic immunologic approaches. An in-depth discussion of these events can be found in reviews of HPV molecular biology.
used in therapeutic immunologic approaches. An in-depth discussion of these events can be found in reviews of HPV molecular biology.
Exposure to HPV appears to occur primarily by intimate sexual contact but does not require intercourse. Other avenues of exposure have been discussed but not verified, such as common pools, hot tubs, and bathrooms. Use of condoms reduces HPV infection but is not fully protective, which is not surprising given that HPV is transmissible through nonpenetrative sexual contact with both male and female partners. Women who practice a homosexual lifestyle are also at risk for HPV exposure. They, like their heterosexual counterparts, require Pap test screening and develop evidence of HPV exposure with positive cervical cytology findings. An abnormal Pap test result in a homosexual woman is no different in its etiology than in a result from a heterosexual woman.
Epidemiologic case studies, often using HPV DNA detection techniques, demonstrate that widespread exposure of the female and male sexually active population has occurred. Data from the CDC National Prevention Information Network indicate that by age 50, at least 80% of women will have acquired genital HPV infection. The majority (74%) of new HPV infections occur among those 15 to 24 years old. In women younger than 25 years, the prevalence of HPV infection ranges between 28% and 46%. The majority of these HPV infections are transient and appear to regress spontaneously. For college-age women, it can be expected that in 70%, the virus will no longer be detectable after 1 year. HPV clearance will increase to approximately 90% by 2 years. The reason for eradication of this viral infection is not known, but the gradual development of an effective cell-mediated immune response to the infection is a leading theory.
Reasons for suspecting that an immune response to HPV infection is the source of regression of disease are well founded but have not yet been proven definitively. Since HPV particles are freed through normal desquamation, there is no cytopathic death involved. Thus, little inflammation occurs to trigger the innate immune system. Women with transient HPV infection are less likely to develop antibody or cell-mediated responses to HPV than women with persistent HPV infections. The humoral response to naturally occurring HPV infections appears to exert little protective effect against HPV persistence or disease. Cell-mediated immune responses are critical to viral clearance once infection is established. Women whose immune systems are compromised, such as organ transplant recipients and HIV-infected individuals, have significantly reduced ability to clear their HPV infections. Data from recently HIV-infected adolescents show that even in women with normal CD4 counts, HPV persistence may be prolonged. Women with low CD4+ cell counts (CD4 count <200/mcL) have the highest prevalence of HPV infection, most commonly harbor HR HPV types, and are at highest risk for persistence of cervical HPV infection. The incidence of CIN is four to five times higher in HIV-positive women. Given these data, the CDC designated CIN 2/3 as conditions defining a stage of early symptomatic HIV infection (category B) and invasive cervical cancer as an AIDS-defining condition (category C).
Interestingly, young women first exposed to HPV will be most likely to manifest their infections by developing CIN 1, which is detected by Pap test and can be confirmed by biopsy. However, the HPV types involved in these lesions are predominately in the high-risk category. Although this finding has not led to a change in the definition of low-risk versus high-risk types, it indicates that clinically detectable cervical cytologic abnormalities most likely represent HR HPV no matter what the degree of morphologic abnormality.
Women with persistent oncogenic HPV infections are at greatest risk of developing cervical precancer or cancer. The longer an HPV infection exists, the less likely the patient will clear it. Other associated factors are age (>30 years), infection with multiple HPV types, immunosuppression, cigarette smoking, and possibly other sexually transmitted diseases (in particular Chlamydia trachomatis).
HPV 16 and 18 have a unique carcinogenic potential. In the ALTS trial, the 2-year cumulative risk for developing CIN 3 or worse for HPV 16–positive women was 30% to 40%. The progression from HPV infection to HPV persistence to CIN 2/3 and ultimately cancer is estimated on average to take about 15 years, although cases of rapid-onset disease exist. This gives ample opportunity for the prevention of cervical cancer in a screened population.
Human Papillomavirus Testing Methods
Because HPV cannot be cultured, its detection depends on the identification of its DNA. Since the advent of polymerase chain reaction (PCR) and other sensitive DNA detection methods, very minute amounts of viral DNA can be detected from infected cells. Generally, these methods require the extraction of the cells’ DNA and then the aggregate DNA is probed specifically for the presence of viral DNA. Using such techniques, although very sensitive, does not allow for identification of what cell in the sample carried the viral DNA. Therefore, contamination from sperm, white cells, or mucus of male origin may lead to false-positive HPV DNA test results. The most common methods for HPV DNA detection are PCR and RNA-DNA hybrid detection, which is the method used in Hybrid Capture II (HC II) (Digene, Gaithersburg, MD). General or consensus primer-mediated PCR assays have enabled screening for a broad spectrum of HPV types in clinical specimens using a single PCR reaction. Following amplification using consensus primers, individual HPV genotypes are identified by using a variety of methods. Using consensus primers in a test format known as real-time quantitative PCR, it is possible to generate viral load (concentration) data
from reaction curves generated by monitoring PCR reaction kinetics in real time.
from reaction curves generated by monitoring PCR reaction kinetics in real time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


