Hirsutism
 |
Hirsutism, defined as excessive male-pattern facial and body hair, affects between 5 and 10% of reproductive age women.1 Hirsutism can be the initial or only sign of androgen excess and usually is a consequence of chronic anovulation. Virilization describes the signs and symptoms of more severe androgen excess, which include deepening of the voice, temporal balding (androgenic alopecia), breast atrophy, changes in body habitus, and clitoromegaly. Virilization is rare and most commonly results from congenital adrenal hyperplasia or androgen-producing tumors of the ovary or adrenal.
Hirsutism is both an endocrine and a cosmetic problem and deserves a concerned and sympathetic response. Excessive hair growth on the face, chest, or abdomen is understandably disturbing and raises a number of concerns and questions about the possibility of underlying disease, effects on sexuality and fertility, and available treatments.
This chapter reviews the biology of hair growth and the causes and pathophysiology of hirsutism, and presents a straightforward, effective approach to the diagnostic evaluation and clinical management.
The Biology of Hair Growth
Hair is a distinguishing characteristic of mammals and serves a wide range of functions, including thermoregulation, physical protection, sensory activity, and social interactions.
Androgens are required for development of sexual hair and sebaceous glands, but numerous other factors are involved, including growth hormone, insulin, insulin-like growth factors, glucocorticoids, estrogen, and thyroid hormone.2,3
Embryology
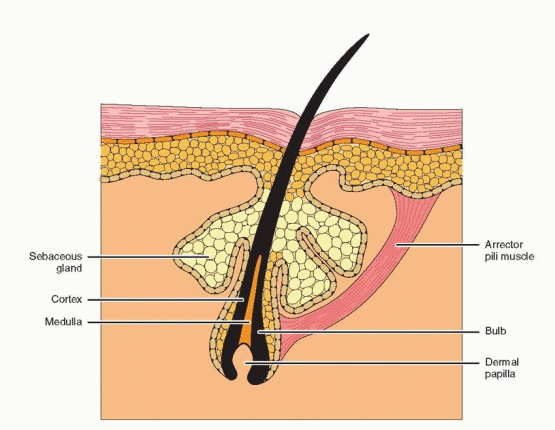
Hair follicles develop at approximately 8-10 weeks of gestation from a small group of epidermal cells overlying undifferentiated mesenchyme. Members of the transforming growth factor beta superfamily, activins and bone morphogenetic proteins in particular, play an important role in the communication between the epithelial and mesenchymal compartments during normal hair follicle development.4,5 Initially, the follicle is composed of a solid column of cells that proliferates from the basal layers of the epidermis and extends downward into the dermis. As the column elongates, it encounters a cluster of mesenchymal cells (the dermal papilla) that envelop its bulbous tip (bulb). The solid epithelial column then hollows to create a hair canal, and the pilosebaceous unit (a hair follicle, sebaceous gland, and arrector pili muscle) is formed. Hair color is determined by pigments produced by melanocytes located in the bulb.
One’s total endowment of hair follicles is determined by 22 weeks of gestation and no new hair follicles develop de novo thereafter. The concentration of hair follicles in facial skin does not differ significantly between the sexes, but does differ between races and ethnic groups. Whereas Asian and Native American women generally have little body hair, women of Mediterranean descent typically have increased amounts of body hair, although serum androgen concentrations are similar in the three groups.6 Differences in hair growth among races and ethnic groups probably also reflect differences in the local levels of 5α- reductase activity, the enzyme that converts testosterone to the more potent and active androgen, dihydrotestosterone (DHT).7
 |
The Hair Growth Cycle
Hair growth is cyclic, rather than continuous, and exhibits three distinct phases, known as telogen (quiescent phase), anagen (growth phase), and catagen (involution phase).2 In the resting phase (telogen), the hair is relatively short and loosely attached to the base (the bulb) of the epithelial canal. As growth (anagen) begins, cells in the epithelial matrix at the base of the hair follicle begin to proliferate, extending downward into the dermis in a column that elongates to approximately 4-6 times its length during telogen. With continued rapid growth, the epithelial column also pushes upward to the skin surface, breaking its tenuous contact with the previous hair, which is shed. The most superficial epithelial cells differentiate to form a keratinized column and growth continues as long as active mitosis persists in the basal epithelial cells. As the growth phase comes to a close, the column rapidly shrinks and the bulb shrivels (catagen) before the hair follicle again enters a quiescent phase (telogen).
The length of hair is determined primarily by the duration of the growth phase. Scalp hair remains in anagen for 2-5 years and spends only a relatively short time in telogen. Elsewhere, such as on the forearm, the hair cycle has a short anagen and a long telogen, yielding a short hair of relatively stable length. The outward appearance of continuous growth or periodic shedding reflects the extent to which hair follicles act in synchrony with others in the area. Typically, scalp hair is asynchronous and, therefore, always appears to be growing; the resting phase of some hairs (approximately 10-15%) is not apparent. If a larger proportion of hairs becomes synchronous and enters telogen simultaneously, noticeable shedding may occur, a process known as telogen effluvium. Although women occasionally may notice and complain of scalp hair loss, the interval of shedding usually lasts no longer than 6-8 months. Growth resumes when asynchrony again becomes established. Telogen effluvium can be precipitated by pregnancy, certain drugs, and by febrile illness.
Hair is categorized as vellus (fine, soft, short, and unpigmented) or terminal (long, coarse, and pigmented).3 The vellus hair that covers the body of infants is called lanugo. Hypertrichosis describes an uncommon condition characterized by a generalized increase in vellus body hair, usually associated with certain drugs (e.g., phenytoin, penicillamine, diazoxide, minoxidil, cyclosporin), systemic illness (e.g., hypothyroidism, anorexia nervosa, malnutrition, porphyria, dematomyositis) or malignancy (as a paraneoplastic syndrome). Hirsutism implies a transformation from vellus to terminal hair.
The Control of Hair Growth
The fate of a hair follicle depends on the health and function of the dermal papilla. Despite major injury to its epithelial component (e.g., freezing, x-rays, or a skin graft), the hair follicle will regenerate and re-grow hair if the dermal papilla survives intact. Serious injury or degeneration of the dermal papilla (e.g., electrolysis or laser hair removal) results in permanent hair loss.
Sexual hair is that which responds to sex steroids and grows primarily on the face, chest, lower abdomen, the pubis, and in the axillae. In androgen-sensitive areas, androgen stimulates hair follicles, inducing the growth of thicker, longer, and darker hairs. Thereafter, the hair exhibits typical cycles of growth, involution, and rest, but does not change in character, even if high androgen levels are not sustained. Because androgen stimulation of hair follicles requires the conversion of testosterone to DHT, the sensitivity of hair follicles to androgens is determined, in part, by the local level of 5α-reductase activity, helping to explain the varying extent of hirsutism observed in women with similar levels of androgen excess.8
Based on data from animal studies and on patterns of human disease, the following summarizes the effects of steroid hormones on hair growth:
Androgens, particularly testosterone, stimulate growth and increase the diameter and pigmentation of hair. Androgens also increase the proportion of time terminal hairs spend in anagen,9 except on the scalp, where androgen decreases the duration of anagen.
Estrogens have actions opposite those of androgens, generally resulting in slower growth of finer and lighter hair.
Progestins have little or no direct effect on hair growth.
Pregnancy, characterized by high levels of both estrogen and progesterone, can induce greater synchrony among hair follicles, leading to periods of growth or shedding.
Observations in studies of the effects of male castration demonstrate an important clinical characteristic of hair growth. Males castrated before puberty do not grow a beard or other sexual hair, but when castrated after puberty is completed, the beard and sexual hair continue to grow, albeit more slowly and with finer caliber hair.
Endocrine disorders can affect the growth of both sexual and nonsexual hair. Hair growth is markedly reduced in individuals with hypopituitarism. Approximately 10-15% of patients with acromegaly also are hirsute. Hypothyroidism sometimes is associated with hair loss on the scalp, the pubis, in the axillae, and, curiously, the lateral third of the eyebrows. Hyperthyroidism generally results in finer hair that is lost easily. Insulin-like growth factor-1 (IGF-1), which stimulates 5α-reductase activity,10 often is increased in women with chronic anovulation, insulin resistance, and hyperinsulinemia.
Hair growth also can be influenced by other factors, such as local skin temperature, blood flow, and edema. Hair grows faster in the summer than in the winter.11 Hair growth also can be observed in association with central nervous system pathology (e.g., encephalitis, cranial trauma, multiple sclerosis), and with certain drugs.
Androgen Production
Hirsutism reflects the interaction between circulating androgen levels and the sensitivity of hair follicles to androgen stimulation. In women, the major circulating androgens (in descending order of serum concentration) are dehydroepiandrosterone sulfate (DHEA-S), dehydoepiandrosterone (DHEA), androstenedione, testosterone, and DHT.12 DHEA-S, DHEA, and androstenedione can be considered pre-hormones because they have little or no intrinsic androgenic activity and require conversion to testosterone to exert androgenic effects.
DHEA-S is produced almost exclusively by the adrenal glands, at a rate ranging between 3.5 and 20 mg/day;13 the normal serum concentration is 100-350 μg/dL in most laboratories. DHEA is produced by both the adrenals (50%) and the ovaries (20%), and from the peripheral conversion of DHEA-S (30%). The production rate of DHEA is between 6 and 8 mg/ day14 and normal serum concentrations range between 1 and 10 ng/mL. Androstenedione production is divided equally between the ovaries and the adrenals; the production rate is
between 1.4 and 6.2 mg/day and the normal serum concentration is 0.5-2.0 ng/mL.15,16 Serum immunoassays for DHEA-S, DHEA, and androstenedione generally reflect the amount of biologically available hormone because none of the three is protein-bound to any significant extent.
between 1.4 and 6.2 mg/day and the normal serum concentration is 0.5-2.0 ng/mL.15,16 Serum immunoassays for DHEA-S, DHEA, and androstenedione generally reflect the amount of biologically available hormone because none of the three is protein-bound to any significant extent.
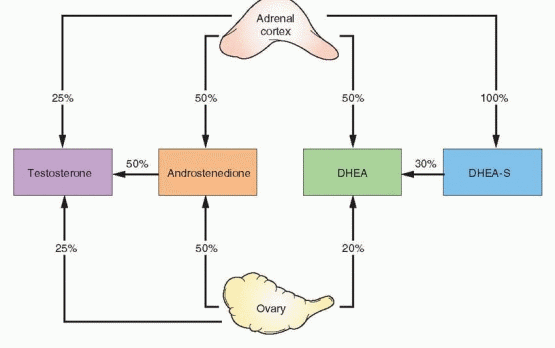 |
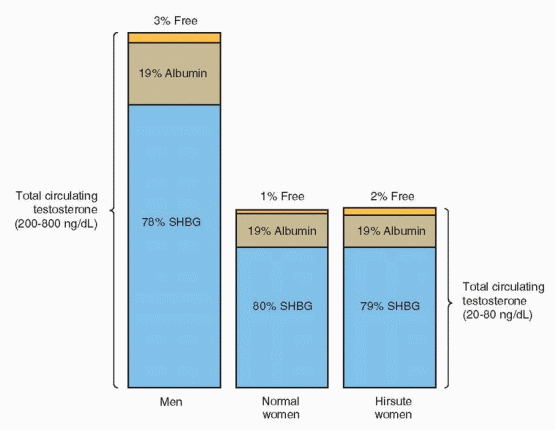
Testosterone production derives from the adrenals (25%), the ovaries (25%), and from peripheral conversion of androstenedione (50%). The production rate ranges between 0.1 and 0.4 mg/day and the normal serum concentration is 20-80 ng/dL; levels do not fluctuate widely, but are lowest during the early follicular phase, and approximately 20% higher at midcycle.14 In normal women, about 80% of circulating testosterone is bound to a beta globulin known as sex hormone-binding globulin (SHBG), another 19% is loosely bound to albumin, leaving only about 1% unbound or free. Routine serum immunoassays for testosterone measure the total testosterone concentration, including both bound and unbound hormone. However, the androgenic actions of testosterone relate primarily to the amount of free hormone and, to a limited extent, to the fraction associated with albumin. Anything that affects the SHBG concentration also affects the concentration of free/active testosterone.
Androgens themselves decrease SHBG production in the liver. Consequently, testosterone binding capacity in men is lower than in normal women; approximately 3% of total testosterone circulates in the free, active form in men. Whereas insulin and glucocorticoids also decrease SHBG levels, estrogens and thyroid hormone increase SHBG production. Therefore, binding capacity is increased in women with hyperthyroidism, in pregnancy, and during treatment with estrogens. In hirsute women, excess androgen production (and hyperinsulinemia, when present) depresses SHBG levels, increasing the amount of free/ active testosterone to approximately 2%, even though the total testosterone level may remain within the normal range. Although specific assays to measure the level of free testosterone are available, they are costly and rarely necessary. The very presence of hirsutism or virilization indicates androgen excess. In hirsute women with “normal” serum total testosterone levels, decreased binding capacity and increased free testosterone can be assumed.
 |
In women with hirsutism, only about 25% of circulating testosterone arises from peripheral conversion, most coming from direct glandular secretion, with the ovary being the primary source of both increased testosterone and androstenedione.17 By far, the most common cause of hirsutism is chronic anovulation and excess androgen production by the ovaries. Adrenal causes of hirsutism are very uncommon.
Although testosterone is the major circulating androgen, DHT is the major nuclear androgen in many androgen-sensitive tissues, including hair follicles and sebaceous glands. DHT is produced only in the periphery, by intracellular conversion of testosterone (via 5α-reductase). Circulating levels of DHT are, therefore, very low and do not reflect the level of 5α-reductase activity.18 3α-androstanediol is the peripheral tissue metabolite of DHT, and its glucuronide conjugate, 3α-androstanediol glucuronide (3α-AG), can be used as a marker of peripheral androgen metabolism.19,20 Serum 3α-AG levels correlate highly with levels of 5α-reductase activity in genital skin and are elevated almost uniformly in hirsute women,21 including those with normal serum androgen levels, indicating that “idiopathic” hirsutism likely results from increased peripheral 5α-reductase activity. However, assays for serum 3α-AG have little clinical utility, primarily because results have no significant impact on the diagnosis and treatment of hirsutism.
After the menopause, the production rate and serum concentration of androstenedione fall by about half, with approximately 80% derived from the adrenals.22 Testosterone production and serum levels also decline, primarily due to the decrease in peripheral production, via the conversion of androstenedione.23,24 Ovarian testosterone production is largely maintained after the menopause, as demonstrated by the 40-50% decrease in serum testosterone levels after oophorectomy in postmenopausal women.25,26 Because the decrease in estrogen production far exceeds that in androgen production after menopause, the postmenopausal ovary is primarily an androgen-producing organ.27 Elevated gonadotropin levels stimulate androgen synthesis in ovarian hilar and stromal cells.28,29 Adrenal androgen production also declines progressively with age; serum DHEA concentrations in women between ages 40 and 50 years are approximately half those in younger women.30
Causes of Hirsutism
The causes of hirsutism include specific endocrine disorders, such as androgen-secreting tumors, classical and nonclassical congenital adrenal hyperplasia (CAH), Cushing syndrome, and the hyperandrogenic insulin-resistant acanthosis nigricans (HAIR-AN) syndrome, as well as disorders of exclusion, including polycystic ovary syndrome (PCOS) and idiopathic hirsutism. In a case series of 873 women presenting with symptoms of androgen excess, the prevalence of these disorders was as follows:31
Diagnosis | Number | Prevalence (%) |
Specific Disorders | ||
Androgen-secreting neoplasm | 2 | 0.23 |
Classical congenital adrenal hyperplasia | 6 | 0.69 |
Nonclassical congenital adrenal hyperplasia | 18 | 2.06 |
HAIR-AN syndrome | 33 | 3.78 |
Disorders of Exclusion | ||
Polycystic ovary syndrome | 716 | 82.02 |
Idiopathic hirsutism | 39 | 4.47 |
Hyperandrogenemia, hirsutism, and normal ovulation | 59 | 6.75 |
Total | 873 | 100.00 |
PCOS is by far the most common cause of androgen excess in women. The diagnostic criteria, clinical features, and treatment of PCOS are considered in depth in Chapter 12. The prevalence of PCOS among populations of hirsute women varies with differences in the diagnostic criteria proposed by the National Institutes of Health Conference on PCOS (NIH, 1990),32 the European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine Consensus Workshop Group (ESHRE/ASRM, 2003),33 and the Androgen Excess Society (AES, 2009).34 All include oligo/anovulation and clinical or biochemical evidence of hyperandrogenism as diagnostic criteria, two of the three (ESHRE/ASRM, AES) regard polycystic ovarian morphology (as defined by ultrasonographic imaging) as a criterion, and all three require exclusion of other specific diagnoses (e.g., congenital adrenal hyperplasia, Cushing syndrome, androgen-secreting tumors, and hyperprolactinemia). In three large case series of women presenting with symptoms of androgen excess, the prevalence of PCOS ranged between 57% and 82%.31,35,36
Although the “consensus” criteria all were developed in an effort to unify opinion and to standardize the diagnosis of PCOS, primarily for purposes of clinical investigation, ironically, they have created more clinical confusion and controversy. Many have objected to the ESHRE/ASRM criteria (also known as the Rotterdam criteria, where the conference was held) because they permit the diagnosis of PCOS in women with polycystic ovarian morphology, in the absence of hyperandrogenism. In contrast, both the NIH and the AES criteria require hyperandrogenism for diagnosis of PCOS. In this chapter, focused on the evaluation and treatment of hirsutism, the term PCOS describes only women with oligo/anovulation associated with hyperandrogenism and having no other specific diagnosis. In truth, there is no evidence that PCOS is a specific endocrine disorder having one unique cause. Rather, it is a common condition with features that develop as a direct consequence of chronic anovulation, which can result from a wide variety of causes. Whereas, in that context, the disorder might be more accurately described as “chronic anovulation with polycystic ovaries,” the term PCOS is firmly entrenched in our scientific and clinical lexicon.
Hyperandrogenic insulin-resistant acanthosis nigricans (HAIR-AN) syndrome has the same clinical features as PCOS, but in the extreme. The primary underlying pathology is severe insulin resistance, with acanthosis nigricans being an epiphenomenon.37,38 A compensatory chronic and severe hyperinsulinemia stimulates a marked increase in ovarian androgen production, via theca cell receptors for insulin and insulin-like growth factor-1 (IGF-1), and induces a marked decrease in serum SHBG concentrations, yielding a large increase in free testosterone levels. In turn, high circulating androgen levels exacerbate the underlying insulin resistance, resulting in a self-propagating positive feedback loop that increases in severity over time, ultimately causing severe hirsutism and, in many, virilization (temporal balding, deepening of the voice, changes in body habitus, clitoromegaly). Ovarian stromal hyperthecosis is a histologic diagnosis, based on the observation of distinct clusters of luteinized thecal cells scattered throughout the ovarian stoma.39 Patients with hyperthecosis typically are obese, severely hirsute, and often virilized, most having serum testosterone concentrations greater than 150 ng/dL and exhibiting severe insulin resistance and hyperinsulinemia.40 It is likely that most, if not all, patients with the HAIR-AN syndrome have ovarian hyperthecosis, but hyperthecosis also can arise in postmenopausal women.41,42 and 43
Idiopathic hirsutism describes hirsute women with regular menstrual cycles and normal serum androgen levels.8,44,45 Although some may have subtle forms of ovarian or adrenal enzymatic dysfunction,46 an increased sensitivity to androgens, mediated by increased peripheral 5α-reductase activity,21 is the most logical explanation. In affected women, normal circulating androgen levels stimulate hair growth. Many women previously assigned a diagnosis of idiopathic hirsutism would now be considered to have PCOS, according to some criteria.33
Congenital adrenal hyperplasia (CAH) is a specific but uncommon cause of hirsutism. The clinical features, diagnostic criteria, and treatment of CAH are discussed in detail in Chapter 10. Whereas females with classical CAH usually are recognized at birth or during early infancy, the nonclassical form of the disorder (also known as late-onset CAH) presents later, at or after puberty, with hirsutism and menstrual irregularity or amenorrhea. In different studies, the prevalence of nonclassical CAH has ranged between 1 and 15%.47,48,49 and 50 The most common cause of both classical and nonclassical CAH is an adrenal 21-hydroxylase (P450c21) deficiency, resulting in excess production of 17α-hydroxyprogesterone (17OHP), which is the substrate for 21-hydroxylase in adrenal cortisol synthesis and a precursor for androgen synthesis (Chapter 9).
Androgen-secreting neoplasms of the ovary or adrenal are a rare cause of androgen excess and hirsutism. Androgen-secreting tumors account for only 5% of all ovarian tumors. Most are Sertoli-Leydig cell tumors, lipid- and theca-cell (stromal) tumors, or hilus-cell tumors, most are associated with frankly elevated serum testosterone concentrations greater than 150-200 ng/dL,51,52 and 53 and most can be imaged by transvaginal ultrasonography. Although some adrenal adenomas secrete testosterone, most androgen-secreting adrenal tumors are carcinomas that secrete DHEA, DHEA-S and cortisol, in addition to testosterone.54
Some women with hirsutism also have mild hyperprolactinemia. Elevated serum prolactin concentrations can be associated with increased serum DHEA-S levels,55,56 prolactin receptors have been identified in the human adrenal, and prolactin can increase adrenal DHEA production in vitro.57 Although DHEA-S is a weak androgen, it can be converted in the periphery to testosterone and, in turn, to DHT. Hirsutism in women with hyperprolactinemia may result directly from prolactin stimulation of adrenal androgen production, but also could result from excessive ovarian androgen production due to chronic anovulation, caused by hyperprolactinemia.
Virilization during pregnancy should raise suspicion for a pregnancy luteoma, which is a hyperplastic mass of luteinized ovarian cells rather than a true tumor. Although most luteomas produce little androgen or have little or no androgenic effect, serum concentrations
of androstenedione, testosterone, and dihydrotestosterone can be increased, sometimes dramatically;58,59 and 60 only approximately one-third of reported pregnancy luteomas have been associated with maternal hirsutism or virilization,58,61 probably because any increase in serum free testosterone is limited by the large increase in sex hormone-binding globulin (SHBG) levels that occurs during pregnancy. Typically, luteomas are solid masses ranging between 6 and 10 cm in size; in approximately half of cases, they are bilateral.59, 62 Pregnancy luteomas typically regress promptly after delivery, suggesting that human chorionic gonadotropin (hCG) plays a role in stimulating or perpetuating their androgen production,63 although most are identified late in gestation, long after the peak in maternal serum hCG concentrations. In contrast, functional androgen-producing theca-lutein cysts (hyperreactio luteinalis) can develop in women with multiple pregnancies, isoimmunized or diabetic mothers, and those with molar pregnancies or gestational trophoblastic disease, all of which are associated with increased maternal serum hCG concentrations. Rarely, mothers with pre-existing hirsutism related to PCOS or ovarian stromal hyperthecosis also can develop theca-lutein cysts and become hirsute or virilize.64,65 and 66 In those who do, serum concentrations of testosterone and androstenedione are elevated.
of androstenedione, testosterone, and dihydrotestosterone can be increased, sometimes dramatically;58,59 and 60 only approximately one-third of reported pregnancy luteomas have been associated with maternal hirsutism or virilization,58,61 probably because any increase in serum free testosterone is limited by the large increase in sex hormone-binding globulin (SHBG) levels that occurs during pregnancy. Typically, luteomas are solid masses ranging between 6 and 10 cm in size; in approximately half of cases, they are bilateral.59, 62 Pregnancy luteomas typically regress promptly after delivery, suggesting that human chorionic gonadotropin (hCG) plays a role in stimulating or perpetuating their androgen production,63 although most are identified late in gestation, long after the peak in maternal serum hCG concentrations. In contrast, functional androgen-producing theca-lutein cysts (hyperreactio luteinalis) can develop in women with multiple pregnancies, isoimmunized or diabetic mothers, and those with molar pregnancies or gestational trophoblastic disease, all of which are associated with increased maternal serum hCG concentrations. Rarely, mothers with pre-existing hirsutism related to PCOS or ovarian stromal hyperthecosis also can develop theca-lutein cysts and become hirsute or virilize.64,65 and 66 In those who do, serum concentrations of testosterone and androstenedione are elevated.
Evaluation of Women with Hirsutism
Accepting that the large majority of women with hirsutism have PCOS or idiopathic hirsutism, the evaluation of hirsute women is aimed at identifying the few having other causes that require additional specific evaluation and/or treatment. As always, evaluation should begin with a careful history and physical examination, which always provide important diagnostic clues. Laboratory investigation and imaging are used primarily to exclude other rare or potentially serious possibilities.
History and Physical Examination
The key elements of the medical history in women with hirsutism include the menstrual history and the age at onset and the rate of progression of hirsutism; the family and medication history also provide important information.
The menstrual history should include age at menarche, the regularity of menses, a characterization of premenstrual molimina, and information regarding any previous pregnancies and methods of contraception. Whereas women with PCOS typically report menstrual irregularity beginning at or soon after menarche, an abrupt departure from a previously established pattern of regular menses suggests another diagnosis. Although the clinical presentation of nonclassical CAH can closely resemble that of PCOS, hirsutism tends to be more severe in women with CAH.67,68 A later age at onset of hirsutism (after age 25) or rapid progression over a period of months suggests an androgen producing neoplasm. It is important to correlate changes in menstrual pattern with changes in weight and to remember that previous hormonal contraception could have obscured or delayed the onset of symptoms of menstrual dysfunction or androgen excess. Hirsutism in childhood usually is caused by classical CAH or an androgen-secreting tumor. Rare genetic causes of hirsutism, such as Y-chromosome mosaicism or incomplete androgen insensitivity, usually present with signs of androgen excess at puberty.
A family history of hirsutism, oligo/amenorrhea, obesity, and infertility are consistent with a familial predisposition to PCOS or, occasionally, nonclassical CAH, which is more common in women with Hispanic, Mediterranean, Slavic, or eastern European Jewish (Ashkenazi) heritage.69 Drugs that can stimulate hair growth include methyltestosterone,
anabolic steroids (e.g., norethandrolone), phenytoin, diozoxide, danazol, cyclosporin, and minoxidil. DHEA or androstenedione, which are available as food supplements, can increase testosterone levels in women and cause hirsutism and acne, even at relatively low doses. The hair growth caused by medications, other than androgens, typically is diffuse and vellus in nature (hypertrichosis).
anabolic steroids (e.g., norethandrolone), phenytoin, diozoxide, danazol, cyclosporin, and minoxidil. DHEA or androstenedione, which are available as food supplements, can increase testosterone levels in women and cause hirsutism and acne, even at relatively low doses. The hair growth caused by medications, other than androgens, typically is diffuse and vellus in nature (hypertrichosis).
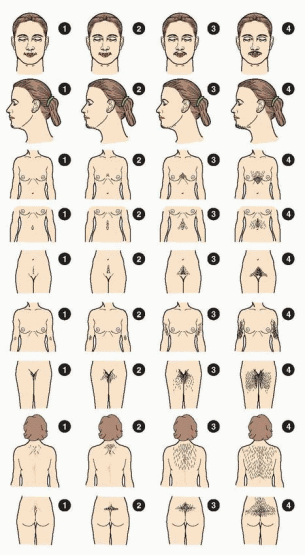
The physical examination should include a calculation of the body mass index (BMI) and document the distribution and extent of hirsutism. The modified Ferriman-Gallwey score is the most common method for grading the extent of hirsutism in clinical investigations.70,71 The method derives from studies in white women and scores hair growth from 0-4 in each of 9 androgen-sensitive areas, including the upper lip, chin, chest, upper and lower abdomen, upper arm, thighs, and the upper and lower back. Scores less than 8, 8-15, and greater than 15 generally indicate mild, moderate, and severe hirsutism, respectively. Approximately 95% of women have a modified Ferriman-Gallwey score less than 8. However, because the distribution of scores is not normally distributed and is skewed far to the left (half having a score of 0), scores of 3 or higher fall outside of the norm. Approximately 22% of women have scores of 3 or higher, 70% of which complain of hirsutism.72 Notably, approximately 15% of women with scores less than 3 also consider themselves hirsute.
Overall, approximately 25% women use some sort of cosmetic treatment for excess hair, such as bleaching, plucking, shaving, waxing, or electrolysis; the frequency of self-treatment correlates positively with the Ferriman-Gallwey score. There are no significant differences between white and black women with regard to the distribution of scores or the proportions that complain of hirsutism or use some method of hair removal.72 Taken together, these data indicate that it is quite normal for most women to have at least some hair growth in androgen-sensitive areas and that a score of 8 or higher reflects significant androgen excess that warrants evaluation. Although the modified Ferriman-Gallwey score is the accepted standard for clinical investigations involving hirsute women, it is difficult to use clinically, primarily because most women who seek medical attention for the complaint already are using one or more methods of hair removal. Moreover, the score is unreliable for women in racial or ethnic groups having relatively little body hair; although less likely to develop hirsutism, they can exhibit other signs of androgen excess, such as acne and thinning or loss of hair. The easiest and most practical way to assess the severity of hirsutism is to determine the methods used to remove hair (e.g., shaving, plucking, waxing) and the frequency of their use, which also provides a clinically relevant measure for assessing the response to treatment.
The physical examination also should note other relevant skin manifestations and any signs of virilization. Acne, seborrhea, and temporal balding are signs of androgen excess. Acanthosis nigricans (a gray or brown velvety discoloration of the skin, most commonly observed at the nape of the neck, the groin and axillae) indicates insulin resistance, and thin skin, striae, or bruising are signs of hypercortisolism. In addition to frontal or crown balding, signs of virilization include deepening of the voice, increased muscle mass, breast atrophy, and clitoromegaly. Clitoral size varies significantly among women73; in one study, the mean length of the glans clitoris was 5.1 ± 1.4 mm and the mean width was 3.4 ± 1.0 mm.74 Clitoromegaly generally is defined by a clitoral length greater than 10 mm or by a clitoral index (length times width) greater than 35 mm2,75 Other relevant physical findings include spontaneous or expressible galactorrhea, suggesting hyperprolactinemia, and abdominal or pelvic masses that may represent an androgen-secreting tumor. The large majority of functional ovarian tumors are palpable.
The physical manifestations of androgen excess generally reflect the extent to which androgen levels are elevated. Hirsutism is the most common complaint associated with androgen excess and essentially all women with hirsutism have an increased production rate of testosterone and androstendione.76 Acne, increased libido, clitoromegaly, and virilization reflect progressively higher serum androgen levels.
 |
Alopecia can be a vexing problem for both patient and clinician. In many cases, alopecia is only temporary, resulting from telogen effluvium induced by some transient change that synchronizes a larger than normal proportion of scalp hair follicles, such as pregnancy or a febrile illness, and resolves after a period of 6-8 months. In a series of 109 consecutive women presenting with a complaint of diffuse alopecia, two-thirds had no clinical evidence of hirsutism or menstrual dysfunction, two had nonclassical CAH, and two had hyperprolactinemia associated with a pituitary adenoma.77 Of the 42 (38.5%) that had elevated serum androgens, 11 were ovulatory and not hirsute, 13 were ovulatory and hirsute, and 18 had both
oligomenorrhea or amenorrhea and hirsutism.77 Women with complaints of alopecia deserve evaluation for hyperandrogenism that can be treated successfully. Laboratory evaluation also should exclude thyroid disorders (serum TSH) and chronic illness. However, because alopecia can reflect increased scalp 5α-reductase activity, normal circulating androgen levels do not necessarily preclude effective treatment.78,79 Up to 60% of women with acne and normal serum androgen concentrations exhibit evidence of increased peripheral 5α-reductase activity and may benefit from treatment for hyperandrogenism.80 Hair loss also is a normal consequence of aging, beginning in both sexes around the age of 50 years.81
oligomenorrhea or amenorrhea and hirsutism.77 Women with complaints of alopecia deserve evaluation for hyperandrogenism that can be treated successfully. Laboratory evaluation also should exclude thyroid disorders (serum TSH) and chronic illness. However, because alopecia can reflect increased scalp 5α-reductase activity, normal circulating androgen levels do not necessarily preclude effective treatment.78,79 Up to 60% of women with acne and normal serum androgen concentrations exhibit evidence of increased peripheral 5α-reductase activity and may benefit from treatment for hyperandrogenism.80 Hair loss also is a normal consequence of aging, beginning in both sexes around the age of 50 years.81
Laboratory Evaluation
The relationship between hirsutism and circulating androgen concentrations is not entirely clear. Whereas some studies have found a correlation between hirsutism and androgen levels,82 others have observed that only 50% of women with mild hirsutism have elevated free testosterone levels, and that 33% of women with moderately elevated free testosterone concentrations have no hirsutism, 40% have mild, and 27% have moderate hirsutism.83 These observations suggest strongly that other factors, such as insulin and individual variations in androgen sensitivity, have substantial influence on the development and severity of hirsutism.
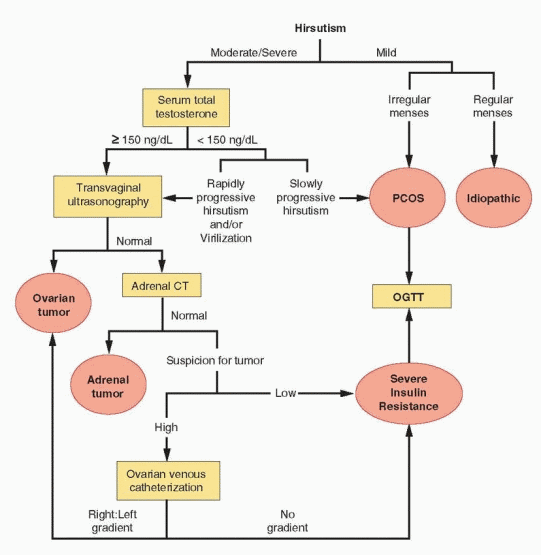
Laboratory evaluation is indicated for many but not all women with hirsutism.84 The primary aim is to identify those having potentially serious endocrine disorders requiring specific treatment (nonclassical CAH, androgen-secreting tumors, Cushing syndrome). Thyroid disorders and hyperprolactinemia should be excluded in women with menstrual dysfunction. Laboratory evaluation is recommended for women with moderate or severe hirsutism, or hirsutism that is sudden in onset, rapidly progressive, or associated with symptoms or signs of virilization.85 Routine laboratory evaluation of women with mild hirsutism is neither necessary nor cost-effective. In women with oligo/amenorrhea, mild hirsutism can be attributed confidently to increased ovarian androgen production resulting from chronic anovulation. In women with regular menses, hirsutism most likely reflects an increased sensitivity to androgens relating to increased peripheral 5α-reductase activity.
The serum total testosterone concentration provides the best overall measure of androgen production and is the only hormone that need be measured in most women with hirsutism who merit evaluation. Testing for nonclassical CAH can be safely reserved for patients with an early onset of hirsutism (pre- or peri-menarcheal onset, including those with premature adrenarche), women with a family history of the disorder, and those in high-risk ethnic groups (Hispanic, Mediterranean, Slavic, or Ashkenazi Jewish heritage). Additional evaluation also is indicated for those with hirsutism having onset before puberty or after age 25, rapidly progressive hirsutism, or hirsutism that is accompanied by signs of virilization or hypercortisolism (Cushing syndrome).
The Serum Testosterone Concentration
Serum testosterone levels (normal 20-80 ng/dL) are elevated in most (70%), but not all, women with chronic anovulation and hirsutism. The total testosterone concentration can be normal in hirsute women because SHBG levels are depressed by androgen and insulin, thereby increasing the amount of unbound or free testosterone. Indeed, free testosterone levels are approximately twice normal (an increase from 1% to 2%) in women with PCOS.86
Laboratory testing for elevated androgen levels should begin with a serum total testosterone concentration. Total testosterone assays measure free testosterone, albuminbound testosterone, and testosterone bound to SHBG. Although the free testosterone level is a more sensitive indicator of androgen excess, direct immunoassays of free testosterone are inaccurate,87 yielding values only 20-60% of those measured by other, more precise methods.88,89 The best method for measuring the free testosterone level is equilibrium dialysis (a laborious, time-consuming, and costly method).87 The free testosterone concentration also can be calculated using equations derived from the laws of mass action, knowing the serum total testosterone, SHBG, and albumin concentrations, and the association constants for the interactions of testosterone with SHBG and albumin.90,91 Calculated values generally correlate well with those determined by equilibrium dialysis, although accuracy varies with the specific assays used to measure total testosterone and SHBG. However, measurement or calculation of the free testosterone level generally is unnecessary, because the total testosterone level readily identifies women who may have an androgen-producing tumor.
A serum total testosterone concentration greater than 150 ng/dL identifies almost all women with a potential androgen-producing tumor.35,51,52,54,92,93 However, because serum testosterone concentrations can vary significantly in women with and without tumors,52 a tumor still should be suspected, and excluded, in women with rapidly progressive hirsutism or signs or symptoms of virilization, even when the serum testosterone concentration is below the threshold value. Nearly all women with PCOS have a testosterone level less than 150 ng/dL, as do all women with idiopathic hirsutism, by definition. The suggested threshold value has very high sensitivity and negative predictive value, indicating that it captures virtually all women with tumors and can effectively exclude the diagnosis.93 The positive predictive value of a serum total testosterone greater than 150 ng/dL is quite low, indicating that few women who meet the criterion will, in fact, have a tumor, primarily because such tumors are very rare; the large majority will have PCOS or hyperthecosis. Taken together, the clinical history (age at onset and rate of progression of hirsutism), physical examination (pelvic masses), and the serum total testosterone concentration will identify women with androgen-producing tumors.
It also is important to remember that testosterone levels are elevated significantly during normal pregnancy. Concentrations are greater than 100 ng/dL during the first trimester and can reach 500-800 ng/dL by term,94 primarily due to the estrogen-induced increase in SHBG. Mother and fetus normally are protected from virilization because free testosterone levels rise only modestly and are rapidly converted to estrogen via placental aromatization. Because testosterone levels normally are lower in postmenopausal women, concentrations greater than 100 ng/dL should raise suspicion for a tumor.
The Serum DHEA-S Concentration
DHEA-S circulates in higher concentration than any other steroid and derives almost exclusively from the adrenal gland. It is, therefore, a direct measure of adrenal androgen activity. The upper limit of normal in most laboratories is approximately 350 mg/dL, but ranges vary among laboratories. DHEA-S serves primarily as a pre-hormone, providing substrate for conversion to testosterone and dihydrotestosterone in the periphery.95
Although the serum DHEA-S concentration would seem useful for identifying women with adrenal causes of hyperandrogenism, the test lacks both sensitivity and specificity for that purpose. DHEA-S levels frequently are not grossly elevated in women with nonclassical CAH or Cushing syndrome, and often are elevated in women with PCOS. Moreover, diagnosis of nonclassical CAH and Cushing syndrome requires other, more specific tests, as discussed below.
The serum DHEA-S concentration is moderately elevated in over half of women with PCOS.96 The reasons remain unclear, despite extensive investigation. Some have argued that the increase in DHEA-S levels results from a 3b-hydroxysteroid dehydrogenase deficiency, induced by chronic anovulation and estrogen stimulation, similar to the mechanism that operates in the fetal adrenal cortex.97,98 Whereas there are data to support such a mechanism,99,100,101,102 and 103 evidence is conflicting.104,105,106,107 and 108 Notably, ACTH levels are not elevated in women with PCOS,109,110 exaggerated adrenal androgen secretion cannot be attributed to any increase in sensitivity to ACTH,96 and ovarian (and estrogen) suppression by treatment with a long-acting gonadotropin-releasing hormone (GnRH) agonist has no consistent effect on DHEA-S levels in women with PCOS.111,112,113 and 114 Although increased adrenal P450c17 17,20 lyase activity could cause adrenal androgen excess,114,115 and 116 the patterns of steroidogenic response to ACTH stimulation in women with PCOS do not support the hypothesis.117 The prevalence of adrenal androgen excess is comparable to that of insulin resistance among women with PCOS, suggesting that hyperinsulinemia might be the cause of increased adrenal androgen production.6 However, insulin infusion studies indicate that insulin does not stimulate, and actually impairs, 17,20 lyase activity in both normal and hyperandrogenic women.118,119 and 120 In sum, no one mechanism explains the moderate adrenal androgen excess commonly observed in women with PCOS.
The serum DHEA-S concentration can be grossly elevated (≥700 mg/dL) in women with rare androgen-secreting adrenal tumors. However, in almost all such patients, serum testosterone levels also are greatly elevated,121 via peripheral conversion of high circulating DHEA-S levels, or because the tumor also secretes testosterone. A serum DHEA-S concentration may be useful in women whose clinical presentation suggests strongly the possibility of a tumor, but the test otherwise has little or no clinical utility in the evaluation of hirsutism.
Evaluation for a Suspected Androgen-Producing Tumor
When the serum total testosterone concentration (≥ 150 ng/dL) or the clinical presentation suggests the possibility of a rare androgen-producing ovarian or adrenal tumor (rapidly progressive hirsutism or symptoms or signs of virilization), evaluation is indicated to exclude the diagnosis or localize the lesion.
Androgen-secreting tumors of the ovary (in decreasing order of prevalence) include Seroli-Leydig tumors, lipid-cell tumors, hilar-cell tumors, and rare androgen-producing theca-cell and Brenner tumors; virtually all are associated with grossly elevated serum total testosterone levels (≥ 150 ng/dL). Occasionally, virilization results from a nonfunctioning tumor due to stimulation of the surrounding stroma.122 Most functioning ovarian tumors are palpable on pelvic examination, but small tumors easily can go unrecognized. Transvaginal ultrasonography can identify ovarian follicles and cysts as small as 3-5 mm in diameter and almost all solid ovarian mass lesions, although very small tumors located in the hilar region still can escape detection.
Adrenal CT imaging is extremely sensitive for detecting the rare androgen-producing adrenal adenoma or carcinoma, when pelvic examination and transvaginal ultrasonography fail to reveal an ovarian tumor.123 Most androgen-secreting adrenal tumors are malignant.124,125 and 126 Adrenal adenomas typically are smaller (<4 cm in diameter) than carcinomas and have smooth borders and characteristically low unenhanced CT attenuation values; irregular margins, necrosis, hemorrhage, or calcification suggest a carcinoma.127 When needed, additional information to help define the nature of an adrenal mass lesion can be obtained
by magnetic resonance imaging (MRI), by functional nuclear imaging with scintigraphy using a labeled cholesterol analog (131I-6-iodomethyl norcholesterol),128 or positron emission tomography (PET) scanning.
by magnetic resonance imaging (MRI), by functional nuclear imaging with scintigraphy using a labeled cholesterol analog (131I-6-iodomethyl norcholesterol),128 or positron emission tomography (PET) scanning.
Findings of bilateral disease require further evaluation to distinguish among the causes, which include metastatic cancer (most commonly from breast, kidney, or lung), adrenal hyperplasia (caused by long-term stimulation by pituitary or ectopic sources of ACTH and by rare forms of ACTH-independent macro- and micronodular disease),129,130 infection (tuberculosis and fungal), hemorrhage, pheochromocytoma, and amyloidosis.
Routine adrenal imaging is not recommended and can be misleading, because nonfunctioning adrenal masses (incidentalomas) are common and their incidental detection demands additional, otherwise unnecessary, evaluation.131 In autopsy studies, the prevalence of incidental adrenal adenomas approaches 10%.132,133 In 2 case series of patients having abdominal CT scanning for a variety of indications, the prevalence of adrenal incidentaloma was 3-4%.134, 135
Incidentally detected adrenal masses require evaluation to determine whether they are functional; up to 15% secrete excess hormones, such as cortisol, catecholamines, and aldosterone.136 The requisite tests include a 24-hour urine collection for fractionated metanephrines and catecholamines (pheochromocytoma), blood samples for fractionated metanephrines, testosterone and DHEA-S (adrenal carcinoma), plasma aldosterone and rennin activity (primary aldosteronism), and an overnight dexamethasone suppression test (Cushing syndrome).131 Fine-needle aspiration (FNA) biopsy may be indicated when there is reason to suspect a malignancy outside of the adrenal gland or in patients undergoing staging evaluation for a known cancer.133,137 Although FNA is a relatively safe procedure, potential complications include adrenal hematoma and abscess, abdominal pain, hematuria, pancreatitis, and pneumothorax.138,139 Because inadvertent FNA of a pheochromocytoma can precipitate an acute hypertensive crisis, the diagnosis always should be excluded by biochemical tests before FNA is performed.140 When testing detects no evidence of hormone function and there is no reason to suspect a cancer, expectant management is appropriate; current recommendations include repeated imaging after 6, 12, and 24 months (to detect evidence of progressive growth), and repeated endocrine evaluation annually (to detect autonomous function not identified at baseline) for at least 4 years.131, 141, 142
Selective ovarian venous catheterization can be considered for the rare patient having no demonstrable ovarian or adrenal mass lesion.53,92,143,144 and 145 However, because the overall clinical utility of the technique is still uncertain,146 the procedure should be reserved only for those in whom a tumor is strongly suspected. An analysis of results obtained in 136 reported patients with hirsutism who had selective ovarian venous sampling yielded a number of important observations.146 A right:left ovarian venous effluent testosterone ratio greater than 1.44 correctly identified 90% of right-sided tumors, and lower values correctly identified 86% of women with left-sided or bilateral lesions. In three women with a left-sided tumor, the left:right testosterone ratio was greater than 15. The differing anatomy of the right (draining into the vena cava) and left ovarian vein (draining into the left renal vein) and the related technical difficulty of catheterization might explain why venous sampling was more effective for identifying right-sided tumors.
When suspicion for a tumor is insufficient to warrant ovarian venous catheterization, or the procedure reveals no significant gradient in testosterone concentrations, the likelihood of an occult ovarian tumor is very small, leaving the HAIR-AN syndrome or stromal hyperthecosis as the most likely cause of severe hyperandrogenism. Both are associated with severe insulin resistance, which can be documented by performing an oral glucose tolerance test including insulin levels, as discussed below. Rarely, open surgical exploration of
the ovaries may be necessary to establish a diagnosis; laparoscopic inspection and biopsy are not sufficient.
the ovaries may be necessary to establish a diagnosis; laparoscopic inspection and biopsy are not sufficient.
Dynamic endocrine evaluation, using dexamethasone, contraceptive steroids, or a GnRH agonist in attempts to isolate adrenal or ovarian androgen production, is not recommended, because results are unreliable and can be misleading.122,147,148 and 149 Ovarian androgen-secreting tumors are sensitive to LH stimulation and thus respond to ovarian suppression and stimulation.150,151 and 152
Insulin Resistance
Insulin resistance is a common feature of women with PCOS and a key component of the HAIR-AN syndrome and ovarian stromal hyperthecosis. Although high circulating androgen concentrations decrease insulin sensitivity, the primary pathology in women with HAIR-AN and hyperthecosis is severe insulin resistance, resulting in grossly elevated insulin levels that stimulate ovarian androgen production in theca cells (via insulin, IGF-1, and hybrid receptors) and markedly decrease SHBG production, thereby greatly increasing the amount of free androgen. Insulin resistance and hyperinsulinemia also explain the occasional elderly woman who presents with severe progressive hirsutism. The problem does not reflect an ovarian response to elevated gonadotropin levels, but the development of hyperinsulinemia and hyperthecosis. Insulin appears to have a direct effect on the severity of hirsutism and a synergistic interaction with testosterone.153
The numerous methods that can be used to assess insulin sensitivity are discussed in the chapter devoted to chronic anovulation and PCOS (Chapter 12) and are summarized only briefly here. The hyperinsulinemic euglycemic clamp technique is the gold standard method for measuring insulin sensitivity but has no practical clinical application because it is time-consuming, labor-intensive, invasive, costly, and requires experienced personnel.154 A number of less complicated, inexpensive, “homeostatic” measures have been described, all based on the fasting glucose and insulin concentrations and using straightforward calculations.155 These include the fasting insulin concentration,156 the fasting glucose/insulin ratio,157 the homeostatic model assessment (HOMA),158,159 the quantitative insulin sensitivity check index (QUICKI),160 and others. As the sheer number of different measures of insulin resistance illustrates, there is currently no uniformly accepted, validated, simple test for measuring insulin resistance in clinical practice. All of the measures have limitations, primarily the lack of a standardized insulin assay,161 and the absence of data indicating that such measures can predict the response to treatment. Moreover, treatment with insulin-sensitizing agents has no important benefits for the treatment of hirsutism.162 Consequently, routine assessment of insulin sensitivity in the evaluation of hirsutism is not recommended.
A baseline 2-hour oral glucose tolerance test (75-g glucose load) is recommended for all women with PCOS,33,163,164 because up to 35% exhibit impaired glucose tolerance (glucose 140-199 mg/dL) and up to 10% have non-insulin-dependent diabetes mellitus (glucose ≥ 200 mg/dL).165 In patients with severe hyperandrogenism having no evidence of an androgen-secreting tumor, the corresponding fasting and 2-hour insulin concentrations can be used to document the degree of insulin resistance, in support of the diagnosis of HAIR-AN syndrome or hyperthecosis; most have grossly elevated insulin levels.40 The 2-hour glucose/insulin ratio (mg/dL/ μU/mL) provides an estimate of insulin sensitivity, with values less than 1.0 indicating insulin resistance. Plasma insulin concentrations that exceed an upper limit of normal or a defined threshold value (e.g., a 2-hour plasma insulin > 100 mU/mL) also have been used as a qualitative test for insulin resistance.
 |
Nonclassical Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia (CAH) is caused by adrenal steroidogenic enzyme defects that result in excessive adrenal androgen production. By far, the most common cause is 21-hydroxylase deficiency; other enzyme defects (e.g., 11β-hydroxylase, 3β-hydroxysteroid dehydrogenase) are relatively rare. In all, the pathophysiology relates primarily to decreased cortisol production, which stimulates a compensatory increase in pituitary ACTH secretion, causing adrenal hyperplasia; increased levels of steroid hormones proximal to the enzyme block seek an alternative metabolic pathway, resulting in increased production of androgens. The disorder is inherited in an autosomal recessive fashion and is discussed in detail in Chapters 9 and 10.
Females with classical CAH (both salt-wasting and simple virilizing forms) present at birth with ambiguous genitalia (adrenogenital syndrome).166,167 and 168 Those with the nonclassical (“late-onset”) form of CAH have normal external genitalia and present later, during childhood or early adolescence, with precocious puberty or as young adults with other signs of hyperandrogenism, such as acne, hirsutism, and menstrual irregularity, very much like those with PCOS.
Whereas it would seem that nonclassical CAH should be excluded specifically in all women with hirsutism, the yield from routine testing is quite low, because the disorder is
uncommon.31,36,169 In the United States, the prevalence of the disorder among white women who present with hirsutism is between 1% and 4%.170 Therefore, specific testing for nonclassical CAH can be reserved for those having an early onset of hirsutism (pre- or perimenarcheal, including girls with premature adrenarche), women with a family history of the disorder, and those in high-risk ethnic groups (Hispanic, Mediterranean, Slavic, or Ashkenazi Jewish heritage).
uncommon.31,36,169 In the United States, the prevalence of the disorder among white women who present with hirsutism is between 1% and 4%.170 Therefore, specific testing for nonclassical CAH can be reserved for those having an early onset of hirsutism (pre- or perimenarcheal, including girls with premature adrenarche), women with a family history of the disorder, and those in high-risk ethnic groups (Hispanic, Mediterranean, Slavic, or Ashkenazi Jewish heritage).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


