Hematologic Abnormalities and Jaundice
Henry C. Lee and Ashima Madan
RED BLOOD CELL DISORDERS
Hematologic problems arise frequently in the newborn period.1 This section is focused on disorders specific to the newborn period. For further discussion of the developmental changes that occur in hematopoiesis, see Chapter 429. Hematologic disorders, including congenital disorders, are discussed in detail in Section 23: Disorders of the Blood.
NUCLEATED RED BLOOD CELLS
Nucleated red blood cells (nRBCs) are not common in older children but can be found frequently in the peripheral blood smear of newborns.8 They represent circulating erythrocyte precursors, or normoblasts, which are normally found in the bone marrow. Healthy term newborns may have some nRBCs in the peripheral blood. However, elevated nRBCs may represent the result of pathologic processes such as relative in utero hypoxia resulting in increased erythropoietin, which stimulates increased production of red blood cells. Although many laboratories report nRBCs in relation to the number of white blood cells (nRBCs/100 WBC), it is probably more informative to express this as an absolute number per unit volume (ie, nRBCs/mm3). A value of 1000 nRBCs/mm3 may be considered the higher limit of normal for a normal term newborn. Preterm newborns typically have higher nRBCs at birth.
An increase in the number of circulating nRBCs can represent conditions of increased erythropoiesis or stress-mediated release of normoblasts from the bone marrow. Increased erythropoiesis may occur in times of chronic hypoxia as in preeclampsia and placental insufficiency. Infants born to smoking mothers have been found to have increased nRBCs.9 Increased erythropoiesis may result from blood loss from any cause, including hemolysis, which occurs in the context of red blood cell isoimmunization. Elevation of nRBCs also occurs in infants of diabetic mothers and in congenital infections.
Hypoxia does not have to be chronic to observe nRBC elevation at birth. Acute stress and subacute stress are also associated with increased circulating nRBCs. Elevated nRBCs in this context may serve as a marker for fetal asphyxia.10,11 Increased numbers of nRBCs correlates with lower umbilical cord pH and therefore may reflect degree and duration of asphyxia.12,13 Chronic asphyxia is typically associated with higher nRBCs than with acute asphyxia events.14
ANEMIA
The mean hemoglobin concentration in the term newborn ranges from 14 to 20 g/100 mL.15 In the first hours after birth, the hemoglobin concentration can rise due to a relative reduction in plasma volume. However, the hemoglobin level soon begins to decline, reaching a “physiologic nadir” at around 2 to 3 months of age. Preterm newborns have lower hemoglobin concentrations at birth and a larger decrease after birth, reaching a nadir at an earlier age, as early as 6 weeks for very-low-birth-weight infants.16 The causes of anemia in the newborn period include increased blood loss or decreased production of red blood cells.
Blood loss may occur before, during, or after birth. Fetal-to-maternal hemorrhage can be detected by the observation of fetal red blood cells in maternal blood by the Betke-Kleihauer test.17 Twin-to-twin transfusion can occur in monozygous twins with monochorionic placentas. This should be suspected when the hemoglobin concentration differs by more than 5 g/100 mL. Placental abruption can be chronic or acute in nature and, when severe, can result in morbidity or death in the absence of rapid resuscitation. Other placental disorders that can lead to significant hemorrhage include placenta previa, in which the placenta implants directly over the cervix and vasa previa, when fetal blood vessels overlie the cervical opening unprotected by the placenta or umbilical cord. Both conditions carry high risk of hemorrhage with vaginal delivery, and optimal outcomes depend on prenatal diagnosis and cesarean delivery.18
Closed hemorrhage into body spaces, which can serve as significant reservoirs of blood, may be associated with birth trauma in the newborn. This is more common in preterm infants. Cephalohematomas are generally self-limited and resolve over several days; larger hematomas may lead to anemia and jaundice. Subgaleal hemorrhage, which can be associated with vacuum extraction, may lead to more extensive anemia because bleeding is not limited by the periosteum. Caution and close monitoring are warranted because shock and coagulopathy may ensue.19 Other areas of “hidden” bleeding include the liver, spleen, adrenal glands, and retroperitoneum. Unexplained symptoms of hypovolemic shock may warrant exploration with abdominal ultrasound.
Anemia can result from blood loss through hemolysis, which produces hyperbilirubinemia and jaundice. Hemolysis may be immune mediated when maternal antibodies are directed against antigens such as those in the Rh or ABO blood groups. Although the Rh(D) antigen has been implicated as causing the most severe cases, hemolytic disease can occur with other “minor” antigens such as the Kell, Duffy, Kidd, and other Rh(c) and Rh(E) antigens. Other causes of hemolysis include membrane defects such as hereditary spherocytosis or elliptocytosis, hemoglobin-opathies such as the thalassemia syndromes, and enzyme abnormalities such as pyruvate kinase deficiency and glucose-6-phosphate dehydrogenase deficiency.20
ERYTHROBLASTOSIS FETALIS
Erythroblastosis fetalis was first described in 1932 as a distinct condition comprising anemia, universal edema of the fetus (hydrops fetalis), and neonatal jaundice.21 The most important of the Rh membrane proteins is the D antigen. Mothers who lack the D antigen are designated as Rh-negative. When blood cells from an Rhpositive fetus leak into the maternal circulation, maternal sensitization to the antigen occurs with subsequent passage of anti-D antibodies into the fetal circulation with resulting hemolysis. Disease is rare during the first pregnancy, but with each subsequent pregnancy, there is increased risk of significant disease.
The incidence of severe erythroblastosis fetalis has decreased substantially with the use of anti-D immune globulin given to Rh-negative mothers who have not yet been sensitized.22,23 Administration of immune globulin should occur at 28 weeks, and an additional dose should be given after birth of an Rh-positive infant.
Mothers who have developed a significant antibody response need close fetal monitoring for possible intervention. Spectrophoto-metric estimation of bile pigment in the amniotic fluid can provide an estimate of the severity of disease.24 Ultrasound has more recently allowed for earlier detection of hydrops and also direct measurement of the fetal hemoglobin level by percutaneous umbilical blood sampling (PUBS).25 This technique also allows for intrauterine blood transfusion in cases of severe fetal anemia.
There is a spectrum of clinical manifestation in Rh hemolytic disease. For mild cases, there may be mild to moderate hyperbilirubinemia after birth. However, even those infants should have hemoglobin monitored after discharge because they are at risk of progressive anemia. In moderate to severe disease, exchange transfusion may be required if phototherapy is not sufficient. In severe cases, affected infants may be significantly hydropic and stillborn.
POLYCYTHEMIA
Neonatal polycythemia is usually defined as a central venous hematocrit greater than 65%, although blood flow may be impaired at a hematocrit as low as 60%. However, most infants with a high hematocrit are asymptomatic. Infants with increased blood viscosity from polycythemia and resulting organ dysfunction from impaired blood flow have hyper-viscosity syndrome.
The incidence of neonatal polycythemia is reported as between 1% to 5% of newborns and depends on the altitude of the study population.15 Polycythemia is less frequent in pre-term births due to lower red blood cell mass.
Neonatal polycythemia is usually caused by either increased intrauterine erythropoiesis or increased transfusion to the fetus or newborn just prior to ligation of the umbilical cord. Conditions associated with intrauterine hypoxia, such as placental insufficiency and maternal smoking, increase the risk of polycythemia. Newborns of mothers with hypertension are also at risk for polycythemia, even outside the context of intrauterine growth restriction.26 In cases of twin-to-twin transfusion syndrome in monochorionic twin pregnancies, the recipient twin is at higher risk of polycythemia.
The hematocrit generally peaks 2 hours after delivery and declines to cord levels at around 12 to 18 hours of age.27 Although capillary hematocrit may suffice as a screening tool, a venous hematocrit should be obtained to confirm the diagnosis and support treatment decisions.27,28
Hyperbilirubinemia is likely to occur from breakdown of the increased red blood cell mass, and therefore, the development of jaundice should be monitored closely. Hypoglycemia may accompany polycythemia and may be aggravated by conditions such as diabetes mellitus and placental insufficiency. Additional organ dysfunction associated with polycythemia and hyperviscosity include neurological symptoms such as hypotonia, irritability, and lethargy, tachypnea and respiratory distress, feeding difficulty, and thrombocytopenia.
Polycythemic infants have an increase in red cell volume but normal plasma volume. Therefore, the recommended treatment is isovolemic partial exchange transfusion.29 The few randomized controlled trials evaluating partial exchange transfusion have been small in size and have not demonstrated a significant impact on long-term outcomes.30 Nevertheless, symptomatic infants with a venous hematocrit above 65% to 70% may benefit from prevention of ongoing injury with partial exchange transfusion. The exchange can best be accomplished by insertion of an umbilical venous catheter. The volume to be exchanged can be calculated using this formula:
volume (mL) = circulating blood volume × [(Hct current − Hct desired)/Hct current]
where the circulating blood volume can be estimated as 90 mL/kg times weight for term infants and 100 mL/kg times weight for preterm infants.31 No significant benefit has been seen for any particular dilution fluid whether it be albumin, plasma, or crystalloid.31 Therefore, normal saline should be used as the dilution fluid because it is low risk and low cost compared to blood-derived products. Glucose and calcium should be monitored during and after the exchange transfusion.
WHITE BLOOD CELL DISORDERS
NEUTROPENIA
Neutropenia is often seen in the newborn period and may be due to decreased production or increased destruction of white cells. It is commonly seen in infants born to mothers with pregnancy-induced hypertension. This neutropenia can be severe with an absolute neutrophil count less than 500/μL but is generally transient and resolves without any specific treatment in the first 3 to 5 days after birth.32,33 There is usually an absence of a left shift in the white blood cell count.34
The neutropenia seen in neonatal sepsis syndromes is a result of accelerated neutrophil use and depleted bone marrow neutrophil storage pools. There is usually a left shift and other morphologic characteristics, such as toxic granulation, vacuolization, and Döhle bodies.32 This neutropenia is also transient, and clinical improvement is generally accompanied by resolution.
When neutropenia lasts more than several days, rarer causes should be investigated. Infants with congenital neutropenia (Kostmann syndrome) have profound neutropenia, often with an absolute neutrophil count of less than 200/μL. There is arrest at the promyelocyte stage in the bone marrow. Kostmann syndrome was initially described in a family with autosomal recessive inheritance, but the majority of other reported cases are sporadic mutations with an autosomal dominant inheritance.32,35 Congenital neutropenia can also be related to immune-mediated destruction with antibodies from either the mother or infant.36 Alloimmune neonatal neutropenia is caused by maternal sensitization to fetal neutrophil antigens. Maternal antibodies are directed against the infant’s neutrophils. This is analogous to alloimmune thrombocytopenia or Rh disease. Neutropenia can last for weeks, and infants are at risk for infection. Possible therapies include intravenous immune globulin, infusion of granulocytes that lack the specific antigen, and granulocyte colony-stimulating factor. For further discussion of granulocyte disorders see Chapter 441.
LEUKEMIAS
Although leukemia is an uncommon disorder in the neonatal period, it is the leading cause of death due to neoplastic disease in the neonate. The characteristics of leukemia in the newborn period also differ from leukemia in later childhood, with unique chromosomal rearrangements and differing prognoses.37 Trisomy 21 and 11q23 translocations are the most common chromosomal alterations seen in association with neonatal leukemias. In general, neonatal leukemia carries a grave prognosis, although recent use of multiagent chemotherapy has been promising.37,38 Acute myelocytic leukemia is more common than acute lymphocytic leukemia.38,39
Leukemia can present as early as in utero with hydrops and polyhydramnios, leading in some instances to stillbirth. Some neonates may present at birth, while others may appear normal and develop hematologic symptoms over the first weeks after delivery. Nodular cutaneous infiltrates or “blueberry muffin spots” may be evident, particularly in acute myelocytic leukemia.37 Other signs and symptoms include hepatosplenomegaly, anemia, and bleeding secondary to thrombocytopenia.
Children with trisomy 21 are at risk for development of leukemia, including a unique form that is seen in the newborn period and can resolve spontaneously without chemotherapy. This condition is called transient leukemia or transient myeloproliferative disorder and affects approximately 10% of newborns with trisomy 21.40 Although it shares many of the features of other leukemias, including varying degrees of hepatosplenomegaly, megakaryoblastic cells in the blood and marrow, thrombocytopenia, and anemia, there is a spontaneous regression over several months. However, some patients are at risk of severe disease and even death in the neonatal period from sepsis, hepatic fibrosis, and cardiopulmonary failure.41 Furthermore, even after spontaneous resolution, a significant proportion will eventually develop another hematologic disorder, most commonly acute megakaryoblastic leukemia.
PLATELET AND COAGULATION DISORDERS
THROMBOCYTOPENIA
Thrombocytopenia in the newborn can be classified as mild (100−150 × 109 platelets/L), moderate (50−100 × 109 platelets/L), and severe (< 50 × 109 platelets/L). Although some petechiae and bruising may be present over the presenting part secondary to pressure during the normal birth process, the platelet count should be checked when more than a few petechiae are present or in any sick infant. Thrombocytopenia occurs frequently in infants admitted to the neonatal intensive care unit: more than 20% of infants compared to less than 1% of healthy newborns.42-44 The low platelet counts seen in sick newborns reflects the severity of illness and resolves as the underlying condition, often sepsis, improves. Thrombocytopenia in a premature infant can be an early sign of sepsis or necrotizing enterocolitis. For patients in the intensive care unit, thrombocytopenia may also be caused as a side effect of medications such as heparin and ranitidine, although it is sometimes difficult to distinguish whether thrombocytopenia is due to medications used to treat sick infants or to the underlying condition.45 Indomethacin, used to treat patent ductus arteriosus, inhibits platelet function, and platelet count should be monitored during its use.
Immune destruction of platelets can occur from maternal antibodies. Mothers with autoimmune thrombocytopenia or (idiopathic thrombocytopenic purpura) can pass antiplatelet IgG through the placenta. Severe thrombocytopenia in infants is rare in these cases but, when present, can be treated with intravenous immune globulin and corticosteroids. However, alloimmune thrombocytopenia, which occurs when maternal antibodies form against fetal platelet antigens, can be associated with severe presentations, including intracranial hemorrhage and even in utero death.46 Potential prenatal therapies include intravenous immune globulin and corticosteroids administered to the mother, as well as in utero platelet transfusions. In Caucasians, human platelet antigen (HPA)-1a is the most common antigen, whereas in Asians, HPA-4 is more common.46 Diagnosis can be made by platelet typing of both parents and the infant. Testing for antiplatelet antibodies should be performed in the baby and the mother because it may be falsely negative in the baby. Cranial ultrasound should be obtained to check for hemorrhage. When platelet transfusion is necessary, washed maternal platelets or HPA-compatible platelets from the blood bank can be used. Intravenous immune globulin may also be used as adjunctive therapy.
Thrombocytopenia can be seen as a result of megakaryocyte disruption in fetal acidosis or hypoxia and in mothers with preeclampsia. Other causes include infection, both bacterial and in congenital viral infections, such as cytomegalovirus, toxoplasmosis, and rubella.44 Various syndromes that can present in the newborn period and are associated with thrombocytopenia include trisomy 13, 18, and 21; Fanconi anemia; and Bernard-Soulier syndrome. Thrombocytopenia absent radii syndrome is a rare, autosomal recessive disorder, presenting with severe thrombocytopenia and bilateral agenesis of radii. Platelet sequestration occurs as a result of localized disseminated vascular malformations in Kasabach-Merritt syndrome.
COAGULATION DISORDERS
Coagulation disorders can present in the newborn period as petechiae, purpura, or internal hemorrhage. Laboratory evaluation can be challenging due to the immature development of the hemostatic factors in newborns, and reference values for older children and adults may not be applicable for tests such as prothrombin time and activated partial thromboplastin time.47,48 Furthermore, premature infants will have higher values than term infants, and different laboratory reagents and systems can have differing results.49 These laboratory “abnormalities” are generally not associated with increased bleeding in most instances.
Much of the hemorrhagic problems occurring in the nursery are secondary to underlying illness such as sepsis and/or disseminated intravascular coagulation. Bleeding from vitamin K deficiency, previously known as hemorrhagic disease of the newborn, is due to decreased vitamin K levels after birth, which results in impaired carboxylation of vitamin K–dependent clotting factors, making them functionally inactive.50 Vitamin K deficiency is suggested by prolonged prothrombin time and activated partial thromboplastin time, in association with normal platelet count and fibrinogen, and can be confirmed with measurement of the specific vitamin K–dependent factors (II, VII, IX, and X). Treatment is with intravenous vitamin K. Routine newborn prophylaxis to prevent vitamin K deficiency using intramuscular vitamin K is optimal. Oral dosing has shown mixed results.50
Inherited conditions can also present with significant bleeding and can be diagnosed during the newborn period.51,52 Contrary to presentations in later life, such as bleeding into a joint or muscle, neonates often have excessive bleeding in response to various iatrogenic conditions such as venipuncture, heel stick, Vitamin K administration, and circumcision.53 Hemophilia A is diagnosed with prolonged activated partial thromboplastin time and decreased factor VIII levels, which should be within normal adult range. However, the diagnosis of hemophilia B may be difficult in milder cases because factor IX and other vitamin K–dependent factors are decreased in newborns, particularly at earlier gestations. In borderline cases, molecular analysis or repeat testing may be necessary at 6 months of age.53 Von Willebrand disease generally does not present in the newborn period because there is a physiologic increase in von Willebrand factor at birth. For further discussion, see Chapter 436.
THROMBOTIC DISORDERS
Thromboembolic events in the perinatal period are rare. Even so, there has been increasing recognition of the potential morbidities associated with hereditary prothrombotic states. Thromboembolic events occur in the newborn period most often as a complication of indwelling vascular catheters. A prothrombotic state may also present as perinatal stroke. Although stroke is a rare event in this population, evidence suggests that some of these events may be related to prothrombotic states such as factor V Leiden and mutations in the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene54 (see Chapters 52 and 552). Other maternal conditions that may increase the risk of thrombosis include diabetes mellitus, systemic lupus erythematosus, and antiphospholipid syndrome. Renal vein thrombosis can occur spontaneously and occurs more frequently in preterm infants.55 Presenting signs are hematuria, flank mass, and thrombocytopenia.
Laboratory testing for evaluation of thrombophilia include assays for known factors associated with prothrombotic states. These include factor V Leiden, prothrombin gene mutation, antithrombin deficiency, proteins C and S deficiencies, and MTHFR mutation.56 Mild deficiency states may prove difficult to detect in the newborn period because many factors are normally below adult levels.57 Follow-up testing at 6 months of age may be useful.
There is no consensus regarding anticoagulant and thrombolytic therapy for newborns. All potential treatments carry the risk of major bleeding. Therefore, supportive care and close observation may be an optimal approach. When medications are used, the therapy will depend on thrombus location, degree of impairment, and cause of thrombosis if known. Unfractionated heparin is monitored by frequent measurement of activated partial thromboplastin time, which guides dosing of heparin boluses and altering of heparin infusion rates. Low molecular weight heparin is becoming a preferred treatment because it is an easier alternative, having more predictable pharmacokinetics and requiring less monitoring via anti-Xa levels.58 Higher doses than those used in adults are generally necessary to achieve therapeutic levels. Adverse effects such as major bleeding appear to occur less frequently than with unfractionated heparin.
Thrombolytic agents act by converting plasminogen to plasmin, and their use in neonates is limited by the relatively low concentrations of plasminogen in newborns. Tissue plasminogen activator is currently the thrombolytic therapy of choice for neonates due to its availability and increased fibrin specificity.59 Due to the potential risk of major bleeding and lack of controlled trials, its use is limited to organ-or life-threatening situations. Oral anticoagulation with warfarin is difficult in newborns because warfarin is a vitamin K antagonist and vitamin K–dependent factors are already low in newborns. The low concentrations of vitamin K in breast milk and the supplementation of vitamin K in formula also make the monitoring and use of warfarin inappropriate in most cases of neonatal thrombosis.59
JAUNDICE
Jaundice, one of the most common conditions encountered in the care of newborn infants, refers to the yellow discoloration of the skin and sclerae resulting from bilirubin deposition in tissues. The condition arises when the rate of bilirubin production exceeds the rate at which bilirubin is eliminated. Newborn infants have a rate of bilirubin formation that is 2 to 3 times higher than that of adults and is attributable mainly to the higher hematocrit and the shorter life span of the red blood cells in the newborn.60 The decrease in bilirubin elimination occurs from the limited ability of the newborn liver to conjugate bilirubin and increased enterohepatic circulation. Although jaundice can result from an increase in either unconjugated (indirect) or conjugated (direct) bilirubin, a rise in the indirect fraction is the most common cause of newborn jaundice and is the focus of this section. The approach to evaluation of a conjugated hyperbilirubinemia is discussed in Chapter 419.
 BILIRUBIN METABOLISM
BILIRUBIN METABOLISM
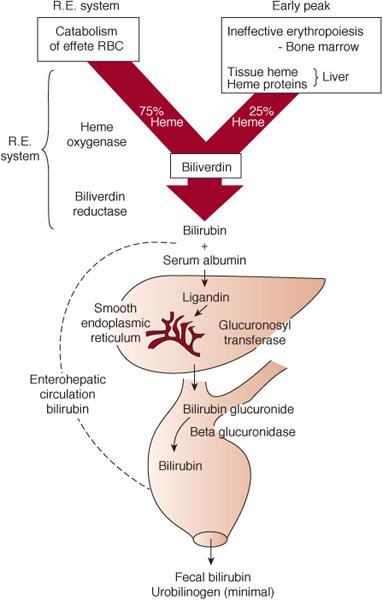
Bilirubin is derived from the catabolism of heme. Approximately 75% of bilirubin is derived from the breakdown of hemoglobin from senescent red blood cells. The remainder arises from ineffective erythropoiesis and from the breakdown of hemoproteins, such as cytochromes, myoglobin, nitric oxide synthase, glutathione peroxidase, and catalase (Fig. 53-1).
Heme is degraded in a 2-step process by the enzyme heme oxygenase resulting in formation of biliverdin and carbon monoxide in equimolar amounts. Carbon monoxide, which diffuses from the cell, binds to hemoglobin in circulating red blood cells to form carboxyhemoglobin (COHb) and is eventually excreted during exhalation (measurable as end-tidal carbon monoxide). Bilirubin is produced from biliverdin by the action of biliverdin reductase. Upon entering the circulation bilirubin binds to albumin and is transported to the liver. Inside the hepatocyte, bilirubin binds to ligandin and subsequently undergoes conjugation to glucuronic acid catalyzed by uridine diphosphate glucuronosyltransferase (UGT) into a water soluble form that is easily excretable. Distribution of bilirubin into tissues depends on its binding to albumin and the serum pH. The greater the binding to albumin and the more alkaline the pH, the more likely bilirubin will remain in circulation until it enters the liver. Conjugated bilirubin is excreted into the intestine via the bile, where it is either deconjugated by enzyme β-glucuronidase and reabsorbed into the circulation (enterohepatic circulation) or converted by bacteria to nonabsorbable breakdown products. Because the newborn infant has less intestinal bacteria, the enterohepatic circulation of bilirubin is active in the newborn and contributes to the increased propensity for jaundice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


