FIGURE 45-1 A: Intraoperative photograph of tumor resection with margin of healthy extensor mass and supinator muscle. B: The completed resection with radial nerve outlined. Sensory branch (bold arrow) and PIN (narrow arrow, retracted by forceps) outlined. PIN was used for reinnervation of flap.
CLINICAL QUESTIONS
- What are the indications for functional free muscle transfer?
- Which muscles are used most often and why?
- What are the preoperative requirements to maximize success?
- Which nerves are used for reinnervation of the transferred muscle?
- What are the indications for myocutaneous flap coverage of the upper limb?
- Which muscles are used most frequently?
- What are the complications of free muscle transfer?
THE FUNDAMENTALS
The one man team is a complete and total myth.
—Don Shula
The absence of muscle function in a hand or upper limb can be devastating. This can be due to trauma, nerve injuries, compartment syndromes, cancers, and congenital deficiencies. Free muscle transfer is performed when (1) there are no other viable reconstructive options available, such as a regional tendon transfer, and (2) both the recipient site and donor muscle have a reasonable chance of successful functional outcome. Many muscles have been used for functional free muscle transfer with variable success. The best donor muscle for free transfer to the upper limb is the gracilis. The latissimus dorsi, pectoralis major, serratus anterior, and rectus abdominis have been used with less success. Restoration of elbow flexion, finger flexion, and finger extension are the most common functional goals of free muscle surgery, and much less commonly free transfers to the deltoid and triceps. This is complex surgery. Whenever possible, simpler solutions, if available, should be pursued.1,2
Free muscle transfer is also performed for coverage of upper limb major soft tissue deficiencies whenever other less complex treatments (skin grafts, regional or pedicle flaps) are not viable. This is most common after major trauma, oncologic resection, and chronic infection. The gracilis, latissimus dorsi, transverse rectus abdominis (TRAM), and serratus anterior muscles are the usual free musculocutaneous flaps used for coverage in the upper limb. Free tissue transfer surgery is best performed with an outstanding team. Simultaneous harvesting of the donor muscle and preparation of the recipient site lessens anesthesia time, surgeon fatigue, and the most likely patient complications. The optimal team consists of highly skilled nurses and/or midlevel providers, therapists, surgical sub-specialists, and cooperative patients and families.
Etiology and Epidemiology
Severe avulsion and rupture injuries of the brachial plexus can result in a poor functional outcome by natural history. Nerve reconstruction by grafting (when there is at least one intact cervical spine nerve root), transfers in the first 1 to 9 months of life, or both are the usual treatment to alter natural history in these infants. When children present late or when nerve surgery fails,3 tendon transfers,4,5 teno-deses,6 and musculoskeletal stabilization procedures are the preferred regional solutions to improve the involved limb function. However, when the injury is so devastating and the donor options are too limited, free muscle transfer may be indicated.7 This is usually to restore elbow flexion and/or to improve hand function8–10 with a functional free gracilis transfer.11 Doi and his team developed the staged double muscle transfer for severe plexus avulsion injuries with (1) free gracilis for elbow flexion and finger extension neurotized by the spinal accessory nerve (SAN) and (2) second free gracilis for finger flexion neurotized by the intercostal nerves (ICNs). This is part of an extensive surgical plan also including sensibility and musculoskeletal stabilization procedures.
Volkmann’s ischemic contracture can result in a devastating loss of flexor hand and wrist function. The preferred surgical solution is contracture release and regional transfers if possible (see Chapters 27 and 37). Free gracilis muscle transfer to the digital flexors with neurotization by the anterior interosseous nerve (AIN) is reserved for severe injuries with no other viable options.12 Similarly, there are upper limb malignancy situations, most commonly with rhabdomyosarcoma, in which tumor resection can be followed by free muscle transfer to restore hand or wrist function.13,14
Mangled upper extremities15,16 and severe, chronic osteomyelitis17 in children are also rare clinical scenarios that can respond favorably to free tissue transfer. These procedures are usually for soft tissue coverage but can include composite and functional transfers. Multiple transfers may be necessary. If feasible, this approach is often preferred to a less functional amputation (Figure 45-2).
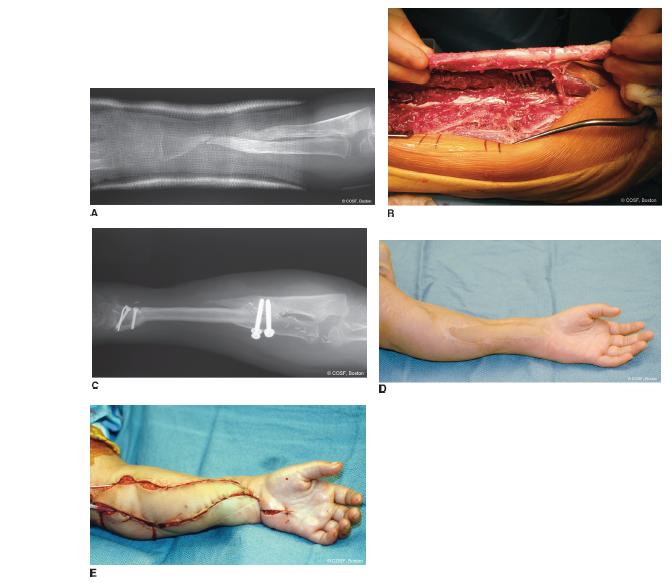
FIGURE 45-2 A: Patient referred for second opinion regarding amputation after numerous surgeries for nonunion forearm fracture from polymicrobial infection. B: Free fibula harvest for single bone forearm skeletal reconstruction after excision of nonviable bone. C: Postoperative radiograph of healed fibula reconstruction. D: Healed forearm with skin paddle from fibula. E: Free gracilis flap for flexor compartment reconstruction of hand and wrist.
Clinical Evaluation
The goal of a free muscle transfer is adequate antigravity strength to provide a functional arc of motion and improved use of the hand and upper limb. There must be (1) adequate passive range of motion of involved joints; (2) a recipient artery, vein, and nerve for a viable, functional transfer; (3) sufficient soft tissue coverage for unimpaired tendon and muscle excursion; (4) reasonable hand sensibility and intrinsic strength; (5) balanced antagonistic muscle strength; (6) a highly motivated patient with long-term vision and the ability to wait for functional results; and (7) no better or easier solutions.18 In our practice, this requires a lot of discussion, therapy, and often preliminary surgery to set the stage for a successful outcome. This is not for the faint of heart; weak of will; or impatient child, family, or surgeon. The message needs to be repeated often, the expected outcome made clear, and the patient’s and family’s ability to do the work and be reasonable tested along the way before diving into these procedures. These free muscle transfers are the ultimate “elective” operation. In the end, functional transfer and limb salvage surgery is not magic. If all goes well, the child will be better but not normal in function.
If there is any doubt about the vascularity of the recipient site, an angiogram is obtained. In trauma and oncology situations, this is almost always done. If there is uncertainty about the viability of a nerve donor, exploration and even nerve biopsy is done before donor-site harvesting.
Surgical Indications
It’s not enough that we do our best; sometimes we need to do what’s required.
—Winston Churchill
As discussed in the previous sections of this chapter, it is imperative that all the less complex solutions be considered first and compared against free tissue transfer. Patient selection is critical. Only when all the prerequisites for functional transfer have been met should surgery be seriously considered. Specifically, free muscle transfers are indicated in the right patient for (1) restoration of elbow flexion (brachial plexopathy); (2) digital flexor function (Volkmann’s ischemic contracture); (3) prehension (bra-chial plexopathy, after oncologic resection); and (4) digital extension (Volkmann’s ischemic contracture, after oncologic resection).1,19,20 These elective procedures can be successfully performed (95% to 98% flap survival) in the child (even age 2 years or younger).21–23
Similarly, free flaps for soft tissue coverage are indicated as a part of limb salvage surgery for major trauma, chronic infection, and oncology care. Again, less complex options must be considered first.24
SURGICAL PROCEDURES
The key is not the “will to win”…everybody has that. It is the will to prepare to win that is important.
—Bobby Knight
The prerequisites for free tissue transfers are a high level of familiarity with the anatomy and performance of these procedures as well as expert microsurgical and hand reconstructive skills. There are no green circle or blue square level procedures here, only black diamond and double black diamond level skill for all members of the team. The thinking and planning part of these procedures strains the brain. The doing part is very hard (“Why do you think microsurgery is a four-letter word?”) and requires a large treasure chest of expert technical skills. The communicating and doctoring part is critical to your patient’s wellbeing and outcome. You need to build yourself and every member of your team up to an elite level if this is to be a part of your career. If not, find some outstanding professional colleagues to whom you can send these complex patients for the best care possible. And then help with the before and after care.
For functional free muscle transfers, a suitable donor nerve is essential to a positive outcome. One of the problems with a brachial plexus injury that has been treated with microsurgery is that there may not be adequate donor nerves for late reconstruction with a free muscle. If all reasonable ipsilateral donors were used in the initial microsurgical neural reconstruction, the only alternatives become the contralateral C7, contralateral pectoral, or ipsilateral phrenic. None of these are universally predictable and have been abandoned by most centers. Besides poor nerve surgery outcome with some avulsion injuries in adults, the dearth of donor nerves is one of the reasons that Doi advocated for early free gracilis transfer for elbow flexion and digital extension; the SAN could be used for neurotization without expending it in the initial nerve reconstruction. Similarly, the ICNs can be preserved for later reconstruction of digital flexion, elbow extension, and hand sensibility.25 The best donor nerves are the SAN, ICNs, and the AIN and PIN for elbow and finger function. The gracilis is the best muscle for upper limb functional transfers. It is expendable; has predictable nerve and blood supply; and contours well for elbow flexion, digital flexion, and digital extension. It can be harvested with a skin paddle or covered with a split-thickness skin graft. Its functional excursion usually results in an improved limb.
 Free Gracilis to Flexor Forearm
Free Gracilis to Flexor Forearm
The more I practice, the luckier I get.
—Jerry Barber, about golf
The ipsilateral gracilis and involved arm are prepped and draped. One team harvests the donor gracilis, while the other meticulously prepares the recipient site. The gracilis team is on the opposite side, so they have easy access to the medial thigh. The donor hip is abducted, externally rotated, and flexed; the knee is flexed. A straight line is drawn from the adductor longus tendon to the tibial tubercle. The gracilis is posterior to this line. The gracilis is either harvested with or without a skin paddle. If a skin paddle is used, an ellipse is created in the proximal one-third, centered over the muscle and posterior to the skin line noted above (Figure 45-3A). Its dimensions should match the required coverage in the upper limb. We often take a paddle for postoperative monitoring as long as it is not too bulky. The paddle needs to be sutured to the underlying muscle to prevent injury to the skin perforators throughout the operation. If no skin paddle is used, the longitudinal proximal incision is made posterior to the gracilis to allow exposure of the neurovascular pedicle from beneath the adductor longus muscle. The vascular pedicles to the adductor longus and magnus are ligated from the profunda to untether the pedicle to the gracilis (Figure 45-3B). The surgeon can choose to incise the entire medial thigh if there is difficulty with exposure and mobilization; or, more commonly, a proximal longitudinal incision over the bulk of the muscle and pedicles and a distal transverse or longitudinal incision over the tibial tubercle and tendon insertion are used. Endoscopic-assisted harvesting is also done by some surgeons.26 The tendon is detached distally and the tendon and entire muscle belly are brought out through the proximal wound with neurovascular pedicle and muscle origin still intact. Electrical stimulation of the muscle confirms contractility. The muscle is brought out to the length, and sutures are placed every 5 cm to mark anatomic length. The color of the muscle and its electrical stimulation response are used to judge vascularity and avoid prolonged ischemia. The muscle is left in moist sponges until the recipient site is ready for transfer.
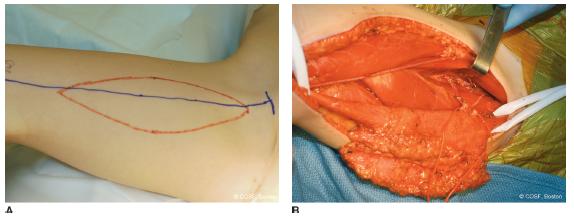
FIGURE 45-3 A: Outline of free gracilis muscle harvest with skin paddle for both coverage and postoperative monitoring. B: Gracilis flap elevation and isolation of neurovascular pedicle to muscle.
The recipient-site preparation includes extensile exposure from the medial distal humerus to the wrist. This is usually through a previous incision for contracture release, nerve decompression, and muscle debridement. Proximally, the brachial artery, median nerve, and superficial and deep veins are isolated and tagged with appropriate-colored elastic loops. The goal is find the right match for arterial, venous, and neural repair. When possible, we go end to end with the veins but end to side with major arteries or end to end with a matching-size artery, such as the interosseous. There must be excellent pulsatile flow (across the room, hit you in your eye sort of flow) from the transected recipient vessel. Do not place your elective transfer at risk by succumbing to using an impaired artery. Keep at it until you get the right one. Do not compromise. The vascular dissection is carried out for maximal excursion and no kinking. Be gentle with your vessels to lessen risk of spasm from iatrogenic trauma. The nerve used is usually the AIN, though at times it can be the flexor digitorum superficialis. Again, preparation of the nerve at the recipient site is quite extensive, so the repair work can begin as quickly as possible after ligation of the gracilis pedicle and the onset of ischemic time for the flap. The medial epicondyle and flexor-pronator origin are prepared for insertion of the origin of the free gracilis. Distally, the flexor digitorum profundus (FDP) tendons are mobilized and transected. Full excursion of the digits to the distal palmar crease should be possible with proximal pulling. The flexor pollicis longus (FPL) is isolated in the same fashion. If possible the gracilis will be split into separate neuromuscular units for independent control. If not, conjoint repair will occur. Then the pedicles are ligated, and the nerve is sharply divided with maximum length. The gracilis is brought to the recipient field, and the ischemia clock is started. A second team can close the leg wound, while the microscopic team completes the transfer.
The muscle is positioned so the pedicles are an easy match. Then the proximal portion is temporarily sutured to the medial epicondyle and flexor-pronator mass. The vein is repaired first if all is going smoothly and the ischemia time is reasonable. Next, the artery is done and finally the nerve. There should be no tension or kinking on any of the neurovascular pedicles. If there is any persistent distal muscle ischemia, it is debrided. The muscle is then brought out to maximum excursion and tension by re-creating the exact 5 cm suture intervals. This will give the best functioning transfer. The wrist and digits are placed in full extension, and the point of repair of the gracilis to the FDP and FPL tendons is marked. The proximal muscle repair is then completed to the medial epicondyle and surrounding soft tissues. The FDP tendons are then repaired to the gracilis tendon or weaved through the muscle depending on the anatomy of each case. The FDP gracilis is done with a group repair, so the digital cascade is maintained. The skin is closed over drain(s) without undue tension. This is either with gracilis skin paddle to the forearm skin or with split-thickness skin graft over the muscle. The arm is protected in a noncon-strictive long-arm splint for 4 weeks. Close monitoring of the vascularity is done in the hospital for 5 to 7 days.
 Free Gracilis to Elbow Flexion
Free Gracilis to Elbow Flexion
Brachial Plexus Injury or Major Trauma
The two-team approach and free muscle harvesting are identical for transfer to the elbow as they are for the forearm as outlined above. The preparation of the anterior arm is extensile from acromion to upper medial forearm. Proximally the gracilis muscle origin is usually attached to the acromion and clavicle rather than the coracoid due to the length differential of the gracilis to the upper arm. The attachment of the gracilis is done by periosteal, transosseous, or osseous anchor sutures to the acromion and clavicle. Distally the gracilis is inserted into the biceps tendon.
Neurotization is either by ICNs (T2-T4 motor branch) or SAN (inferior portion cranial nerve XI in a brachial plexus injury).27–31 Three-stage reconstruction is performed with nerve graft contralateral arm, then free gracilis for elbow flexion, then biceps tenodesis for finger flexion32; or direct neurotization from the motor branch of the musculocutaneous in major trauma with biceps loss or no other alternatives.15,33,34 The ICNs are dissected free with a curvilinear incision or longitudinal incision centered on T2-T4. If the SAN is used, it is dissected free in the supraclavicular region under the trapezius, and the inferior branches used are passed beneath the clavicle for direct repair (see Chapters 21 and 37). The goal with both the ICNs and SAN neurotization is to do a direct neural repair without a graft. Vascular repair is usually end to end to a matching side branch of the brachial artery or end to side to the brachial artery itself.
The gracilis muscle is always marked with sutures every 5 cm when it is out to maximum tension. These markings are used to determine the optimal length of muscle in setting the distal insertion point. The distal gracilis and the biceps tendon are marked to create this maximum tension with repair. The proximal attachment of the muscle is attached to the acromion and lateral clavicle. Then the vascular repairs are carried out, usually the veins first followed by the arterial repair. Finally, the nerve repair is done. The proximal attachment is then completed with care taken to avoid compression or kinking of the neurovascular pedicles. The distal insertion is performed in elbow flexion at optimal length by the previous markings. The skin is closed over a drain without tension. The distal muscle and tendon are closed directly. Proximally, either the skin paddle closure (the usual for us) or split-thickness skin graft is used to prevent vascular compression with too tight a closure. A shoulder spica (Figure 45-4) or posterior long-arm splint/cast with access to the skin paddle is placed with a supportive sling. Immobilization is for 4 weeks.

FIGURE 45-4 Shoulder spica cast protection for free gracilis to biceps in child with failed recovery of biceps by nerve reconstruction with grafts at an outside institution. The arrow identifies the cast window, allowing for postoperative monitoring of flap viability.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


