Background
Ovarian hyperstimulation syndrome is an iatrogenic complication of controlled ovarian stimulation. Early ovarian hyperstimulation syndrome occurs during luteal phase of controlled ovarian stimulation within 9 days after human chorionic gonadotropin trigger and reflects an acute consequence of this hormone on the ovaries. Late ovarian hyperstimulation syndrome occurs 10 or more days after human chorionic gonadotropin trigger and reflects increased endogenous human chorionic gonadotropin levels following pregnancy. Human chorionic gonadotropin stimulates granulosa-lutein cells to produce vascular endothelial growth factor messenger RNAs, which in turn raises serum vascular endothelial growth factor concentration and increases vascular permeability in women with ovarian hyperstimulation syndrome. Efforts to reduce the incidence and severity of ovarian hyperstimulation syndrome after oocyte retrieval, and in particular primary prevention efforts, are vital to prevent thrombogenesis and other serious complications.
Objective
The objective of the study was to compare the efficacy of letrozole, an aromatase inhibitor, with aspirin in primary prevention of early ovarian hyperstimulation syndrome and to compare vascular endothelial growth factor levels between groups.
Study Design
Participants in this prospective randomized trial included 238 participants undergoing cryopreservation of the whole embryos after oocyte retrieval with at least 1 of the following high-risk factors for ovarian hyperstimulation syndrome: oocyte retrieval ≥25; estradiol level ≥5000 pg/mL on the day of human chorionic gonadotropin administration; and clinical or ultrasonographic evidence of ovarian hyperstimulation syndrome on the day of oocyte retrieval, such as ultrasonographic evidence of ascites. After human chorionic gonadotropin triggering, experimental (119 cases) and control (119 cases) groups received letrozole and aspirin, respectively, for 5 days. The 5 categories of ovarian hyperstimulation syndrome include no, yes-mild, yes-moderate, yes-severe, and yes-critical. The primary outcome was the incidence and severity of early ovarian hyperstimulation syndrome. The secondary outcome included vascular endothelial growth factor level both on the second and seventh day after the human chorionic gonadotropin trigger, and clinical and laboratory features of ovarian hyperstimulation syndrome symptoms.
Results
The incidence of ovarian hyperstimulation syndrome was significantly higher in women receiving aspirin, compared with letrozole (90.2% vs 80.4%, P = .044). Moderate and severe ovarian hyperstimulation syndrome was also higher in the aspirin group, 45.1%, compared with the letrozole group, 25.0% ( P = .002). Moreover, the duration of luteal phase was shortened in letrozole group compared with aspirin group (8.1 ± 1.1 days vs 10.5 ± 1.9 days, P < .001). The vascular endothelial growth factor level was significantly higher in the letrozole-treated group than aspirin-treated group (0.49 ± 0.26 vs 0.42 ± 0.22, P = .029).
Conclusion
Letrozole was more effective than aspirin in decreasing the incidence of moderate and severe early-onset ovarian hyperstimulation syndrome. Our results indicate that ovarian hyperstimulation syndrome might be caused through a luteolytic effect rather than through modulation of vascular endothelial growth factor, racing by a decline in estradiol and termination of early-onset ovarian hyperstimulation syndrome in advance in high-risk women with cryopreservation of the whole embryos.
Ovarian hyperstimulation syndrome, an iatrogenic complication of controlled ovarian stimulation occurring during luteal phase or early pregnancy, is exclusively associated with an exaggerated ovarian response to exogenous gonadotropin stimulation. The incidence of moderate to severe ovarian hyperstimulation syndrome is 3.1–8.0% for in vitro fertilization cycles but can reach to 20.0% for high risk women.
The disease severity of ovarian hyperstimulation syndrome can be classified as mild, moderate, severe, and critical according to clinical manifestations and laboratory findings. Compared with the patients with mild ovarian hyperstimulation syndrome, more organs are affected in the severe and critical subsets.
Lyons et al were the first to describe 2 distinct patterns of ovarian hyperstimulation syndrome: the early and late forms. Early ovarian hyperstimulation syndrome occurs in the luteal phase of controlled ovarian stimulation within 9 days after human chorionic gonadotropin trigger and reflects an acute consequence of exogenous human chorionic gonadotropin on ovaries. On the other hand, late ovarian hyperstimulation syndrome occurs 10 days after a human chorionic gonadotropin trigger and is the consequence of an increase of endogenous human chorionic gonadotropin levels following pregnancy.
Ovarian hyperstimulation syndrome prevention is a multistage process and can be classified into 2 main categories: primary and secondary. The key step of primary ovarian hyperstimulation syndrome prevention is to recognize the preexisting risk factors and individualize the ovarian stimulation protocol appropriately.
Secondary prevention, on the other hand, is extended to patients who have mounted an exaggerated response to controlled ovarian stimulation and aims to prevent progression to ovarian hyperstimulation syndrome, for example, through cryopreservation of embryos. However, even with embryo cryopreservation, patients are still exposed to exogenous human chorionic gonadotropin, and early ovarian hyperstimulation syndrome cannot be avoided completely.
Although human chorionic gonadotropin has no direct vasoactive properties, it stimulates granulosa-lutein cells to produce vascular endothelial growth factor/vascular endothelial growth factor receptor-2 messenger RNAs, which in turn raises serum vascular endothelial growth factor concentration and increases vascular permeability in ovarian hyperstimulation syndrome. Soares et al pointed out that expression of vascular endothelial growth factor/vascular endothelial growth factor receptor-2 messenger RNAs correlates with enhanced vascular permeability, and both peak at 48 hours after human chorionic gonadotropin injection.
Recently the administration of letrozole during the luteal phase of in vitro fertilization cycles offers another therapeutic modality for patients at high risk of ovarian hyperstimulation syndrome because letrozole drastically reduces estradiol levels by blocking human aromatase in a potent, specific, and reversible way. Indeed, 2 randomized controlled trials that evaluated administration of letrozole during luteal phase in in vitro fertilization cycles concluded that letrozole drastically decreased estradiol concentration, preventing ovarian hyperstimulation syndrome.
In this regard, letrozole may offer a promising selection for patients at high risk for ovarian hyperstimulation syndrome who cryopreserve their embryos aiming to reduce the potential risk that high estradiol concentrations pose. It has been known that vascular endothelial growth factor is the major mediator of ovarian hyperstimulation syndrome. However, these 2 randomized controlled trials had not tested the vascular endothelial growth factor level during the luteal phase after oocyte retrieval under letrozole treatment, especially during the decline in estradiol concentration observed following letrozole administration.
Low-dose aspirin has been recommended as a regimen to prevent or reduce the severity of ovarian hyperstimulation syndrome symptoms. Here we designed a prospective randomized study to investigate whether letrozole treatment after oocyte retrieval is more effective in preventing early ovarian hyperstimulation syndrome development than traditional aspirin preventive administration in women at high risk for ovarian hyperstimulation syndrome. Our secondary aim was to compare vascular endothelial growth factor levels between treatment groups both on the second and seventh day after the human chorionic gonadotropin trigger.
Materials and Methods
This was a prospective, randomized trial conducted at the Reproductive Medicine Center of the First Affiliated Hospital, Sun Yat-sen University. This trial was registered at ClinicalTrials.gov (registration number NCT02670304 ) and was approved by the Institutional Review Board of The First Affiliated Hospital, Sun Yat-sen University. Written informed consent was obtained from all of the participants prior to the trial.
Study participants
The participants were recruited from the Reproductive Medicine Center of the First Affiliated Hospital, Sun Yat-sen University from January 2012 to August 2013. The participants were potentially eligible for the trial if they were at high risk of developing ovarian hyperstimulation syndrome with cancellation of fresh embryo transfer and cryopreservation of the whole embryos after oocyte retrieval.
For inclusion, participants had at least one of these high-risk criteria: number of oocyte retrieval ≥25; estradiol level ≥5000 pg/mL on the day of human chorionic gonadotropin administration; and clinical or ultrasonography proven ovarian hyperstimulation syndrome on the day of oocyte retrieval, such as ultrasonographic evidence of ascites.
The couples were given counseling regarding the risk and symptom of ovarian hyperstimulation syndrome, and all agreed to cryopreserve the whole embryos. Screening could be initiated at any time during the controlled ovarian stimulation cycle because the risk of ovarian hyperstimulation syndrome may become apparent around the time of human chorionic gonadotropin trigger, whereas the randomization day was fixed to the day of oocyte retrieval. For example, on the day of the human chorionic gonadotropin trigger, some participants may have had an estradiol level ≥5000 pg/mL or ultrasonography evidence of ascites and the risk of ovarian hyperstimulation syndrome was apparent, and then the randomization was made on the day of oocyte retrieval.
Participants were excluded if they had contraindications to letrozole, including severe liver and renal dysfunction. If coasting, which is defined as withholding gonadotropin stimulation during controlled ovarian stimulation resulting in atresia of small follicles, or other preventive measures for managing ovarian hyperstimulation syndrome had been applied, the patient would not enter the trial. Participants could be allowed to enter the study only once.
Study design and drug regimen
Participants underwent gonadotropin-releasing hormone agonist protocols, which was use of other than a midluteal long gonadotropin-releasing hormone agonist (Daphiline Beaufour, IPSEN, Paris, France; 0.8–1.0 mg, subcutaneously) suppressive protocol or short gonadotropin-releasing hormone agonist (Daphiline Beaufour, IPSEN; 0.05–0.1 mg, subcutaneously) suppressive protocol.
In each protocol, gonadotropins (Gonal-F; Laboratories Serono SA, Geneva, Switzerland) were administered in variable doses, depending on individual age and/or ovarian responsiveness in previous cycles, and further adjusted according to serum estradiol levels and vaginal ultrasound measurement of follicular diameter, obtained every 2 or 3 days. Final oocyte maturation was achieved by administration of 250 μg recombinant human chorionic gonadotropin (Ovidrel; Laboratories Serono SA) once at least 3 follicles reach a diameter ≥17 mm on ultrasound. Oocytes were aspirated under transvaginal ultrasonography guiding approximately 36 hours after human chorionic gonadotropin injection (human chorionic gonadotropin plus 2). No luteal support was given.
Participants with high-risk ovarian hyperstimulation syndrome undergoing cryopreservation of the whole embryos after oocyte retrieval were randomly assigned into 2 groups according to a computer-generated randomization list. The experimental group received letrozole (Femara, 2.5 mg twice daily; Novartis, Basel, Switzerland) treatment from the day of oocyte retrieval (human chorionic gonadotropin plus 2) for 5 days, whereas the control group received aspirin (Bayer; 100 mg daily, plus folic acid tablets as placebo of letrozole) for 5 days. Participants and physicians were blinded to the study group assignments throughout the trial.
Endocrine determination
Patient characteristics, including age, antral follicle count, basal follicle-stimulating hormone, luteinizing hormone, estradiol levels, and body mass index were collected from the database. The baseline hormone values were obtained on the third day of menstrual cycle, 3 months before the treatment cycle. On the day of oocyte retrieval (human chorionic gonadotropin plus 2) and the fifth day (human chorionic gonadotropin plus 7) after administration of letrozole or aspirin, the blood samples were taken from the ulnar vein of each participant to determine vascular endothelial growth factor and estradiol level.
Vascular endothelial growth factor was quantified by an enzyme-linked immunosorbent assay approach using human vascular endothelial growth factor enzyme-linked immunosorbent assay kits (Ray Biotech, Inc, Norcross, GA). For vascular endothelial growth factor, detectability was 10 pg/mL, and the intraassay and interassay coefficients of variation were <10% and <12%, respectively.
Serum samples were subjected to chemiluminescent microparticle immunoassays to determine estradiol levels using commercially available kits and a c4000 Architect system (Abbott Diagnostic Division, Abbott Laboratories, Chicago, IL). Estradiol was additionally quantitated using the Abbott Architect Estradiol Assay 200 (revised 2004) 1-step chemiluminescent microparticle immunoassays. Assay sensitivity was <10 pg/mL for estradiol, and the intraassay and interassay coefficients of variation were <5.6% and <4.9%, respectively.
Classification of ovarian hyperstimulation syndrome
The severity of ovarian hyperstimulation syndrome was evaluated according to clinical manifestations and laboratory findings as previously reported. A brief description of the classification of ovarian hyperstimulation syndrome severity is shown in Table 1 . Ascites and pleural effusion were monitored by ultrasound and recorded for each participant on the day of oocyte retrieval (human chorionic gonadotropin plus 2) and the fifth day (human chorionic gonadotropin plus 7) after administration of letrozole or aspirin. Biochemical values such as hematocrit, white blood cell count, blood urea nitrogen, creatinine, liver enzymes, serum potassium, and sodium were measured as well.
| OHSS stage | Clinical features | Laboratory features |
|---|---|---|
| Mild | Abdominal bloating/discomfort | No important laboratory alterations |
| Mild nausea/vomiting | ||
| Diarrhea | ||
| Enlarged ovaries | ||
| Moderate | Mild features + | Elevated hematocrit (>41%) |
| Ultrasonographic evidence of ascites | Elevated WBC (>15*10 9 /L) | |
| Hypoproteinemia | ||
| Severe | Mild and moderate features + | Hemoconcentration (hematocrit >55%) |
| Clinical evidence of ascites | WBC >25*10 9 /L | |
| Hydrothorax | Creatinine clearance <50 mL/min | |
| Severe dyspnea | Creatinine >115 μmol/L | |
| Oliguria/anuria | Na+ <135 mmol/L | |
| Intractable nausea/vomiting | K+ >5.0 mmol/L | |
| Tense ascites | Elevated liver enzymes | |
| Low blood/central venous pressure | ||
| Rapid weight gain (>1 kg in 24 h) | ||
| Syncope | ||
| Severe abdominal pain | ||
| Venous thrombosis | ||
| Critical | Anuria/acute renal failure | Worsening of findings |
| Arrhythmia | ||
| Thromboembolism | ||
| Pericardial effusion | ||
| Large pleural effusion | ||
| Arterial thrombosis | ||
| Adult respiratory distress syndrome |
Liver dysfunction is defined as elevated liver enzyme, including alanine aminotransferase >80 U/L. Renal dysfunction was defined as creatinine >115 μmol/L or creatinine clearance rate <50 mL/min. Electrolyte imbalance was defined as sodium < 135 mmol/L and potassium >5.0 mmol/L. Elevated white blood cell count was defined as >15 × 10 9 /L. Hemoconcentration was defined as hematocrit >0.55. Evidence of ascites includes ultrasonographic detection of ascites or clinical indication of ascites. Patients were followed up until menstruation.
Outcome measures
The primary outcome measure was the incidence and severity of early ovarian hyperstimulation syndrome. The 5 categories of ovarian hyperstimulation syndrome include no, yes-mild, yes-moderate, yes-severe, and yes-critical. The secondary outcomes included vascular endothelial growth factor level and clinical and laboratory features of ovarian hyperstimulation syndrome symptoms.
Statistical analysis
To achieve 80% power to detect a minimally important absolute difference of 20% between the letrozole and the aspirin groups with respect to the rate of early-onset ovarian hyperstimulation syndrome (from 60% to 80%) at an alpha level of 0.05, a minimum of 83 participants should be needed for each study group. To account for a 5% rate of loss to follow-up, at least 175 participants were required for the present trial.
Data analyses were performed using SPSS 13 package program (SPSS Inc, Chicago, IL). Categorical data were presented as number and percentage. Continuous variables were given as mean ± SD and were tested using the Student t test if the variables presented normal distribution. Statistics was given as median and range when the assumption of normality was violated. Nonparametric test (Wilcoxon Signed Ranks Test) was applied to compare median between groups. Pearson’s χ 2 test was used as well when appropriate. Fisher’s exact test and the Yates correction for continuity was used to complete the χ 2 test when appropriate.
Ordinal logistic regression analysis (5 categories of ovarian hyperstimulation syndrome served as dependent variables) was also used for the association with clinical variables and ovarian hyperstimulation syndrome after letrozole or aspirin treatment.
All analyses of significance were 2 sided, and a value of P < .05 was considered statistically significant.
Results
Study participants
From January 2012 to August 2013, 238 women who were eligible and willing to participate in the trial were randomly assigned into 2 groups to receive either letrozole (119 women) or aspirin (119 women) ( Figure 1 ) treatment. The follow-up rate of the primary outcome was 100%.
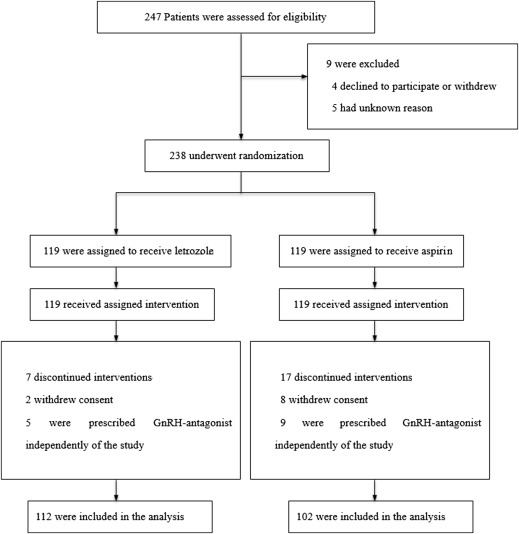
The patient characteristics of both groups were similar ( Table 2 ), with no statistically significant difference in age, body mass index, antral follicle count, basal follicle-stimulating hormone, luteinizing hormone level, and mean number of oocytes retrieved. The basal estradiol level of aspirin group was significantly higher than that of letrozole group (34.1 ± 15.8 vs 30.3 ± 11.4 pg/mL, P = .038). The total gonadotropin dosage of aspirin group was significantly higher than that of the letrozole group (2029.3 ± 662.1 vs 1624.9 ± 426.6 IU, P < .001).
| Letrozole (n = 119) | Aspirin (n = 119) | P value | |
|---|---|---|---|
| Female age, y | 29.1 ± 3.5 | 30.0 ± 3.7 | .061 |
| PCOS, n, % | 19 (16.0) | 16 (13.4) | .583 |
| Duration of infertility, y | 4.1 ± 2.2 | 3.9 ± 2.1 | .452 |
| BMI, kg/m 2 | 20.6 ± 3.2 | 20.7 ± 2.1 | .836 |
| AFC | 16.2 ± 5.6 | 16.9 ± 5.0 | .306 |
| Cause of infertility, n, % | |||
| Tubal factor | 36 (30.3) | 31 (26.1) | .471 |
| Male factor | 23 (19.3) | 29 (24.4) | .347 |
| Endometriosis | 9 (7.6) | 7 (5.9) | .605 |
| PGD for chromosomal abnormality | 6 (5.0) | 7 (5.9) | .775 |
| PGD for thalassemia of both partners | 7 (5.9) | 10 (8.4) | .450 |
| Multiple factors | 32 (26.9) | 26 (21.8) | .365 |
| Unexplained infertility | 6 (5.0) | 9 (7.6) | .424 |
| Basal FSH, IU/L | 5.3 ± 1.2 | 5.2 ± 1.0 | .562 |
| Basal LH, IU/L | 3.7 ± 1.9 | 3.4 ± 1.3 | .091 |
| Basal E2, pg/ml | 30.3 ± 11.4 | 34.1 ± 15.8 | .038 |
| Total Gn, IU | 1624.9 ± 426.6 | 2029.3 ± 662.1 | .003 |
| E2 on HCG day ≥5000, pg/mL, n, % | 78 (65.5) | 89 (74.8) | .119 |
| Oocytes, n | 30.0 ± 7.8 | 29.9 ± 6.1 | .985 |
Here the serum estradiol level >5000 pg/mL was classified as 5000 pg/mL because the samples would not be diluted any further. As a result, there was no comparison between the 2 groups regarding the estradiol levels on the day of human chorionic gonadotropin administration.
A total of 5283 in vitro fertilization retrieval cycles occurred in the time frame of the study at our center. The differences between our study group and other patients treated during this time frame who did not have high-risk factors for ovarian hyperstimulation syndrome are shown in Table 3 .



