Background
The ability to provide safe and effective pharmacotherapy during obstetric complications, such as preterm labor or postpartum hemorrhage, is hampered by the systemic toxicity of therapeutic agents leading to adverse side effects in the mother and fetus. Development of novel strategies to target tocolytic and uterotonic agents specifically to uterine myocytes would improve therapeutic efficacy while minimizing the risk of side effects. Ligand-targeted liposomes have emerged as a reliable and versatile platform for targeted drug delivery to specific cell types, tissues or organs.
Objective
Our objective was to develop a targeted drug delivery system for the uterus utilizing an immunoliposome platform targeting the oxytocin receptor.
Study Design
We conjugated liposomes to an antibody that recognizes an extracellular domain of the oxytocin receptor. We then examined the ability of oxytocin receptor–targeted liposomes to deliver contraction-blocking (nifedipine, salbutamol and rolipram) or contraction-enhancing (dofetilide) agents to strips of spontaneously contracting myometrial tissue in vitro (human and mouse). We evaluated the ability of oxytocin receptor–targeted liposomes to localize to uterine tissue in vivo, and assessed if targeted liposomes loaded with indomethacin were capable of preventing lipopolysaccharide-induced preterm birth in mice.
Results
Oxytocin receptor–targeted liposomes loaded with nifedipine, salbutamol or rolipram consistently abolished human myometrial contractions in vitro, while oxytocin receptor–targeted liposomes loaded with dofetilide increased contraction duration. Nontargeted control liposomes loaded with these agents had no effect. Similar results were observed in mouse uterine strips. Following in vivo administration to pregnant mice, oxytocin receptor–targeted liposomes localized specifically to the uterine horns and mammary tissue. Targeting increased localization to the uterus 7-fold. Localization was not detected in the maternal brain or fetus. Targeted and nontargeted liposomes also localized to the liver. Oxytocin receptor–targeted liposomes loaded with indomethacin were effective in reducing rates of preterm birth in mice, whereas nontargeted liposomes loaded with indomethacin had no effect.
Conclusion
Our results demonstrate that oxytocin receptor–targeted liposomes can be used to either inhibit or enhance human uterine contractions in vitro. In vivo, the liposomes localized to the uterine tissue of pregnant mice and were effective in delivering agents for the prevention of inflammation-induced preterm labor. The potential clinical advantage of targeted liposomal drug delivery to the myometrium is reduced dose and reduced toxicity to both mother and fetus.
Click Supplemental Materials under article title in Contents at ajog.org
Introduction
Complications arising from preterm birth (PTB) are the leading cause of death among children age <5 years, accounting for nearly 1 million deaths in 2013, while postpartum hemorrhage (PPH) is the leading cause of maternal mortality worldwide, accounting for up to 27.1% of maternal deaths. Given that both can arise from dysregulation of uterine contractility, the need exists for safe and effective clinical interventions capable of modifying myometrial contractions to improve treatment of women in preterm labor, to induce or facilitate labor and to prevent or treat PPH, without adverse off-target effects on either the mother or fetus.
When a woman presents with preterm labor, attempts are often made to halt contractions by administering tocolytics that inhibit or block components of the contraction cascade. A recent study proposed that “the ideal tocolytic agent should be myometrium-specific, easy to administer, inexpensive, effective in preventing PTB and improve neonatal outcomes, with few maternal, fetal, and neonatal side effects, and without long-term adverse effects.” Standard therapy varies from country to country, but tocolysis may involve the administration of calcium channel blockers, such as nifedipine (NIF); β 2 -adrenergic receptor agonists, such as salbutamol (SAL); an oxytocin receptor (OTR) antagonist, such as atosiban; or a prostaglandin synthetase inhibitor, such as indomethacin (IND). Unfortunately, the systemic administration of these therapies and lack of specificity means that large doses need to be administered to achieve a therapeutic effect at the target tissue, the myometrium. Maternal side effects of β 2 -adrenergic receptor agonists include tremors, heart palpitations, and tachycardia, as well as myocardial ischemia and pulmonary edema. NIF has been associated with fewer side effects, however approximately 1% of women experience a severe side effect and a further 1% experience mild adverse side effects. Atosiban is associated with the lowest side effect risk but the efficacy of this agent is disputed. Usefulness of IND is limited by fetal side effects, such as premature closure of the ductus arteriosus. Achieving targeted drug delivery to the myometrium would reduce the quantity of drug required to achieve therapeutic efficacy, reduce the likelihood of maternal and fetal side effects, and would therefore represent a significant advancement for maternal-fetal medicine.
Targeted liposomes have emerged as a platform for achieving the delivery of drugs to specific tissues. Liposomes are artificial vesicles that range in size from 50-1000 nm, and are composed of ≥1 phospholipid bilayers. Liposomes are able to encapsulate both lipophilic and/or hydrophilic drugs, and are nontoxic and biodegradable with minimal immunogenicity. Liposomal encapsulation improves the pharmacokinetics of drugs, particularly if the liposome surface is PEGylated, which reduces uptake by the reticuloendothelial system and prolongs half-life. This has led to the development of liposomal-based preparations of various agents, including doxorubicin, amphotericin B, daunorubicin, and verteporfin. Ligand-targeted liposomes offer the potential for site-specific delivery of drugs to designated cell types or organs in vivo that selectively express specific cell surface cognate receptors. Although many types of targeting molecules are available, such as peptides/proteins and carbohydrates, the coupling of antibodies to the liposome surface to create immunoliposomes has many advantages. One advantage of using antibodies is their stability and higher binding avidity because of the presence of dual binding sites. For example, liposomes coated with antibodies to intercellular adhesion molecule (ICAM)-1 have been developed for the treatment of inflammatory diseases. Administration of ICAM-1-targeted immunoliposomes loaded with an analgesic agent demonstrated specific localization and therapeutic efficacy exclusively in peripheral inflammatory tissue. All control groups (free drug solution, empty nontargeted liposomes, drug-loaded nontargeted liposomes, and empty ICAM-1-targeted immunoliposomes) showed no significant therapeutic response.
The aim of this study was to develop a means of targeting therapeutic agents to uterine myometrial tissue, to allow therapeutic modification of myometrial contractions in obstetric settings, such as preterm labor, labor induction, and PPH. The expression of the OTR is significantly up-regulated in myometrial cells approaching term. Here we report the development of OTR-targeted PEGylated immunoliposomes loaded with traditional tocolytics, such as NIF and SAL, as well as rolipram (ROL), a phosphodiesterase (PDE)4 inhibitor and potent inhibitor of myometrial contractions. Moreover, we report enhancement of human myometrial contraction duration in vitro through liposomal delivery of dofetilide (DOF), a hERG channel blocker that increases myometrial contraction duration, demonstrating that this delivery platform can be used to either inhibit or enhance contractions in human myometrial tissue. We demonstrate that intravenously (IV) administered OTR-targeted liposomes localize specifically to the uterine tissue of pregnant mice in vivo. Finally, using an inflammatory mouse model of PTB (lipopolysaccharide [LPS] administration), we show that OTR-targeted liposomes loaded with IND are effective in preventing PTB, while IND-loaded nontargeted liposomes have no effect.
Materials and Methods
Myometrial tissue acquisition
Human studies
These studies were performed in Newcastle, Australia, and were approved by the Hunter and New England Area Human Research Ethics Committee, adhering to guidelines of the University of Newcastle and John Hunter Hospital, Newcastle, Australia (02/06/12/3.13). All participants gave informed written consent. Collection of myometrial samples (5 × 5 × 10 mm) occurred from the lower uterine segment of term singleton pregnancies. All women were examined clinically, and those with signs of infection were excluded. Women were undergoing term elective cesarean delivery and were not in labor (NIL); the clinical indications for elective NIL cesarean delivery were previous cesarean delivery or previous third-/fourth-degree tear. All participants ranged from 37-40 completed weeks of gestation. Following delivery of the placenta, all women immediately received 5 U of oxytocin (Syntocinon) into an IV line, which was administered as standard care. Myometrial biopsies were excised 3-5 minutes after oxytocin administration, thus all samples were briefly exposed to oxytocin. After biopsy, myometrial samples were dissected from connective tissue and washed in ice-cold physiological saline.
Mouse in vitro studies
Mouse uterine horns were dissected from pregnant CD1 Swiss mice (8-10 weeks of age) at term gestation prior to the onset of labor (fetal gestation day [GA] 18). Mouse studies were approved by the University of Newcastle Animal Ethics Committee (A-2014-400/A-2014-429). All mice were housed under SPF/PC2 conditions under a 12-hour light-day cycle and had food and water available ad libitum.
Liposome manufacture
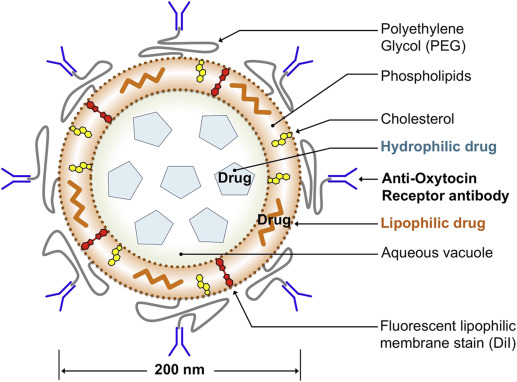
Liposomes containing NIF, SAL, ROL, DOF (each at approximately 4 mg/mL), or IND (approximately 5.5 mg/mL), as determined by high-performance liquid chromatography, were manufactured as previously outlined. Liposomes were composed of 1,2-distearoyl-sn-glycero-2-phosphocholine (DSPC) and cholesterol in a molar ratio of 2:1, and contained 1,2-distearoyl- sn -glycero-3-phospho-ethanolamine-N-[maleimide (polyethylene glycol)-2000] (DSPE-PEG(2000) maleimide) at 1.5 mol percent of DSPC as a coupling lipid (Avanti Polar Lipids). The resulting multilamellar dispersions were reduced in size and lamellarity to approximately 200 nm in diameter by high-pressure extrusion. The activated liposome suspension was then mixed with thiolated polyclonal anti-OTR antibody (catalog no. ab115664; Abcam, Cambridge, MA), which was prepared by first conjugating 25 μg of OTR antibody with a heterobifunctional reagent N-succinimidyl-3-(2-pyridyldithio) propionate ( Figure 1 ). The OTR antibody recognizes an extracellular domain of the human OTR. Nontargeted liposomes were coated with rabbit IgG. All liposomes incorporated the membrane stain 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) for fluorescent detection. Unconjugated antibody and nonencapsulated drug was removed by centrifugal filtration of the liposomes through a 100-kd molecular weight filter (Amicon Ultra-15). Amicon Ultra-15 filters were washed with Milli-Q H 2 O before 500 μL of liposome suspension was loaded into the filter reservoir. Liposomes were diluted with 5 mL of sterile 0.9% saline and centrifuged at 4000 x g until retentate volume was <250 μL. Liposomes were then resuspended in a further 5 mL of 0.9% saline and centrifuged until retentate volume was <250 μL. Filtered liposomes were then collected, transferred to a fresh Eppendorf tube, and redispersed to an original volume of 500 μL.
The size distribution of the liposomal dispersion was determined by dynamic laser light scattering (Zetasizer Nano S, ATA Scientific). Encapsulation efficiency was determined by disrupting the vesicles with ethanol and evaluating drug concentration using high-performance liquid chromatography. Quantification of the amount of antibody associated with liposomes was determined using the CBQCA protein assay (ThermoFisher Scientific Inc, Waltham, MA), using bovine serum albumin for the preparation of the standard curve.
Myometrial contractility studies
Myometrial strips were set up as previously described. Briefly, NIL human myometrial samples, or uterine horns obtained from pregnant CD1 Swiss mice, were dissected into strips (10 × 2 × 2 mm) and suspended in organ baths containing 30 mL of physiological saline solution (PSS) containing 120 mmol/L of NaCl, 5 mmol/L of KCl, 25 mmol/L of NaHCO 3 , 1 mmol/L of KH 2 PO 4 , 1.2 mmol/L of MgSO 4 , 2.5 mmol/L of CaCl 2 , and 11 mmol/L of glucose, and continuously gassed with carbogen (95% O 2 , 5% CO 2 ) at pH 7.4. Strips were connected to a Grass FT03C force transducer (Grass Instruments, Quincy, MA) and 1 g passive tension applied (1 g was calibrated to equal 1 V). PSS was replaced 5 times during the first hour, with strips retensioned to 1 g passive tension following each wash. Thereafter strips were maintained at 37°C until spontaneous rhythmic contractions developed. Data were digitized using a MacLab/8E data-acquisition system and contraction status visualized in real time using Chart software (ADInstruments, Dunedin, New Zealand). For each strip a contraction baseline was acquired to serve as reference.
To administer liposome treatments, 600 μL of PSS buffer was carefully extracted from an organ bath and transferred to an Eppendorf tube. The appropriate volume of liposome preparation (mixed by inversion) was pipetted into the PSS to predilute the liposomes. The total volume of prediluted liposomes (600 μL PSS + liposomes) was then carefully reinjected back into the appropriate organ bath. Final concentrations of each drug were: NIF 7.7 μmol/L, SAL 9.25 μmol/L, ROL 19.4 μmol/L, and DOF 3.0 μmol/L. Doses were based on prior investigations of the nonencapsulated drug (in vitro contraction assays using human myometrium). Where treated tissue was not washed, tissue strips remained in the presence of the liposomes for the duration of the assay. Where washout studies were performed, organ baths were twice drained of buffer and refilled with 37°C PSS. Human tissue strips were washed after 1 hour and 25 minutes whereas mouse tissue strips were washed after 15 minutes.
Tension generated by tissue strips is indicated in the results and representative contraction traces. The effect of treatments is interpreted relative to the pretreatment contraction baseline, which consisted of ≥3 contractions of consistent frequency and amplitude.
In vivo biodistribution study
Timed-mated CD1 Swiss pregnant mice were injected with drug-free, DiI-labeled preparations of either nontargeted or OTR-targeted liposomes on fetal GA 17 and 18 at 4:00 pm . Mice that labored overnight were euthanized on the morning of day 19 (9:00-11:00 am ) by CO 2 asphyxiation. Maternal internal organs of interest (heart, brain, liver, lung, kidney, uterus, and mammary tissue) were harvested and transferred to a Petri dish along with a sacrificed neonate. The Petri dish was loaded into an in vivo imaging system (IVIS-100) (Xenogen, Alameda, CA) and a light image captured. Tissues were then imaged under conditions appropriate for the detection of DiI (excitation: 554 nm; emission: 583 nm; filter: DsRed; exposure: 4 seconds; field of view: 10; binning: 4). Organs were imaged 17-19 hours after the second injection, following labor. Background signal was subtracted from the detected signal to produce the final fluorescence image. Fluorescence signal is reported as radiance (p/s/cm 2 /sr). The radiance range was kept constant across all images (min = 2.0 × 10 8 ; max = 1.8 × 10 9 ).
PTB study
Time-mated pregnant CD1 Swiss mice were administered 0.7 μg/g LPS from Escherichia coli (0111:B4) (Sigma-Aldrich) via intraperitoneal (IP) injection at 12:00 pm on GA 15 (1-time injection). LPS dose had been previously optimized to result in PTB rates of 50-70%. Total IP injection volume was 150 μL in saline.
At 4:00 pm on GA 15, mice began receiving daily IV injections of IND free-drug or liposomal preparations according to assigned treatment groups. Treatment groups are indicated in Table 1 . Total IV injection volume was 150 μL. Mice were monitored for onset of labor every 6 hours. Treatments were repeated daily at 4:00 pm until all mice labored. Term gestation was 19-22 days. Mice that labored within 48 hours of receiving LPS (GA 17) were deemed to have labored preterm.
| Group | One-time IP injection (12:00 pm on GA 15, 150 μL) | Daily IV injections (4:00 pm , GA ≥15, 150 μL) |
|---|---|---|
| 1 | Saline | Saline |
| 2 | 0.7 μg/g LPS | 50% DMSO |
| 3 | 0.7 μg/g LPS | 1.0 mg/kg/d IND in 50% DMSO |
| 4 | 0.7 μg/g LPS | 2.0 mg/kg/d IND in 50% DMSO |
| 5 | 0.7 μg/g LPS | OTR-targeted, drug-free liposomes in saline |
| 6 | 0.7 μg/g LPS | 2.0 mg/kg/d IND via nontargeted liposomes in saline |
| 7 | 0.7 μg/g LPS | 2.0 mg/kg/d IND via OTR-targeted liposomes in saline |
Statistical analyses
For contraction traces, LabChart software (ADInstruments) was used to determine the area under the curve (AUC) ( g tension × seconds) for the 30 minutes prior to treatment (pretreatment) and 30 minutes after treatment (posttreatment). AUC before and after treatment was compared by 2-tailed paired t test (GraphPad Prism).
For DOF studies, contraction plateau duration (seconds) was determined for 4 contractions pretreatment and posttreatment using LabChart software. Plateau duration was determined as the time between the point of highest amplitude and point where contraction force declined sharply. Contraction duration data were obtained for 3 individual tissues (n = 3 women). Pretreatment and posttreatment measurements (n = 12 each) were compared by 2-tailed unpaired t test.
Average radiance (p/s/cm 2 /sr) was determined for each organ of interest using Living Image software (v2.5). Where fluorescence was detected, regions of interest were applied automatically (contour). Where detection was low or absent, regions of interest were specified manually (circles or squares) to tightly encompass the tissue being analyzed. Data were tested for normality by the Shapiro-Wilk normality test (GraphPad Prism). Average radiance for each organ/tissue was compared between treatment groups (n = 4 animals per group) by 1-way analysis of variance (ANOVA) with multiple comparisons (Holm-Sidak) (GraphPad Prism).
For the preterm labor studies, rate of PTB was compared between treatment groups by χ 2 analysis. Time (hours) between LPS injection and labor was calculated. Data were transformed (Y = Y 2 ) to obtain normal distribution (D’Agostino and Pearson normality test) and analyzed by 1-way ANOVA with multiple comparisons (Tukey). Data recorded for number of pups for term deliveries were normally distributed (Shapiro-Wilk normality test) and analyzed by 1-way ANOVA with multiple comparisons (Tukey). Preterm deliveries did not yield any viable pups.
Consumables and reagents
Mice were supplied by the University of Newcastle Animal Support Unit. NIF (catalog no. 1075), SAL hemisulfate (catalog no. 0634), ROL (catalog no. 0905), and DOF (catalog no. 3757) were purchased from Tocris (Bristol, United Kingdom). IND (catalog no. L2630) was purchased from Sigma-Aldrich Pty Ltd (Sydney, Australia). Anti-OTR antibody (ab115664) was purchased from Abcam. Other miscellaneous reagents were purchased from Sigma-Aldrich Pty Ltd and ThermoFisher Scientific Inc.
Materials and Methods
Myometrial tissue acquisition
Human studies
These studies were performed in Newcastle, Australia, and were approved by the Hunter and New England Area Human Research Ethics Committee, adhering to guidelines of the University of Newcastle and John Hunter Hospital, Newcastle, Australia (02/06/12/3.13). All participants gave informed written consent. Collection of myometrial samples (5 × 5 × 10 mm) occurred from the lower uterine segment of term singleton pregnancies. All women were examined clinically, and those with signs of infection were excluded. Women were undergoing term elective cesarean delivery and were not in labor (NIL); the clinical indications for elective NIL cesarean delivery were previous cesarean delivery or previous third-/fourth-degree tear. All participants ranged from 37-40 completed weeks of gestation. Following delivery of the placenta, all women immediately received 5 U of oxytocin (Syntocinon) into an IV line, which was administered as standard care. Myometrial biopsies were excised 3-5 minutes after oxytocin administration, thus all samples were briefly exposed to oxytocin. After biopsy, myometrial samples were dissected from connective tissue and washed in ice-cold physiological saline.
Mouse in vitro studies
Mouse uterine horns were dissected from pregnant CD1 Swiss mice (8-10 weeks of age) at term gestation prior to the onset of labor (fetal gestation day [GA] 18). Mouse studies were approved by the University of Newcastle Animal Ethics Committee (A-2014-400/A-2014-429). All mice were housed under SPF/PC2 conditions under a 12-hour light-day cycle and had food and water available ad libitum.
Liposome manufacture
Liposomes containing NIF, SAL, ROL, DOF (each at approximately 4 mg/mL), or IND (approximately 5.5 mg/mL), as determined by high-performance liquid chromatography, were manufactured as previously outlined. Liposomes were composed of 1,2-distearoyl-sn-glycero-2-phosphocholine (DSPC) and cholesterol in a molar ratio of 2:1, and contained 1,2-distearoyl- sn -glycero-3-phospho-ethanolamine-N-[maleimide (polyethylene glycol)-2000] (DSPE-PEG(2000) maleimide) at 1.5 mol percent of DSPC as a coupling lipid (Avanti Polar Lipids). The resulting multilamellar dispersions were reduced in size and lamellarity to approximately 200 nm in diameter by high-pressure extrusion. The activated liposome suspension was then mixed with thiolated polyclonal anti-OTR antibody (catalog no. ab115664; Abcam, Cambridge, MA), which was prepared by first conjugating 25 μg of OTR antibody with a heterobifunctional reagent N-succinimidyl-3-(2-pyridyldithio) propionate ( Figure 1 ). The OTR antibody recognizes an extracellular domain of the human OTR. Nontargeted liposomes were coated with rabbit IgG. All liposomes incorporated the membrane stain 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) for fluorescent detection. Unconjugated antibody and nonencapsulated drug was removed by centrifugal filtration of the liposomes through a 100-kd molecular weight filter (Amicon Ultra-15). Amicon Ultra-15 filters were washed with Milli-Q H 2 O before 500 μL of liposome suspension was loaded into the filter reservoir. Liposomes were diluted with 5 mL of sterile 0.9% saline and centrifuged at 4000 x g until retentate volume was <250 μL. Liposomes were then resuspended in a further 5 mL of 0.9% saline and centrifuged until retentate volume was <250 μL. Filtered liposomes were then collected, transferred to a fresh Eppendorf tube, and redispersed to an original volume of 500 μL.
The size distribution of the liposomal dispersion was determined by dynamic laser light scattering (Zetasizer Nano S, ATA Scientific). Encapsulation efficiency was determined by disrupting the vesicles with ethanol and evaluating drug concentration using high-performance liquid chromatography. Quantification of the amount of antibody associated with liposomes was determined using the CBQCA protein assay (ThermoFisher Scientific Inc, Waltham, MA), using bovine serum albumin for the preparation of the standard curve.
Myometrial contractility studies
Myometrial strips were set up as previously described. Briefly, NIL human myometrial samples, or uterine horns obtained from pregnant CD1 Swiss mice, were dissected into strips (10 × 2 × 2 mm) and suspended in organ baths containing 30 mL of physiological saline solution (PSS) containing 120 mmol/L of NaCl, 5 mmol/L of KCl, 25 mmol/L of NaHCO 3 , 1 mmol/L of KH 2 PO 4 , 1.2 mmol/L of MgSO 4 , 2.5 mmol/L of CaCl 2 , and 11 mmol/L of glucose, and continuously gassed with carbogen (95% O 2 , 5% CO 2 ) at pH 7.4. Strips were connected to a Grass FT03C force transducer (Grass Instruments, Quincy, MA) and 1 g passive tension applied (1 g was calibrated to equal 1 V). PSS was replaced 5 times during the first hour, with strips retensioned to 1 g passive tension following each wash. Thereafter strips were maintained at 37°C until spontaneous rhythmic contractions developed. Data were digitized using a MacLab/8E data-acquisition system and contraction status visualized in real time using Chart software (ADInstruments, Dunedin, New Zealand). For each strip a contraction baseline was acquired to serve as reference.
To administer liposome treatments, 600 μL of PSS buffer was carefully extracted from an organ bath and transferred to an Eppendorf tube. The appropriate volume of liposome preparation (mixed by inversion) was pipetted into the PSS to predilute the liposomes. The total volume of prediluted liposomes (600 μL PSS + liposomes) was then carefully reinjected back into the appropriate organ bath. Final concentrations of each drug were: NIF 7.7 μmol/L, SAL 9.25 μmol/L, ROL 19.4 μmol/L, and DOF 3.0 μmol/L. Doses were based on prior investigations of the nonencapsulated drug (in vitro contraction assays using human myometrium). Where treated tissue was not washed, tissue strips remained in the presence of the liposomes for the duration of the assay. Where washout studies were performed, organ baths were twice drained of buffer and refilled with 37°C PSS. Human tissue strips were washed after 1 hour and 25 minutes whereas mouse tissue strips were washed after 15 minutes.
Tension generated by tissue strips is indicated in the results and representative contraction traces. The effect of treatments is interpreted relative to the pretreatment contraction baseline, which consisted of ≥3 contractions of consistent frequency and amplitude.
In vivo biodistribution study
Timed-mated CD1 Swiss pregnant mice were injected with drug-free, DiI-labeled preparations of either nontargeted or OTR-targeted liposomes on fetal GA 17 and 18 at 4:00 pm . Mice that labored overnight were euthanized on the morning of day 19 (9:00-11:00 am ) by CO 2 asphyxiation. Maternal internal organs of interest (heart, brain, liver, lung, kidney, uterus, and mammary tissue) were harvested and transferred to a Petri dish along with a sacrificed neonate. The Petri dish was loaded into an in vivo imaging system (IVIS-100) (Xenogen, Alameda, CA) and a light image captured. Tissues were then imaged under conditions appropriate for the detection of DiI (excitation: 554 nm; emission: 583 nm; filter: DsRed; exposure: 4 seconds; field of view: 10; binning: 4). Organs were imaged 17-19 hours after the second injection, following labor. Background signal was subtracted from the detected signal to produce the final fluorescence image. Fluorescence signal is reported as radiance (p/s/cm 2 /sr). The radiance range was kept constant across all images (min = 2.0 × 10 8 ; max = 1.8 × 10 9 ).
PTB study
Time-mated pregnant CD1 Swiss mice were administered 0.7 μg/g LPS from Escherichia coli (0111:B4) (Sigma-Aldrich) via intraperitoneal (IP) injection at 12:00 pm on GA 15 (1-time injection). LPS dose had been previously optimized to result in PTB rates of 50-70%. Total IP injection volume was 150 μL in saline.
At 4:00 pm on GA 15, mice began receiving daily IV injections of IND free-drug or liposomal preparations according to assigned treatment groups. Treatment groups are indicated in Table 1 . Total IV injection volume was 150 μL. Mice were monitored for onset of labor every 6 hours. Treatments were repeated daily at 4:00 pm until all mice labored. Term gestation was 19-22 days. Mice that labored within 48 hours of receiving LPS (GA 17) were deemed to have labored preterm.



