Gestational Trophoblastic Neoplasms
Andrew John Li
Gestational trophoblastic neoplasia (GTN) encompasses a broad spectrum of benign and malignant tumors derived from the trophoblast of the human placenta. While they are rare in incidence, they have the potential to become rapidly fatal diseases that afflict young women in their peak reproductive years.
Traditionally, GTN is divided into three histologic categories: hydatidiform mole, invasive mole (chorioadenoma destruens), and choriocarcinoma. Partial hydatidiform moles and placental site trophoblastic tumors (PSTTs) are further recognized as histologically and clinically separate entities under the broad classification of GTN.
Despite the apparent diversity of GTN, these diseases are all derived from the human placental trophoblast and the paternal genome, with an occasional maternal contribution. Human chorionic gonadotropin (hCG) is secreted by these neoplasms and serves as a sensitive tumor marker that correlates well with the clinical course for all but PSTT.
In 1956, metastatic gestational choriocarcinoma, the most malignant form of these diseases, was shown to be curable by chemotherapy. Now, studies demonstrate the curability of most of these patients, although individualization of therapy remains fundamentally important.
This chapter will review this unusual spectrum of human neoplasms and discuss important concepts regarding diagnosis, management, and surveillance for women with GTN.
Histology
Patients may be treated for malignant GTN on the basis of clinical, radiographic, and hCG level determinations without a definitive histologic diagnosis. For this reason, the generic term of gestational trophoblastic neoplasia is useful, especially when treating patients with metastatic disease that is not readily accessible for pathologic evaluation. Except for PSTT, the initial histologic features of any lesion identified as GTN are less important than the clinical data and hCG level.
Hydatidiform Mole
Two distinct types of molar gestations are recognized: partial and complete hydatidiform moles, both of which have distinct cytogenetic origins, pathologic features, and clinical behavior. While it is not as clear whether partial hydatidiform mole represents a form of GTN or an extreme form of hydropic degeneration of the placenta in a chromosomally abnormal pregnancy, it should be considered as a variant of the complete hydatidiform mole with risk of malignant sequelae. Most patients with primary molar gestations do not require adjuvant chemotherapy and may be monitored after therapeutic evacuation with serial hCG level determinations until either spontaneous regression occurs or the patient develops criteria of malignant sequelae.
Partial Hydatidiform Mole
Approximately 1% of pregnancies have a triploid karyotype and resolve in spontaneous abortion; partial hydatidiform moles represent a subset of these pregnancies with histologic features similar to the complete hydatidiform mole. A comparison of karyotypic, pathologic, and clinical
features of partial and complete hydatidiform moles is shown in Table 63.1. Partial moles are often associated with identifiable fetal parts or amniotic membranes. Grossly, the placenta demonstrates a mixture of normal and hydropic villi. Microscopic features include normal and hydropic chorionic villi with focal mild hyperplasia of trophoblastic elements. Scalloping of the hydropic villi is common, with trophoblastic inclusions in the stroma. Fetal vessels are frequently observed with nucleated fetal erythrocytes within the vessels. Normal amniotic membranes are often identified, even if a fetus is not found.
features of partial and complete hydatidiform moles is shown in Table 63.1. Partial moles are often associated with identifiable fetal parts or amniotic membranes. Grossly, the placenta demonstrates a mixture of normal and hydropic villi. Microscopic features include normal and hydropic chorionic villi with focal mild hyperplasia of trophoblastic elements. Scalloping of the hydropic villi is common, with trophoblastic inclusions in the stroma. Fetal vessels are frequently observed with nucleated fetal erythrocytes within the vessels. Normal amniotic membranes are often identified, even if a fetus is not found.
TABLE 63.1 Complete and Partial Hydatidiform Moles | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Partial moles are almost always associated with one haploid maternal and two haploid paternal sets of chromosomes. Presumably, this results from dispermic fertilization of a haploid ovum or fertilization of a haploid ovum with a diploid sperm.
Women with partial hydatidiform mole usually have a clinical diagnosis of spontaneous abortion or missed abortion. Often, hydropic villi are not identified on ultrasound, and the diagnosis is not suspected until after evacuation of the pregnancy. Initial hCG levels are lower than those seen in patients with complete hydatidiform mole, and prompt postevacuation regression of hCG levels usually occurs. Unlike patients with complete moles, who have a 10% to 30% incidence of malignant sequelae, fewer than 5% of the patients with partial moles develop criteria requiring chemotherapy. Regardless of this low risk of malignant sequelae, all women with partial hydatidiform moles should undergo hCG surveillance after evacuation, similar to that recommended for patients with complete hydatidiform mole. If there is any doubt that the products of a conception are molar, hCG monitoring should be done.
Complete Hydatidiform Mole
Complete hydatidiform mole is identified macroscopically by edema and swelling of virtually all chorionic villi without identifiable fetal parts or amniotic membranes. Hydropic villi are usually 1 to 3 cm in diameter, giving the gross appearance of grapelike vesicles. Microscopically, the chorionic villi are hydropic with marked interstitial edema. Fetal vessels are absent in the stroma of the villi. Proliferation of cytotrophoblast and syncytiotrophoblast is observed. Regardless of the degree of trophoblastic proliferation, all patients should be followed in similar fashion. All hydatidiform moles secrete hCG, and this marker is used to monitor regression after evacuation.
Complete moles are almost uniformly diploid with paternal chromosomal markers. Most are 46XX, although a minority will demonstrate a 46XY karyotype. The most common origin of complete hydatidiform mole is fertilization of an empty egg by a haploid sperm followed by reduplication, although some may result from dispermic fertilization of an empty egg.
Unlike women with partial hydatidiform moles, approximately one third to one half of these patients have uterine enlargement greater than expected for gestational dates. Fetal heart tones are absent. Patients often present with vaginal bleeding and spontaneous abortion of the atypical hydropic vesicles. Theca lutein cysts are detected clinically in approximately 20% of patients with complete moles. Pulmonary decompensation, pregnancy-induced hypertension, and hyperthyroidism are occasionally observed. The clinical diagnosis of molar gestation is supported by a characteristic mixed echogenic “snowstorm” image filling the uterus on an ultrasound scan (Fig. 63.1).
Invasive Mole
Invasive moles are histologically identical to complete moles but with invasion into the myometrium without intervening endometrial stroma. Invasive moles usually are diagnosed within 6 months of molar evacuation. Untreated invasive moles tend to invade the uterine wall locally, which can result in uterine perforation and hemorrhage. Direct vascular invasion and metastasis may also occur. Rarely, biopsies of distant metastases reveal the hydropic villi of invasive mole instead of solid sheets of anaplastic cells consistent with choriocarcinoma.
The identification of an invasive mole from uterine curettings may be difficult unless there is sufficient myometrium to document direct myometrial invasion.
Choriocarcinoma
Choriocarcinoma is a highly anaplastic malignancy derived from trophoblastic elements. No chorionic villi are identified. Grossly, the tumor has a red, granular appearance on cut section with focal, often extensive, central necrosis
and hemorrhage. Histologically, the lesion consists of mixed syncytioblastic and cytotrophoblastic elements with numerous abnormal mitoses, multinucleated giant cells, and extensive areas of necrosis and hemorrhage. Choriocarcinoma rapidly invades the myometrium and uterine vessels, and systemic metastasis results from hematogenous embolization. The lung and vagina are the most common sites of metastases, with secondary dissemination to the central nervous system (CNS), kidney, liver, and gastrointestinal tract.
and hemorrhage. Histologically, the lesion consists of mixed syncytioblastic and cytotrophoblastic elements with numerous abnormal mitoses, multinucleated giant cells, and extensive areas of necrosis and hemorrhage. Choriocarcinoma rapidly invades the myometrium and uterine vessels, and systemic metastasis results from hematogenous embolization. The lung and vagina are the most common sites of metastases, with secondary dissemination to the central nervous system (CNS), kidney, liver, and gastrointestinal tract.
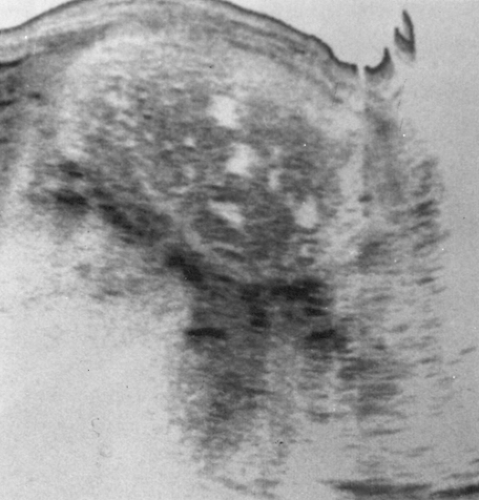 Figure 63.1 A longitudinal ultrasound reveals a hydatidiform mole. The mixed echoic pattern is caused by hydropic villi and focal intrauterine hemorrhage. |
Choriocarcinomas may develop after any type of pregnancy. Approximately 50% of cases are preceded by hydatidiform mole, and the remaining are equally distributed between a normal antecedent term gestation and abortion or ectopic pregnancy. Gestational choriocarcinoma has been observed several years after the last known pregnancy. Spontaneous regression of the primary uterine site has been well documented from autopsy series of patients before the development of effective chemotherapy.
Placental Site Trophoblastic Tumor
PSTTs are locally invasive neoplasms derived from intermediate cells of the placenta. These rare neoplasms are composed of a monomorphic population of intermediate cytotrophoblast cells that secrete human placental lactogen (hPL) and relatively small amounts of hCG. There is typically local myometrial invasion with rare systemic metastases. Diagnosis is typically made by exclusion, and PSTTs should be considered with disease refractory to standard chemotherapy. Hysterectomy is the therapy of choice.
Incidence and Epidemiology
Approximately 3,000 cases of hydatidiform mole and 500 to 750 cases of malignant GTN are diagnosed in the United States each year. Hydatidiform mole is identified in approximately 1 in 1,500 to 2,000 pregnancies in the United States. There is a marked geographical variation in the incidence of this disease, with rates 5- to 15-fold higher in the Far East and Southeast Asia than in the Western industrialized nations. Some of this variation may be accounted for by the methodology of studies reporting the incidence of molar gestation, as many data were reported from the experience at referral centers and may overestimate the true incidence of molar pregnancies in the general population. Racial differences may also account for some of the geographic variations; Japanese immigrants to Hawaii have an incidence of molar gestation intermediate between that of native Hawaiians and native Japanese. Nutritional factors may also be important in the development of hydatidiform mole, including deficiencies of protein or animal fat and fat-soluble carotene.
Known risk factors for hydatidiform mole include previous molar pregnancies and maternal age. Women with
a history of hydatidiform mole have a four to five times higher risk of developing a subsequent molar gestation. Women at the extremes of reproductive age are also at increased risk of developing a hydatidiform mole. Several studies have confirmed that risk increases with advanced maternal age, and others have suggested an increased risk for younger women or adolescents. The impact of paternal age on the incidence of hydatidiform mole is difficult to separate from the effect of maternal age.
a history of hydatidiform mole have a four to five times higher risk of developing a subsequent molar gestation. Women at the extremes of reproductive age are also at increased risk of developing a hydatidiform mole. Several studies have confirmed that risk increases with advanced maternal age, and others have suggested an increased risk for younger women or adolescents. The impact of paternal age on the incidence of hydatidiform mole is difficult to separate from the effect of maternal age.
The incidence of partial hydatidiform mole is unknown. Presumably, many are undiagnosed because of insufficient histologic analysis of tissue from spontaneous and induced abortions. Some pathologists are not familiar with the diagnosis of partial hydatidiform mole, and karyotyping is seldom performed on material obtained from spontaneous abortions. One report reclassified approximately 10% of all moles in their studies as partial hydatidiform moles on the basis of histologic analysis.
Invasive mole follows approximately 10% to 15% of complete hydatidiform moles. In the United States, choriocarcinoma will follow in approximately 1 in 40 moles, 1 in 5,000 ectopic pregnancies, 1 in 15,000 abortions, and 1 in 150,000 normal pregnancies.
Albeit rare, GTN may also present in ectopic pregnancies and in twin gestations. The incidence of molar disease following an ectopic pregnancy is reported as 1.5 per 1,000,000 pregnancies, and only case reports and small case series have been reported. Twin pregnancy, consisting of a complete hydatidiform mole and coexistent normal fetus, is even more rare, with 30 cases identified during an 18-year period where 7,200 cases of GTN were registered at the Sheffield Trophoblast Centre. Diagnosis is difficult, and ultrasound findings in early pregnancy may be misleading. In an analysis of the major series reporting experience with twin mole/viable fetal pregnancies, successful pregnancy resulted in only 29% of cases; furthermore, an increased risk of complications, such as preeclampsia and hemorrhage, was identified.
Management
The basic principles in management of the patient with hydatidiform mole include establishment of the diagnosis, evacuation of the molar gestation, and close surveillance of hCG level after evacuation. Patients with complete hydatidiform moles frequently present with spontaneous abortion of hydropic villi, which are pathognomonic for molar pregnancy. Absent fetal heart tones, uterine enlargement different from that expected for the gestational age, and a markedly elevated hCG level are all clinical indications that may suggest a diagnosis of hydatidiform mole. Ultrasound is now the diagnostic method of choice for evaluating patients with suspected hydatidiform mole. Ultrasound demonstrates a characteristic image of multiple echogenic regions within the uterus corresponding to hydropic villi and focal intrauterine hemorrhage.
Evaluation of the patient before evacuation of the hydatidiform mole is directed toward preparing the patient for evacuation, obtaining baseline hCG level information, and screening for associated hyperthyroidism. The following studies are recommended:
Complete physical and pelvic examinations
Complete blood count
Blood chemistries, including renal, hepatic, and thyroid function tests
Baseline serum hCG level
Type and screen
Chest radiograph
Pelvic ultrasound.
Suction dilation and curettage (D&C) offers a safe, rapid, and effective method of evacuation of hydatidiform mole in most patients. Some patients who do not desire preservation of reproductive function may benefit from primary hysterectomy for evacuation of hydatidiform mole and concurrent sterilization. However, these patients must be closely followed after hysterectomy, as malignant sequelae may still develop. Hysterotomy or induction of labor for molar evacuation is not recommended.
Suction D&C for evacuation of hydatidiform mole has a low complication rate in patients with uterine sizes corresponding to <16 weeks gestation. Oxytocic agents are administered after cervical dilation and partial evacuation to aid in postoperative hemostasis. Patients with excessive uterine enlargement have a higher risk of pulmonary complications associated with D&C, which may be related to trophoblastic deportation, preeclampsia, fluid overload, anemia, and hyperthyroidism. In patients with hydatidiform mole complicated by uterine enlargement >16 weeks of gestation, baseline arterial blood gases should be obtained preoperatively with an electrocardiogram, radionucleotide-gated heart pool scan for ejection fraction, and a valvular function assessment by cardiac ultrasound. Evacuation should be performed with a laparotomy set and facilities for central hemodynamic monitoring. Patients who are Rh negative should receive the Rh immune globulin vaccine to protect future pregnancies against Rh sensitization.
Primary hysterectomy is a reasonable alternative for termination of molar gestation in patients with hydatidiform mole who have completed childbearing and desire sterilization. Hysterectomy reduces the incidence of malignant sequelae after evacuation of hydatidiform mole from approximately 20% after suction D&C to <5% after hysterectomy. However, this does not eliminate the need for careful follow-up or complete hCG surveillance after termination of hydatidiform mole, as malignant GTN may develop even after hysterectomy. Concurrent surgical extirpation of the adnexa should be considered as with hysterectomy for benign indications.
Theca lutein cysts are clinically detected in approximately 20% of patients with molar gestations. These cysts are thin-walled and highly vascular and develop as a response to ovarian hyperstimulation from the high hCG levels produced by hydatidiform moles. They typically regress spontaneously over several weeks following molar evacuation. It is preferable to avoid operation or ovarian manipulation in patients with uncomplicated theca lutein cysts. Rarely, because of abdominal distension and respiratory compromise, they may require aspiration by ultrasonic guidance. Enlarged cysts may undergo torsion, infarction, or rupture, and oophorectomy should be considered in these circumstances.
Prophylactic short courses of methotrexate or dactinomycin chemotherapy have been considered at the time of molar evacuation in the past, as they may decrease the incidence of malignant sequelae in patients with high-risk features. However, prophylactic chemotherapy does not eliminate the chance of subsequent malignancy or the need for hCG surveillance. Routine prophylactic chemotherapy at the time of molar evacuation for patients with uncomplicated hydatidiform mole is not recommended if reliable hCG surveillance is available.
Surveillance following Molar Evacuation
Following the wide availability of sensitive hCG assays, histologic grading of molar tissue after evacuation of hydatidiform mole has assumed less importance in predicting the potential for malignant postmolar sequelae. Several sensitive hCG assays are available, measuring the β subunit of hCG by radioimmunoassay or by radioimmunometric assay. These assays are able to detect hCG levels elevated above the baseline variations of pituitary gonadotropins. These sensitive hCG assays should be used to monitor patients with GTN after evacuation of hydatidiform mole and during therapy of patients with malignant GTN. Urinary or serum pregnancy screening tests should not be used to follow patients with GTN, because the assays do not have sufficient sensitivity to permit detection of minimal elevations of hCG levels.
The recommendations for postmolar follow-up include determination of serum β-hCG levels every 1 to 2 weeks after evacuation until the hCG level is normal; determination of the hCG level 2 to 4 weeks after first normal level to confirm spontaneous hCG regression; and hCG surveillance every 1 to 2 months for 6 months after the first normal hCG level.
Most women with molar pregnancies undergo hCG level regression after evacuation to normal limits and require no further therapy. Strict contraception is recommended during hCG surveillance to avoid an intercurrent pregnancy that would interfere with monitoring. hCG elevation of an early normal pregnancy may potentially mask the hCG rise associated with postmolar malignant GTN.
Although an early report implicated oral contraceptives as increasing the risk for the development of postmolar malignant GTN, subsequent investigators found no significant increase in the risk of malignant GTN associated with the use of oral contraceptives after molar evacuation. Furthermore, oral contraceptives are considered the contraception agent of choice by most experts. After completion of 6 months of hCG level surveillance with normal results, patients may attempt pregnancy if desired. Because patients are at a four- to fivefold increased risk of recurrent molar pregnancy, they should undergo early screening of future pregnancies with ultrasound to exclude recurrent molar gestations. Figure 63.2 illustrates an algorithm for diagnosis and follow-up of patients with hydatidiform mole.
False-Positive Human Chorionic Gonadotropin Tests
The sensitivity and specificity of the serum hCG level makes it an ideal tumor maker in both diagnosis and surveillance of GTN. However, hCG, a glycoprotein comprised of an α and β subunit, is linked to eight oligosaccharides,
which promotes significant heterogeneity in its structure. Free subunits, degraded molecules, molecules with irregular side chains, and fragments of hCG are present in sera of women in pregnancy, with trophoblastic disease, and with nontrophoblastic neoplasms. Furthermore, hCG variants that include hyperglycosylated hCG (hCG-H), nicked hCG, hCG missing the β subunit C-terminal peptide, free β subunit, and nicked free β subunit may also be present. While all professional laboratory hCG assays utilize antibodies to different sites on the β subunit, variations in antibody use may lead to measurements of different hCG-related molecules.
which promotes significant heterogeneity in its structure. Free subunits, degraded molecules, molecules with irregular side chains, and fragments of hCG are present in sera of women in pregnancy, with trophoblastic disease, and with nontrophoblastic neoplasms. Furthermore, hCG variants that include hyperglycosylated hCG (hCG-H), nicked hCG, hCG missing the β subunit C-terminal peptide, free β subunit, and nicked free β subunit may also be present. While all professional laboratory hCG assays utilize antibodies to different sites on the β subunit, variations in antibody use may lead to measurements of different hCG-related molecules.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


