Endometriosis
 |
Endometriosis is a benign disease defined by the presence of endometrial glands and stroma outside of the uterus and is associated with both pelvic pain and infertility. The ectopic endometrial tissue usually is located in the pelvis but can appear anywhere in the body. The disease exhibits a broad spectrum of clinical signs and symptoms, is prone to progression and recurrence, and often presents vexing clinical management problems for women and their physicians. The pathogenesis and natural history of endometriosis remain poorly understood, but investigations employing modern molecular methods are yielding new insights into the mechanisms of the disease and suggesting new approaches to its diagnosis and treatment.
The Epidemiology of Endometriosis
The true overall prevalence of endometriosis is unknown, primarily because surgery is the only reliable method for diagnosis and generally is not performed on women without symptoms or physical findings that strongly suggest the disease; accordingly, estimates vary with the indication for surgical treatment. The prevalence of asymptomatic endometriosis is 1-7% in women seeking elective sterilization, 12-32% among women of reproductive age with pelvic pain, 9-50% in infertile women, and approximately 50% among teens with chronic pelvic pain or dysmenorrhea.1,2,3,4,5 and 6 The overall prevalence of endometriosis in reproductive aged women probably is between 3% and 10%.7,8,9 and 10
The mean age at time of diagnosis of endometriosis ranges between 25 and 35 years.11,12 Endometriosis is rare in premenarcheal girls but may be identified in half or more of adolescents and young women under age 20 with complaints of chronic pelvic pain or dyspareunia.3,4,13 Most cases in young women under age 17 are associated with müllerian anomalies and cervical or vaginal obstruction.14 Fewer than 5% of women who require surgery for endometriosis are postmenopausal and most of those have received estrogen
therapy.15,16 The prevalence of asymptomatic endometriosis may be somewhat lower in Blacks and higher in Asians than in White women.6,17
therapy.15,16 The prevalence of asymptomatic endometriosis may be somewhat lower in Blacks and higher in Asians than in White women.6,17
Early menarche and short menstrual cycles have been associated with increased risk for endometriosis.7,17 The correlation between the risk of disease and the volume or duration of menses is less consistent.7,18,19 Interestingly, the prevalence of endometriosis is inversely related to body mass index.12,17,20 Pregnancy has a protective effect that decreases with time; whereas risk decreases with parity and prolonged periods of lactation, risk increases with the number of years since last childbirth.21,22 Assorted epidemiologic studies have suggested that heavy consumption of alcohol and caffeine also may increase risk and that regular exercise and smoking may decrease risk for endometriosis.7,17 Primate data have suggested that exposure to polychlorinated biphenyl (PCB) or dioxin may be linked with endometriosis, but studies in women have yielded inconsistent results.23 Other data suggest that exposures in utero can play a role in development of the disease; the incidence of endometriosis is increased in women having prenatal exposure to diethylstilbestrol.24
Pathogenesis of Endometriosis
Although the classic peritoneal lesions of endometriosis were first described in the 1800s, the disease forever will be linked to John Sampson who, in 1921, described a series of perforating hemorrhagic ovarian cysts he called “chocolate cysts,” coining the term “endometriosis” to describe the peritoneal implants he first envisioned as seedlings derived from disease in the ovary.25 There is no generally accepted thesis regarding the origin of endometriosis. Several pathogenic mechanisms have been proposed, including retrograde menstruation and implantation, coelomic metaplasia, direct transplantation, and vascular dissemination. No one mechanism explains all cases of endometriosis and each probably contributes, at least to some extent.
The retrograde menstruation and implantation theory holds that endometrial tissue shed during menstruation is transported via the fallopian tubes into the peritoneal cavity where it implants on the surfaces of pelvic organs. Sampson’s classic paper proposing that endometriosis is ‘due to menstrual dissemination of endometrial tissue into the peritoneal cavity’ was published in 1927.26 Several lines of evidence support the implantation theory as the primary mechanism involved in the pathogenesis of endometriosis.
When laparoscopy is performed during menses, blood in the peritoneal fluid can be observed in 75-90% of women with patent fallopian tubes.27,28 and 29
Viable endometrial cells recovered from the peritoneal fluid during menses can be grown in cell culture28,30 and can attach to and penetrate the mesothelial surface of the peritoneum.31,32
Endometriosis is more prevalent in women with obstructing müllerian anomalies than in women with malformations that do not obstruct menstrual outflow.33
The incidence of endometriosis is increased in women with an early menarche, short menstrual cycles, or menorrhagia.7,17,18,34
Endometriosis is observed most commonly in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligaments, the posterior uterus, and the posterior surface of the broad ligaments.35,36 and 37
Although the evidence for the implantation theory may seem convincing, the coelomic metaplasia theory offers an alternative explanation for the same observations. The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change in mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura). The induction theory is a variation on the same theme and envisions that coelomic metaplasia is induced by exposure to menstrual effluent or other stimuli. In his original paper, Sampson himself allowed that foci of peritoneal endometriosis also might be ‘due to some specific irritant present in the cyst contents which stimulates the peritoneal endothelium to a metaplasia with the development of endometrial tissue typical both in structure and function.’25 A number of observations suggest that endometriosis results from spontaneous or induced coelomic metaplasia, at least in some cases.42
Endometriosis has been described in a premenarcheal girl,43 in women who never have menstruated,44,45 and also occurs in adolescent girls having had relatively few menstrual cycles.46
Because intact endometrial cells have no access to the thorax in the absence of an anatomical defect, the implantation theory cannot explain cases of pleural and pulmonary endometriosis (almost all of which occur on the right side).47 Metaplasia in the pleura (derived from the coelomic epithelium, like the peritoneum and the müllerian ducts), induced by steroid hormones or chemical stimuli released by degenerating endometrial cells into the peritoneal fluid (which communicates with the thoracic cavity via the right hemi-diaphragm), is the more plausible explanation.
Metaplasia in misintegrated coelomic epithelium (adjacent to the mesenchymal limb buds during early embryogenesis)48,49 can explain endometriosis in unusual peripheral sites like the extremities (thumb, thigh, knee).50,51 and 52
Rare cases of endometriosis have been observed in men treated with high doses of estrogen (urinary bladder, abdominal wall).53,54 and 55
Ovarian surface epithelium and stromal cells, co-cultured with estradiol in a three-dimensional collagen gel lattice, form endometrial glands and stroma.56
Eutopic (inside the uterus) and ectopic (outside the uterus) endometrium are distinctly different, both morphologically and functionally, which is difficult to reconcile with the notion that endometriotic implants represent autotransplants of normal endometrial tissue.57
Other mechanisms are invoked to explain cases of extrapelvic endometriosis.58,59 Although coelomic metaplasia might explain endometriosis in the pelvis, the thoracic cavity,47 the urinary and digestive tracts,60,61 the inguinal canal,62 and the umbilicus,63 evidence indicates that vascular or lymphatic dissemination of endometrial cells also may be involved.64,65,66 and 67 Alternatively, circulating stem cells originating from the bone marrow might differentiate into endometriotic tissue in various locations.68 Inadvertent direct transplantation of endometrial tissue at the time of cesarean section, other pelvic surgery, or episiotomy repair seems the most plausible explanation for endometriosis found in abdominal scars69,70 and in the perineum.71
Regardless whether pelvic endometriosis results from implantation of viable endometrial tissue regurgitated into the peritoneal cavity at time of menses or from coelomic metaplasia induced by hormones or other chemical stimuli derived from degenerated endometrial cells, several key questions remain. Why does endometriosis develop only in some women when retrograde menstruation occurs in most women? What explains the widely varying presentations of the disease? Why is there such a poor correlation between the extent of disease and the severity of associated symptoms? Studies of the immune function and genetics of women with endometriosis are suggesting answers.
The Immunobiology of Endometriosis
Endometriosis has been associated with headaches, arthalgias and myalgias, allergies, eczema, hypothyroidism, fibromyalgia, chronic fatigue syndrome, and susceptibility to vaginal candidiasis,72 suggesting a possible link between endometriosis and autoimmune disease. A cross-sectional survey of members of the Endometriosis Association found that members with endometriosis had a higher prevalence of hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosis, Sjogren syndrome, and multiple sclerosis, compared with the published rates in the general U.S. female population; allergies and asthma also were more common.73 Although provocative, the results of the study were highly susceptible to recall and ascertainment bias. Others have found no association between endometriosis and autoimmune disease.74
A higher prevalence of antinuclear antibodies has been reported for women with endometriosis.75, 76 The most common autoantibodies identified have been directed against endometrial antigens,77,78,79,80,81 and 82 including transferrin and laminin-1, which also is found in embryonic tissues.77,83 Such immunologic autoreactivity probably results from inflammation and develops as a consequence of chronic local tissue destruction.72
Endometriosis is associated with changes in both cellular and humoral immunity, suggesting that impaired immune function may contribute to the development of the disease. Altered immune function may predispose some women to develop endometriosis, or influence the severity of disease in affected women. Numerous immune-mediated mechanisms have been implicated. Although the peritoneal fluid of women with endometriosis contains increased numbers of immune cells, evidence suggests that their actions do more to promote the disease than to prevent it.
Macrophages are a key element of the innate immune response, the part of the immune system that is not antigen-specific and does not involve immunologic memory. Macrophages defend the host by recognition, phagocytosis, and destruction of offending microorganisms and also serve as scavengers, helping to clear apoptotic cells and cellular debris. Macrophages secrete a variety of cytokines, growth factors, enzymes, and prostaglandins that help to mediate their own functions while stimulating the growth and proliferation of other cell types. Macrophages are a normal inhabitant of the peritoneal fluid and their numbers and activity are much increased in women with endometriosis.84,85,86 and 87 Instead of acting as scavengers to eliminate ectopic endometrial cells, activated peritoneal macrophages and circulating monocytes in women with endometriosis appear to promote the disease by secreting growth factors and cytokines that stimulate proliferation of ectopic endometrium and inhibit their scavenger functions.88,89
Natural killer (NK) cells are another important component of the innate immune system and function in two ways. NK cells have receptors for immunoglobulin G (IgG) and kill IgG-bound cells in a process known as antibody-dependent cellular cytotoxicity. NK cells also have killer-activating and killer-inhibiting receptors that, when occupied, direct or inhibit cytotoxic activity. Whereas studies of the numbers of peritoneal NK cells in women with endometriosis have yielded conflicting results,87,90,91 those investigating NK cell function have consistently observed decreased cytotoxic activity, which is most pronounced in women with advanced stages of the disease.91,92 and 93 One of the mechanisms responsible appears to involve over-expression of killer-inhibiting receptors in both peripheral and peritoneal cells in women with endometriosis.94,95
Lymphocytes mediate the acquired immune response. B lymphocytes mature in the bone marrow and secrete immunoglobulins, which are antigen-specific antibodies directed against extracellular microorganisms. T lymphocytes help B cells to make antibodies and also eliminate intracellular pathogens by activating macrophages and by killing virus-infected
or malignant cells. T cells are of two types, cytotoxic/suppressor T cells (involved in the cellular immune response) and helper T cells (involved in the humoral immune response). The numbers of both T cell types are increased in the peritoneal fluid of women with endometriosis and in the stroma of ectopic endometrium.87,96,97
or malignant cells. T cells are of two types, cytotoxic/suppressor T cells (involved in the cellular immune response) and helper T cells (involved in the humoral immune response). The numbers of both T cell types are increased in the peritoneal fluid of women with endometriosis and in the stroma of ectopic endometrium.87,96,97
Cytokines and growth factors are a large family of soluble proteins and glycoproteins secreted by leukocytes and other cells into the extracellular environment where they act on the same (autocrine) or nearby cells (paracrine), serving as messengers both within and outside the immune system in regulation of chemotaxis, mitosis, angiogenesis, and differentiation. Whereas an impaired cellular immune response may result in ineffective clearance of refluxed endometrial cells from the peritoneal cavity, cytokines and growth factors appear to promote implantation and growth of ectopic endometrium by facilitating its attachment to the peritoneal surface and by stimulating proliferation and angiogenesis.
Interleukin-1 (IL-1) is a cytokine involved in inflammatory and immune responses and is secreted by activated monocytes and macrophages, T and B lymphocytes, and NK cells. IL-1 has been identified in the peritoneal fluid of women with endometriosis and IL-1 receptor expression is increased in endometriosis-derived stromal cells.91,92,98 IL-1 may promote the development of endometriosis by stimulating the release of angiogenic factors (vascular endothelial growth factor, interleukin-6, interleukin-8)99,10° and by helping endometrial cells that enter the peritoneal cavity to escape immunosurveillance by inducing the release of a soluble form of intercellular adhesion molecule-1 (ICAM-1) from endometriotic cells that competes for immune recognition sites on NK and other immune cells.101,102
Interleukin-8 (IL-8) is a potent angiogenic cytokine produced by mesothelial cells, macrophages, endometrial and other cells. Peritoneal fluid levels of IL-8 are elevated in women with endometriosis and correlate with the severity of disease.103 IL-8 is expressed in endometriotic implants and is up-regulated by IL-1.100,104 IL-8 stimulates adhesion of endometrial stromal cells to extracellular matrix proteins, matrix metalloproteinase activity, and endometrial stromal cell proliferation in a dose-dependent manner, all of which may help to promote the implantation and growth of ectopic endometrium.105,106 and 107
Moncyte chemotactic protein-1 and RANTES (regulated on activation, normal T-cell expressed and secreted) are two chemo-attractant cytokines that recruit macrophages into the peritoneal cavity. Both are secreted by a variety of leukocytes and by mesothelial and endometrial cells, and production of both is increased in ectopic endometrium.108,109 and 110 In women with endometriosis, peritoneal fluid concentrations are increased and correlate with the severity of disease.111,112 IL-1 up-regulates monocyte chemotactic protein-1 expression in eutopic endometrial epithelial cells in women with endometriosis and in cultured ectopic endometrial cells,113,114 an action further stimulated by estrogen.115 RANTES production by endometriotic implants is stimulated by other peritoneal fluid cytokines.98
Tumor necrosis factor-α (TNF-α) is an inflammatory cytokine produced by activated lymphocytes, macrophages, and NK cells, among others. TNF-α is expressed in eutopic endometrial epithelial cells and is up-regulated by IL-1. Peritoneal fluid concentrations are increased in women with endometriosis and correlate with the stage of the disease. The observations that TNF-α increases adherence of cultured stromal cells to mesothelial cells suggests it may facilitate attachment of ectopic endometrium to the peritoneum in women with endometriosis.
To implant and grow, ectopic endometrium must establish a blood supply. Vascular endothelial growth factor (VEGF) is an important mediator of local angiogenesis produced by monocytes and macrophages. The growth factor stimulates proliferation of vascular endothelial cells and also acts as a chemo-attractant for monocytes. VEGF is produced primarily in endometrial glands and is up-regulated by a variety of factors including estrogen and IL-1. Peritoneal fluid VEGF concentrations are increased in women with endometriosis
and are highest in advanced stages of the disease. VEGF also is expressed in endometriotic lesions, more so in active red lesions than in inactive “powder-burn” implants.
and are highest in advanced stages of the disease. VEGF also is expressed in endometriotic lesions, more so in active red lesions than in inactive “powder-burn” implants.
Summary
A wide variety of immunologic abnormalities has been described in women with endometriosis. The peritoneal fluid of affected women contains increased numbers of immune cells, but instead of acting to efficiently remove refluxed endometrial debris from the peritoneal cavity, they appear to promote the disease via two basic mechanisms.
Defects in cellular immune mechanisms (mediated by macrophages and NK cells) impair normal recognition and clearance of refluxed endometrial debris via the immune/inflammatory response, thereby affording endometrial cells and tissue fragments the opportunity to attach to peritoneal mesothelial cells and to invade into the extracellular matrix where they can persist and proliferate. Immune cells in the peritoneal fluid of women with endometriosis also secrete a wide variety of humoral factors (cytokines and growth factors) that stimulate attachment and proliferation of ectopic endometrium and local angiogenesis.
Altered immune function may thus predispose to the development of endometriosis or to more severe disease. Although it is not yet clear whether the immunologic abnormalities observed in women with endometriosis are the cause or the consequence of the disease, they almost certainly play an important role in its pathogenesis.
The Genetics of Endometriosis
In both humans116,117 and 118 and nonhuman primates,119 endometriosis tends to cluster within families, suggesting that genetic factors probably influence susceptibility to developing endometriosis. The disease frequently is observed in monozygotic and dizygotic twin pairs120,121 and 122 and exhibits a similar age of onset in affected non-twin sisters.123 Endometriosis is six to seven times more prevalent among the first-degree relatives of affected women than in the general population.124,125,126 and 127 All of these observations suggest that endometriosis has a genetic foundation and that a predisposition to the disease is inherited as a complex genetic trait for which the phenotype reflects interactions between allelic variants of susceptibility genes and environmental factors.128,129 Gene-expression profiling has identified candidate susceptibility genes relating to implantation failure, infertility, and progesterone resistance.130,131
Molecular Mechanisms
Genes predisposing to the development of endometriosis might include any that direct the molecular processes controlling the survival of detached endometrial cells, their attachment to and invasion of peritoneal surfaces, proliferation, neovascularization, or the inflammatory response. The ectopic endometrium of women with endometriosis exhibits abnormal expression of numerous gene products that are relevant to the pathogenesis of the disease. Compared to normal endometrium, ectopic endometrial implants produce excessive amounts of estrogen, prostaglandins, and cytokines.132,133 and 134 Similar, but more subtle, abnormalities are observed in the eutopic endometrium of women with endometriosis. These observations suggest that abnormalities intrinsic to the endometrium of women who develop endometriosis predispose to cell survival, ectopic implantation, proliferation, and chronic inflammation.
Retrograde menstruation occurs in most women, but endometriosis develops in only a few. The survival of refluxed endometrial debris might result from immune dysfunction, as discussed above, or may reflect a molecular abnormality in the eutopic endometrium of women with endometriosis,135 as discussed below. In any case, eutopic endometrial cells from women with endometriosis are resistant to apoptosis, the normal but complex generegulated physiologic process of programmed cell death that contributes to endometrial breakdown and turnover during the late secretory and menstrual phases of the cycle.136,137 Ectopic endometrium appears even more resistant to apoptosis.138 Resistance to apoptosis may improve the survival of endometrial cells entering the peritoneal cavity and also help to explain why ectopic endometrium is resistant to macrophage-mediated immune surveillance and clearance.
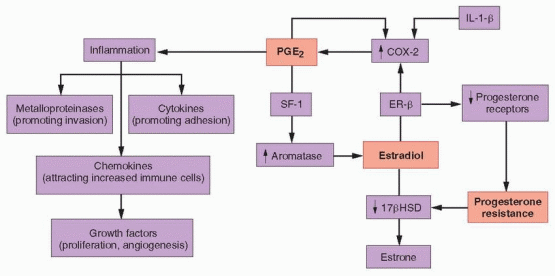
In normal women, levels of endometrial estrogen and prostaglandin E2 (PGE2) production are low because activity of the enzymes aromatase (mediating estrogen synthesis) and cyclooxygenase-2 (COX-2, mediating prostaglandin synthesis) are low. Moreover, during the secretory phase of the cycle, progesterone stimulates 17β-hydroxysteroid dehydrogenase (17βHSD) activity, which converts estradiol to the less potent estrogen, estrone.10 In women with endometriosis, aromatase and COX-2 activity are increased in eutopic endometrium, and greatly elevated in ectopic endometrial tissue implants. Tissue levels of estradiol are high, due to increased aromatase and decreased 17βHSD activity, and tissue levels of PGE2 are increased because COX-2 activity is elevated.10 The eutopic and ectopic endometrium of women with endometriosis thus differs from normal endometrium in at least three distinct and important ways, exhibiting 1) high local estrogen production, 2) high local prostaglandin production, and 3) resistance to the actions of progesterone.
Estrogen Production
There is little question that estrogen plays an important role in the pathogenesis of endometriosis. With rare exception, the disease arises only after menarche and regresses after menopause.139 Estrogen stimulates endometriosis, whereas aromatase inhibitors cause its regression.140 Substantial evidence indicates that both estrogen production and metabolism are altered in endometriosis in ways that promote the disease.10,141,142
Estrogen in women with endometriosis derives from three major sources. As in normal women, estrogen is secreted by the ovary into the circulation and released directly into the peritoneal cavity at ovulation, and is produced in adipose and skin via conversion of circulating androgens. However, in women with endometriosis, substantial amounts of estrogen also are synthesized locally, in endometriotic tissue, which expresses a complete set of steroidogenic enzymes, including aromatase.143
The overproduction of estrogen in endometriotic stromal cells is linked with another of the functional abnormalities observed in the tissue, high local production of prostaglandins. PGE2 stimulates the expression of all of the genes encoding the steroidogenic enzymes required for synthesis of estradiol from cholesterol in endometriotic stromal cells, including, in particular, STAR (encoding the steroidogenic acute regulatory protein, STAR) and CYP19A1 (encoding aromatase).143 Stromal cells in both normal and ectopic endometrium express all of the PGE2 receptor subtypes (EP1, EP2, EP3, and EP4).141 In endometriotic stromal cells, activation of the EP2 receptor (by PGE2) increases intracellular levels of cyclic adenosine monophosphate (cAMP), which induces STAR and CYP19A1 expression.141, 143,144 PGE2 or a cAMP analog increases STAR and aromatase levels and activity in endometriotic stromal cells, but not in normal endometrial stromal cells.134,143,144 These observations indicate that PGE2– and cAMP-dependent steroidogenesis in endometriotic stromal cells requires downstream effectors that are absent or opposed by other inhibitory mechanisms in normal endometrial stromal cells, which exhibit no steroidogenic activity.
The key downstream effector molecule in endometriotic tissue is steroidogenic factor-1 (SF-1), which is absent in normal endometrium. In endometriotic stromal cells exposed to PGE2, SF-1 binds to and assembles an enhancer transcriptional complex, which interacts with the promoters of STAR and CYP19A1 and induces their expression.143 In normal endometrial stromal cells, the absence of any steroidogenic response to PGE2 can be attributed to the absence of SF-1 and to the presence of transcriptional inhibitors of the STAR and CYP19A1 gene promotors. These repressors include the Wilms’ tumor 1 transcription factor (WT1),145 and CCAAT/enhancer binding protein β (C/EBPβ), levels of which are much higher in normal endometrium than in endometriotic tissue. In the absence of SF-1, a transcriptional complex of inhibitors binds to and suppresses steroidogenic promoters in endometrial cells.143
The key downstream effector molecule in endometriotic tissue is steroidogenic factor-1 (SF-1), which is absent in normal endometrium. In endometriotic stromal cells exposed to PGE2, SF-1 binds to and assembles an enhancer transcriptional complex, which interacts with the promoters of STAR and CYP19A1 and induces their expression.143 In normal endometrial stromal cells, the absence of any steroidogenic response to PGE2 can be attributed to the absence of SF-1 and to the presence of transcriptional inhibitors of the STAR and CYP19A1 gene promotors. These repressors include the Wilms’ tumor 1 transcription factor (WT1),145 and CCAAT/enhancer binding protein β (C/EBPβ), levels of which are much higher in normal endometrium than in endometriotic tissue. In the absence of SF-1, a transcriptional complex of inhibitors binds to and suppresses steroidogenic promoters in endometrial cells.143
Prostaglandin Production
Prostaglandins are locally produced hormones involved in inflammation and pain. Both PGE2 and prostaglandin F2a (PGF2a) are overproduced in the uterine and endometriotic tissues of women with endometriosis.146 PGF2a stimulates both vasoconstriction and myometrial contractions, resulting in pain and dysmenorrhea. As in most cells, production of prostaglandin H2 (PGH2) in the myometrium, endometrium, and endometriotic tissue is catalyzed by cyclooxygenase (COX), which has two isoforms.147 COX-1 primarily drives basal prostaglandin synthesis and COX-2 is involved in inflammation. PGH2 is metabolized to other prostaglandins by cell-specific enzymes; in the uterus, PGH2 is converted to PGF2a (by prostaglandin F synthase) and PGE2 (by prostaglandin E synthase).146
In both ectopic and eutopic endometrium in women with endometriosis, COX-2 is upregulated to a greater extent than in endometrial stromal cells from disease-free women,148,149 resulting in increased PGE2 production that induces local estrogen synthesis (as discussed above) and causes inflammation, resulting in pain134,144,150,151; prostaglandin E synthase activity also may be increased.10 COX-2 expression and PGE2 production in uterine and endometriotic tissues are stimulated by IL-1β, PGE2 (an autocrine action), VEGF, and estradiol (via estrogen receptor β).10,152,153 and 154 Altogether, these complementary and redundant mechanisms maintain high levels of PGE2 production in endometriotic tissue.
The high levels of PGE2 in endometriotic tissue induce a classical inflammatory response, characterized by increased production of cytokines, metalloproteinases, and chemokines. Increased levels of inflammatory cytokines (IL-1β, IL-6 and TNF-a) promote adhesion of shed endometrial tissue to peritoneal surfaces, and proteolytic membrane metalloproteinases promote their implantation.110,130,132,155,156,157 and 158 Increased levels of chemokines (monocyte chemoattractant protein 1, IL-8, and RANTES) attract increased numbers of granulocytes, NK cells, and macrophages,109,110,130,132,155,156,159 and auto-regulatory positive feedback loops perpetuate the process.
Progesterone Resistance
Whereas estrogen clearly aggravates endometriosis, the effects of progesterone are less clear. Progesterone stimulates proliferation of normal endometrial stromal cells during the secretory phase, at least transiently.10 Although progestins can be effective for relieving pain in women with endometriosis,160,161 a variety of selective progesterone receptor (PR) modulators having mixed agonistic and antagonistic actions also are.162,163 Moreover, endometriotic tissue produces substantial amounts of progesterone and contains much lower levels of PR than normal endometrium.144,164
In the normal menstrual cycle, progesterone induces differentiation of both endometrial epithelial and stromal cells.165,166 and 167 In the epithelium, progesterone stimulates glycodelin production, and in the stroma, progesterone induces decidualization and stimulates prolactin production. Progesterone also stimulates prolactin production in endometriotic stromal cells, but to a significantly lesser extent, suggesting some degree of progesterone resistance.168 In the normal endometrium, progesterone acts as an anti-estrogen in a paracrine fashion, by stimulating retinoic acid production in the stroma,169,170 and 171 which then induces 17βHSD activity in the epithelium,172,173,174 and 175 resulting in the conversion of estradiol to the less potent estrogen, estrone. However, in endometriotic stromal cells, progesterone does not induce retinoic acid production,176,177 and epithelial 17βHSD activity remains low; tissue estradiol levels are elevated, because high local aromatase activity drives production and low 17βHSD activity impairs its metabolism.168
Gene-expression profiling of the endometrium of women with and without endometriosis has identified a number of genes linked to the actions of progesterone that are downregulated during implantation when progesterone levels normally peak, such as glycodelin, suggesting that the eutopic endometrium of women with endometriosis also is progesterone-resistant.130, 131 Abnormalities in PR regulation might explain the phenomenon.164 During the normal menstrual cycle, levels of both PR-A and PR-B increase progressively during the proliferative phase and decrease after ovulation, indicating that estradiol stimulates PR production, but in endometriotic tissues, PR-A levels are much reduced and PR-B is undetectable.10 Decreased production of a binding protein required for PR function also may contribute to progesterone resistance.178
Epigenetic Changes
The very high levels of SF-1 observed in endometriotic tissue (> 12,000 times greater than in normal endometrium) appear related to a cyosine-phosphate-guanine (CpG) island at its promoter, which is heavily methylated in normal endometrial stromal cells and unmethylated in endometriotic stromal cells.179 Whereas an inhibiting transcription factor binds to the methylated SF-1 promoter, preventing its interaction with transcriptional activators, a stimulating transcription factor binds to the unmethylated SF-1 promoter in endometriotic cells, and activates it.180
The more than 100-fold higher levels of ER-β in endometriotic tissue, compared to normal endometrium, also are associated with hypomethylation of a CpG island, at the promoter
region of the ER-β gene, causing high levels of expression. In endometriotic stromal cells, ER-β occupies the ER-a promoter and suppresses its activity, yielding high levels of ER-β to bind to the PR promoter, which down-regulates PR expression.181
region of the ER-β gene, causing high levels of expression. In endometriotic stromal cells, ER-β occupies the ER-a promoter and suppresses its activity, yielding high levels of ER-β to bind to the PR promoter, which down-regulates PR expression.181
 |
Summary
The working molecular model of the pathogenesis of endometriosis centers on increased survival of refluxed endometrial cells, (resistance to apoptosis) and functional abnormalities in eutopic and ectopic endometrial cells (high local production of estrogen and prostaglandins, progesterone resistance) originating from epigenetic changes (hypomethylation of the promoters for SF-1 and ER-β, all combining to induce a chronic inflammatory response in a feed-forward, self-perpetuating cycle.
High local production of PGE2 stimulates aromatase expression (via SF-1), resulting in increased local production of estradiol, which stimulates COX-2 activity (via ER-β), thereby maintaining the stimulus for increased PGE2 production. PGE2 also induces an inflammatory reaction, with increased local production of cytokines (promoting adhesion), metalloproteinases (promoting invasion), and chemokines (attracting increased numbers of immune cells, which secrete growth factors that stimulate proliferation and angiogenesis). Increased expression of ER-β suppresses PR expression, causing progesterone resistance, manifested as decreased 17βHSD activity, which decreases metabolism of high local estradiol levels generated via increased aromatase activity.
Ultimately, inflammatory and immune responses, angiogenesis, and apoptosis are altered in ways that promote the survival, attachment, and proliferation of ectopic endometrial tissue.
Mechanisms of Pain
Pain is the most common symptom associated with endometriosis. The mechanisms involved are difficult to determine, for a number of reasons. Pain itself is hard to measure, especially when it is chronic. The hormonal environment influences the perception of pain. The placebo effect on pain is substantial and varies among studies. Chronic pelvic pain has a tendency to involve surrounding organ systems over time. The perception and tolerance of pain also vary widely among women.
The pain associated with endometriosis has been attributed to three primary mechanisms.
The direct and indirect effects of focal bleeding from endometriotic implants.
The actions of inflammatory cytokines in the peritoneal cavity.
Irritation or direct infiltration of nerves in the pelvic floor.
Among these, neural irritation or invasion has received the majority of recent attention. Tender nodularity in the cul-de-sac and along the uterosacral ligaments has approximately 85% sensitivity and 50% specificity as a clinical criterion for the diagnosis of deeply infiltrating endometriosis.182 Severe dysmenorrhea and deep dyspareunia are commonly associated symptoms; those having disease adjacent to or within the rectal wall also may have dyschesia.183 The intensity of pain associated with deeply infiltrating endometriosis relates to the depth of penetration and to the proximity or direct invasion of nerves.182,184,185 and 186
Whereas neural inflammation or invasion might explain the pain of women with deeply infiltrating endometriosis, it cannot be the mechanism that produces pain in women who have only superficial disease. The pain associated with mild disease more likely relates to inflammation resulting from cyclic focal bleeding in and around peritoneal implants, or from the actions of inflammatory cytokines released by the larger numbers of macrophages and other immune cells in the peritoneal fluid of women with endometriosis. However, there is no relationship between stage, site, or the morphologic characteristics of pelvic endometriosis and pain.187 The explanation for why many women with advanced endometriosis have little or no pain and those with mild disease may have incapacitating pain remains unclear. The cause may relate to the fact that severe disease is generally chronic and may be less metabolically active. There also is evidence to suggest that persistent neural input from endometriotic tissues may cause central sensitization of the nociceptive system (neurons that receive painful stimuli), manifested by somatic hyperalgesia (increased sensitivity to pain) and areas of referred pain in some women with endometriosis.188
One additional mechanism that may be involved in the pain associated with endometriosis relates to evidence that the hormonal milieu influences pain perception. Numerous studies have examined measures of pain perception across the menstrual cycle. A meta-analysis including 16 such studies concluded that somatic sensory pain thresholds and tolerance are near their lowest levels just prior to and during menses.189
Mechanisms of Infertility
Endometriosis is strongly associated with infertility; between 20% and 40% of infertile women have the disease. Numerous observations support a causal relationship between endometriosis and infertility:
Infertile women are more likely than fertile women to have moderate to severe disease8; the prevalence of minimal endometriosis in infertile women with normal and azoospermic male partners is comparable.190
Although reduced to a similar extent in untreated women with minimal and mild endometriosis and women with unexplained infertility, monthly fecundity decreases further with increasing severity of disease.191,192,193,194 and 195
Monthly fecundity of women with minimal and mild endometriosis receiving treatment with exogenous gonadotropin stimulation and intrauterine insemination (partner sperm), is less than half that observed in women without the disease.196
Monthly fecundity achieved with donor sperm insemination in women with minimal and mild endometriosis is significantly lower than in women with a normal pelvis.197,198 and 199
Overall, the success rates achieved with in vitro fertilization (IVF) in women with endometriosis (all stages) are approximately half those observed in women with tubal disease.200
Taken together, these observations support the conclusion that endometriosis decreases fertility to an extent that correlates roughly with the severity of disease.
The subfertility associated with endometriosis has been attributed to two primary mechanisms: 1) distorted adnexal anatomy that inhibits or prevents ovum capture after ovulation,
and 2) excess production of prostaglandins, metalloproteinases, cytokines, and chemokines, resulting in chronic inflammation that impairs ovarian, tubal, or endometrial function, leading to disorders of folliculogenesis, fertilization, or implantation. The first mechanism offers a logical explanation for infertility in women with advanced stages of endometriosis. The second mechanism may operate in women with milder disease, but whether minimal and mild endometriosis even should be regarded as a cause of infertility remains controversial.
and 2) excess production of prostaglandins, metalloproteinases, cytokines, and chemokines, resulting in chronic inflammation that impairs ovarian, tubal, or endometrial function, leading to disorders of folliculogenesis, fertilization, or implantation. The first mechanism offers a logical explanation for infertility in women with advanced stages of endometriosis. The second mechanism may operate in women with milder disease, but whether minimal and mild endometriosis even should be regarded as a cause of infertility remains controversial.
There is reasonably good experimental evidence that endometriosis decreases fertility when it results in grossly distorted pelvic anatomy. In monkeys with peritoneal autografts of adipose tissue or endometrium, cumulative pregnancy rates were significantly lower in animals that developed moderate or severe endometriosis (12%) than in others with minimal or mild disease or in controls (40%), and none of the animals with ovarian adhesions conceived.203 Decreased fertility in women with advanced endometriosis also could result from premature depletion of the ovarian follicular pool (due to ovarian surgery or destruction).204,205 and 206
Evidence that endometriosis causes abnormalities of follicular development, tubal transport, or endometrial function is relatively weak, deriving from observations of increased apoptosis in the granulosa cells of women with endometriosis,206 adverse effects of peritoneal fluid from women with endometriosis on sperm motility207 and tubal ciliary function in vitro,208 and abnormalities in the expression of markers of endometrial receptivity209 that could result from an intrinsic progesterone resistance.10,210,211
Results of the many observational studies of IVF outcomes in women with endometriosis have varied, but offer some useful insights. A 2002 meta-analysis of observational studies concluded that infertile women with endometriosis were less likely to achieve success than women with tubal factor infertility (OR = 0.56, CI = 0.44-0.70); outcomes were worse in severe than in mild disease.200 The ovarian response to gonadotropin stimulation in women with endometriosis was less robust than in women with tubal disease; both the peak estradiol concentration and number of oocytes retrieved were lower. Fertilization and implantation rates also were decreased, compared to those in women with all indications for IVF or to women with isolated tubal factor infertility.200 The results further suggested that the adverse effects of endometriosis on fertility were not related solely to anatomical factors. Compared directly, women with severe endometriosis had a lower peak estradiol level, oocyte yield, pregnancy rate, and implantation rate than women with mild disease. In contrast, fertilization rates in women with advanced endometriosis were higher than in those with mild disease, possibly because women with severe endometriosis have a more chronic disease that is less metabolically active.200 Results of the analysis remain controversial, because subsequent studies have yielded conflicting data, observing outcomes not significantly different from those in women with unexplained infertility.212,213 Data from the 2007 U.S. National Report on Assisted Reproductive Technology (ART) indicate that live birth rates for patients with endometriosis (34.3%) were comparable to those for women with diagnoses of tubal factor (30.7%), male factor (35.8%), and unexplained infertility (31.8%).214
The overall lower implantation and pregnancy rates observed in women with endometriosis after IVF could reflect poor oocyte quality and subsequent embryogenesis or decreased endometrial receptivity. Studies of IVF outcomes in donor oocyte recipients offer a means to distinguish the two possibilities. Donor oocytes from healthy women yield similar results in recipients with and without endometriosis, but oocytes from women with endometriosis yield poorer results than those from healthy donors in disease-free recipients.215,216,217,218 and 219 Embryos derived from oocytes retrieved from women with endometriosis also have fewer blastomeres and exhibit a higher incidence of arrested and abnormal development than those derived from women without the disease.218,220 Taken together, these observations suggest that the lower implantation and pregnancy rates observed in women with endometriosis more likely result from abnormalities of oocyte quality and subsequent embryogenesis than from decreased endometrial receptivity.
A wide assortment of studies has been aimed at identifying differences in the follicular environment in women with and without endometriosis that might explain the presumed poor oocyte quality in women with the disease. Studies have compared the numbers and types of resident immune cells, the production of various hormones, growth factors, and cytokines, and the expression of numerous genes in follicular fluid and cultured granulosa cells obtained from women with and without endomtriosis, but no consistent differences have been observed.221
Although the outcomes of donor oocyte IVF cycles in women with and without endometriosis do not suggest that the disease has important adverse effects on endometrial receptivity, gene-expression profiling has identified a number of gene products that may be abnormally up- or down-regulated in the endometrium of women with endometriosis during the putative implantation window, including various cell adhesion molecules, matrix metalloproteinases, transcription factors, growth factors, enzymes, and steroid hormone receptors.130,131,222 If endometriosis does have adverse effects on endometrial receptivity, evidence suggests they can be overcome by IVF treatment regimens in most women.
Retrospective studies have observed an increased risk for early pregnancy loss in women with endometriosis.223,224 and 225 However, in appropriately controlled studies, miscarriage rates in untreated women with endometriosis have been in the ranges normally expected and no higher than in treated women.226,227 and 228
Diagnosis of Endometriosis
Classically, the diagnosis of endometriosis requires histologic evidence of ectopic endometrial glands and stroma, but a tissue diagnosis generally is unnecessary because the physical characteristics of the disease are well-described and easily recognized. Unfortunately, despite substantial advances in our understanding of the pathogenesis of endometriosis, there is not yet any reliable noninvasive alternative to laparoscopy for diagnosis of the disease.
Clinical Diagnosis
The clinical symptoms of endometriosis include dysmenorrhea, pain, dyspareunia, cyclic bowel or bladder symptoms, subfertility, abnormal bleeding, and chronic fatigue. A 2008 cross-sectional survey of 1,000 women with endometriosis found that dysmenorrhea (79%) and pain (69%) were the most common symptoms leading to diagnosis.229 Comparing women with minimal and mild endometriosis to those with more advanced stages of disease, dyspareunia was significantly more common in women with limited disease (51% vs. 39%), whereas subfertility (22% vs. 30%) and an ovarian mass (7% vs. 29%) led to a diagnosis more often in those with advanced endometriosis.229 Interestingly, the time to diagnosis was similar among all women. A large case-control study conducted in the U.K. comparing the prevalence of specific symptoms in women with and without endometriosis observed that a greater proportion of women with endometriosis had abdominal/pelvic pain, dysmenorrhea, or menorrhagia (73% vs. 20%).230 Compared to controls, women with endometriosis had increased risks of abdominal/pelvic pain (OR = 5.2, CI = 4.7—5.7), dysmenorrhea (OR = 8.1, CI = 7.2-9.3), menorrhagia (OR = 4.0, CI = 3.5-4.5), subfertility (OR = 8.2, CI = 6.9-9.9), dyspareunia and/or postcoital bleeding (OR = 6.8, CI = 5.7-8.2) ovarian cysts (OR = 7.3, CI = 5.7-9.4), and for diagnosis of irritable bowel syndrome (OR = 1.6, CI = 1.3-1.8) and pelvic inflammatory disease (OR = 3.0, CI = 2.5-3.6).230 These data demonstrate that whereas specific symptoms are associated with endometriosis, the same symptoms are not
uncommon in women without the disease. They also reveal that endometriosis can coexist with or be misdiagnosed as irritable bowel syndrome or pelvic inflammatory disease. It is not surprising that diagnosis can be delayed, often for a period of years.231
uncommon in women without the disease. They also reveal that endometriosis can coexist with or be misdiagnosed as irritable bowel syndrome or pelvic inflammatory disease. It is not surprising that diagnosis can be delayed, often for a period of years.231
Dysmenorrhea and pain that are new in onset, progressive, or severe strongly suggest, but do not reliably predict endometriosis.232 The dysmenorrhea associated with endometriosis often begins before onset of menstrual flow and usually persists throughout menses, sometimes even beyond. The pain usually is diffuse, located deep in the pelvis, dull and aching, and may radiate to the back and thighs or be associated with rectal pressure, nausea, and episodic diarrhea.233 Pain may be more common, severe, and associated with dyspareunia and painful defecation in women with deeply infiltrating disease involving the cul-de-sac and rectovaginal septum.183,234,235 and 236 Dyspareunia associated with endometriosis usually is new in onset and most intense with deep penetration immediately prior to menstruation.237,238 One-half to two-thirds of women with endometriosis and pain have inter-menstrual pain.187
The severity of endometriosis does not correlate with the number and severity of symptoms; women with advanced disease may have few or no symptoms and those with minimal or mild disease may have incapacitating pain.187,237,238 However, in women with deeply infiltrating endometriosis, the severity of pain generally correlates with the depth and volume of disease.183,234,237 Extra-pelvic endometriosis may be associated with a wide assortment of cyclic symptoms that reflects the organs involved (abdominal scars,239,240 the gastrointestinal and urinary tracts,61,241,242 and 243 the diaphragm,244 the pleura,245 and peripheral nerves246).
Physical findings in women with endometriosis vary widely and, when present, relate to the location and extent of disease.237 The external genitalia are typically normal. Occasionally, speculum examination may reveal characteristic blue-colored implants or red proliferative lesions that bleed on contact, both usually in the posterior fornix. Whereas deeply infiltrating endometriosis involving the recto-vaginal septum frequently is palpable, it is not often visible and may have no obvious signs.235 The uterus often is retroverted and can exhibit decreased mobility or fixation. Women with ovarian endometriomas can have a tender, fixed, adnexal mass. Focal tenderness, thickening, induration, and nodularity of the uterosacral ligaments are the most common, and frequently the only, physical finding.247,248 Physical examination has its greatest diagnostic sensitivity when performed during menstruation, but even then a normal examination does not exclude the diagnosis.249 Overall, compared to the gold standard surgical diagnosis of endometriosis, physical examination has relatively poor sensitivity, specificity, and predictive value.250
CA-125
CA-125 is a cell surface antigen expressed by derivatives of the coelomic epithelium (including the endometrium) and is well-established as a useful marker for the monitoring of women with epithelial ovarian cancer. Levels of CA-125 often are elevated in women with advanced endometriosis,251,252 and 253 but also during early pregnancy and normal menstruation, and in women with acute pelvic inflammatory disease or leiomyomata. Serum CA-125 concentrations vary somewhat across the menstrual cycle; in general, levels are highest during the menstrual phase and lowest during the midfollicular and periovulatory phases of the cycle.249,254,255 However, studies of cycle-dependent assay sensitivity and reproducibility have yielded conflicting results, so there is no one best time to perform the test.250
Serum CA-125 has been advocated as a screening test for diagnosis of endometriosis, but a meta-analysis including 23 studies using surgically diagnosed disease as the gold standard concluded that the marker performs rather poorly.256 Cutoff values that provide 90% overall specificity have less than 30% sensitivity, and if adjusted to achieve even 50% sensitivity,
specificity falls to 70%. As a screening test for advanced stages of endometriosis, values associated with 90% specificity have less than 50% sensitivity.256 Overall, the serum CA-125 concentration does not have the necessary sensitivity to be an effective screening test for the diagnosis of endometriosis.
specificity falls to 70%. As a screening test for advanced stages of endometriosis, values associated with 90% specificity have less than 50% sensitivity.256 Overall, the serum CA-125 concentration does not have the necessary sensitivity to be an effective screening test for the diagnosis of endometriosis.
Serum CA-125 levels may have some value in the preoperative evaluation of women known or suspected to have advanced disease. In one study involving 685 women having surgery for endometriosis, the mean serum CA 125 concentrations were 19, 40, 77, and 182 IU/mL in women with minimal, mild, moderate, and severe disease, respectively; preoperative bowel preparation was suggested for women with levels over 65 IU/mL (upper limit normal 35 IU/mL), as they were more likely to have dense omental adhesions, ruptured endometriomas, or cul-de-sac obliteration.257 Serum CA-125 levels also may be helpful for differentiating ovarian endometriomas from other benign cysts, especially when combined with transvaginal ultrasonography.258,259 Whereas the serum CA-125 generally is not a reliable predictor of the effectiveness of medical therapy,260,261 a sustained elevation of serum CA-125 after surgical treatment predicts a relatively poor prognosis.262,263
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


