Physiologic changes in the gastrointestinal system in pregnancy
Gastrointestinal physiology is poorly studied during pregnancy even though gastrointestinal symptoms are the cause of a significant number of patient questions and concerns. Nonspecific symptoms of vomiting, epigastric pain, abdominal cramps, and diarrhea can occur frequently in pregnancy. Motor dysfunction of the gastrointestinal system and increasing uterine size are believed to be contributing factors. These gestational problems are self-limiting and most do not affect maternal or fetal outcome. Their importance in obstetric practice is to differentiate them from the diseases with similar presentation requiring diagnosis and management.
Oral cavity
The oral cavity undergoes a variety of changes during pregnancy. Many of these changes have been attributed to changes in the hormonal milieu. Changes in both salivary gland secretion and connective tissue changes have been described [1]. Pregnancy alters salivary composition, specifically decreasing pH and sodium content while increasing protein content. These changes are thought to be secondary to an increase in circulating estrogens [2]. Estimates of salivary secretion suggest that there is no overall increase in salivary volume.
Connective tissue changes occurring throughout the body during pregnancy can also be seen upon examination of the oral cavity. The most prominent connective tissue findings are gingival hyperplasia that may occur in 30–75% of pregnant women [1]. This presents as increased erythema and edema of the gingival tissue that some refer to as “pregnancy gingivitis.” The most prominent sites of occurrence include the marginal and interdental papillae [1]. Pregnancy gingivitis may be more evident due to an exaggerated inflammatory response that occurs in the presence of periodontal disease. Changes in both progesterone and estrogen have been shown to alter the bacterial microflora, increasing the percentage of Prevotella intermedia [3]. In addition, changes in microflora are associated with altered secretion of lysosomal enzymes that may enhance tissue destruction and gingival bleeding [4]. Many of these changes regress after completion of pregnancy. Therapeutic interventions for these findings are not generally recommended during pregnancy.
Gastrointestinal motility
Our current understanding of gastrointestinal motility in pregnancy is limited. Esophageal function has been studied mainly in relationship to gastroesophageal reflux which is reported at some point in up to 80% of pregnant women [5]. Lower esophageal sphincter (LES) tone gradually declines during pregnancy and reaches a nadir at 36 weeks, declining by as much as 50% [6]. While progesterone is mainly felt to be responsible for the decrease in LES tone [6], estrogen has not been excluded from the etiology, possibly exerting a priming effect [5]. The increased intra-abdominal pressure produced by the expanding uterus also adds to the decrease in esophageal sphincter tone. Esophageal motility is not altered during pregnancy [7]. The combined effects of these changes increase the likelihood of gastroesophageal reflux.
The effects of pregnancy on gastric emptying are conflicting. Available studies suggest that gastric emptying may be more affected during labor than at other times in gestation. Gastric motility is mediated by a variety of inputs that enhance and delay emptying. The composition of oral intake is responsible for a variety of paracrine signals that modulate gastric motility. Foods high in fat content are known to delay gastric emptying. Studies evaluating clearance of water at various periods during the first through third trimester suggest that gastric emptying is delayed [8]. In contrast, other studies evaluating a liquid saline meal suggest that more rapid emptying occurs during pregnancy than in nonpregnant individuals [9]. These differences reinforce the role of paracrine signaling in gastric motility. Other studies evaluating the time from ingestion to small bowel entry suggest that gastric motility is delayed mainly in individuals with symptoms of gastroesophageal reflux [8]. Patients who were asymptomatic had gastric emptying times comparable with those of nonpregnant individuals.
Few studies are available evaluating small and large intestinal motility in normal pregnancy, despite the role it might have on the frequently reported symptoms of irritable bowel and constipation in pregnancy. A variety of techniques have been used to evaluate small intestinal transit times with significantly different results. Parry [10], using radiographic examination to evaluate the transit of a rubber balloon filled with mercury, demonstrated small intestine mean transit times of 58 and 52 hours in a group of pregnant and nonpregnant women respectively. Wald [11], in contrast, determined small intestinal transit times to be in the range of 2 hours during the third trimester of pregnancy and only 90 minutes during the postpartum period by measuring exhaled hydrogen after ingestion of the nonabsorbable carbohydrate lactulose. Lawson [12] showed a similar delay in small intestinal transit during pregnancy compared to post partum. In addition, Lawson noted slower rates during the second and third trimesters compared to the first trimester in the handful of patients examined.
In summary, clinical observation and most available studies indicate that gastrointestinal motility during pregnancy is diminished. Studies evaluating the effects of progesterone on smooth muscle activity clearly demonstrate a decrease in contractility and tone, suggesting that the decreased motility most likely has a hormonal basis.
Nausea and emesis
Nausea and vomiting during the first trimester of pregnancy is so frequent that it is caricatured across most cultures as the initial sign of pregnancy. Nausea is reported in up to 80% of women during their first trimester and can persist in a subset of individuals throughout pregnancy. A variety of theories have been proposed to explain these common symptoms. Goodwin [13] has suggested that the symptoms are either a direct chemical stimulus from placental products or are due to resetting of the stimulus threshold in the vestibular or gastrointestinal systems. The evidence to support these theories is presented.
Both human chorionic gonadotropin (HCG) and estrogens have been implicated as the chemical mediators of nausea and emesis during pregnancy. There is temporal association of HCG level with severity of symptoms during normal pregnancy. Also, complicated pregnancies, associated with higher HCG levels, are frequently affected with greater symptomatology. Nausea is common in patients taking oral contraceptive pills with a similar dose–response relationship. The temporal relationship of estrogens and nausea and vomiting in pregnancy supports this theory.
A change in vestibular or gastrointestinal sensitivity has been another explanation for increased nausea and vomiting in pregnancy. Studies have demonstrated that individuals prone to motion sickness are likely to be more symptomatic with pregnancy [14]. Changes in serum osmolarity have been shown to induce vestibular-mediated nausea and vomiting [15].
Changes in gastric rhythmic activity have more recently been implicated in the causation of nausea in pregnancy. Normal gastric myoelectric activity results in slow wave propagation from the proximal body to the distal antrum at a rate of 3 cycles per minute (cpm). Rhythm disturbance, either increased or decreased slow wave propagation, is associated with nausea. Koch and colleagues demonstrated that women with normal slow wave activity (3 cpm) were less likely to complain of nausea during pregnancy [16]. In contrast, individuals with high or slower rates were more likely to complain of nausea. Jednak [17] demonstrated that protein-dominant meals were associated with decreased symptoms and corrected slow wave dysrhythmias. Carbohydrate- or fat-dominant meals had no effect on symptoms or slow wave dysrhythmias. These studies suggest that meal composition may also affect symptomatology.
Clinical studies have shown that oral tissues are affected by pregnancy and several oral lesions may have an impact on pregnancy outcomes. A few commonly seen oral lesions are discussed below.
Aphthous ulcers
Aphthous ulcers (also known as canker sores) are painful open sores inside the mouth or upper throat caused by a break in the mucous membranes. There is no evidence for an association between aphthous stomatitis and pregnancy; the importance for the obstetric provider is to recognize underlying systemic illnesses that may be associated with recurrent aphthae. Celiac sprue, gluten-sensitive enteropathies, inflammatory bowel disease, vitamin and mineral deficiencies, particularly B vitamins (1, 2, 6 and 12) iron, folic acid and zinc, HIV infection, Behçet’s syndrome, neutropenia of any cause may be associated with oral ulcers. Sodium lauryl sulfate, a toothpaste detergent, has also been linked to recurrent aphthous stomatitis (RAS). Herpetiform ulcers have similar appearance and presentation.
Management is generally palliative, with use of topical analgesics; topical or oral corticosteroids may be indicated for severe recalcitrant disease. Identification and treatment of underlying disorders are important, especially with nutritional deficiency as replacement of a deficient vitamin or mineral should result in prompt resolution. Treatment with thalidomide has shown benefit in RAS associated with HIV infection; however, its only FDA-approved uses are for multiple myeloma and erythema nodosum leprosum and its use in pregnancy is clearly contraindicated.
Periodontal disease
Gingivitis in pregnancy has been reported at prevalence rates of 40–100% [18]. The cause of pregnancy-induced gingivitis is likely multifactorial and includes pregnancy-related physiologic vascular and inflammatory changes. Clinically, the gingival tissue appears smooth, swollen, and dark red and bleeds easily. Hyperplasia of interdental papillae may be noted.
Periodontitis is characterized by gingival inflammation with accompanying loss of supportive connective tissues, including alveolar bone. Recent studies have suggested that periodontal inflammation is associated with an increased risk of preterm birth, as well as low birthweight and pre-eclampsia [19–21]. However, in a study involving 823 pregnant women, treatment of periodontitis was not shown to significantly alter rates of preterm birth, low birthweight or fetal growth restriction [22].
Optimal oral hygiene and removal of dental plaque can reduce gingival swelling, erythema and bleeding. Regular exams and cleaning should be continued through pregnancy.
Pyogenic granuloma
Pyogenic granuloma, previously known as epulis gravidarum, is a benign growth on the oral mucosa and can occur in up to 5% of pregnancies. It usually occurs buccally on the upper anterior teeth and is painless. It can be colored from red to pink and may be smooth in surface or lobulated. The lesion can grow rapidly, but is rarely greater than 2 cm in diameter. It resolves spontaneously after delivery. Surgical resection, if needed, should only be done after delivery as recurrence is extremely common when the granuloma is removed during pregnancy.
Gastroesphageal reflux
Background and epidemiology
Gastroesphageal reflux disease (GERD) is common in pregnancy. It is estimated that 30–50% of pregnant women experience heartburn and that in some populations the incidence may be as high as 80% [23]. Reported risk factors for GERD in pregnancy include increasing gestational age, parity, and a history of heartburn [24]. While most pregnant women with GERD experience symptoms for the first time in pregnancy, some may suffer from an exacerbation of pre-existing disease [25]. Most patients have a benign disease course, with only a few experiencing GERD-related complications such as gastrointestinal bleeding or stricture formation. In general, symptoms begin at the end of the first trimester, worsen through the remainder of pregnancy, and then resolve promptly after delivery.
Pathophysiology
The pathogenesis of GERD in pregnancy is likely multifactorial. Decreased sensitivity of the basal LES to hormonal, pharmacologic, and physiologic stimuli may cause gestational GERD [26]. Other proposed mechanisms include decreased esophageal peristalsis, esophageal dysmotility and delayed gastric emptying due to hormonal and mechanical changes [27].
The role of increased intra-abdominal pressures due to the expanding gravid uterus in the development of GERD is controversial. Loss of the intra-abdominal segment of the LES with loss of the normal pressure gradient between the intrathoracic and intra-abdominal segments has been shown in pregnancy and proposed as a possible factor contributing to gestational GERD [27]. However, in one study, nearly 40% of pregnant women without heartburn were also found to have loss of the intra-abdominal LES [28]. In addition, it remains unknown whether the LES can compensate in pregnancy as it does in other states of increased abdominal pressure, such as cirrhosis, wherein LES pressures rise proportionately to increasing intra-abdominal pressure [5,25,29].
Clinical presentation
The presenting symptoms of GERD in pregnancy are similar to those of the general population. Patients complain most frequently of pyrosis (heartburn) and regurgitation. Other reported symptoms include indigestion, epigastric pain, waterbrash (sour taste in the mouth), anorexia and nausea and vomiting [25]. Although symptoms may be intense, complications such as esophagitis, gastrointestinal bleeding, and stricture formation are rare due to the short duration of acid exposure [25,27].
Diagnosis and evaluation
Diagnosis of GERD is based upon symptoms. Radiographic evaluation by upper GI series is avoided in most circumstances due to the risks of radiation exposure to the fetus. Upper endoscopy is generally not necessary but can be considered in refractory cases, severe dysphagia and gastrointestinal bleeding. Odynophagia (painful swallowing) refractory to acid-reducing medications should be evaluated by upper endoscopy because infectious esophagitis (e.g. Candida esophagitis) may occur in pregnancy even in immunocompetent hosts [30] and can masquerade as GERD.
Treatment
Treatment of GERD in pregnancy should follow a “step-up” approach. Patients with mild symptoms are often adequately treated with therapeutic lifestyle modifications. These include strict abstinence from tobacco and alcohol. Patients should also avoid late-night meals, recumbency after eating, and trigger foods (e.g. spicy or sour foods, carbonated beverages, coffee). Eating several small meals throughout the day and elevating the head of the bed by 6 inches with blocks (and not simply propping up the upper body with pillows which may actually exacerbate symptoms) may provide additional benefit [5].
For patients who fail conservative measures, antacids are first-line pharmacologic therapy. It is estimated that 30– 50% of women take antacids for the control of heartburn symptoms during pregnancy [5]. Aluminum, magnesium, and calcium-based antacids (Al(OH)3, Mg(OH)2, and CaCO3) are all considered safe for the treatment of GERD in pregnancy. However, aluminum-containing antacids can cause constipation, and magnesium-containing antacids can cause diarrhea [31]. Patients on supplemental iron should be advised to not take iron and antacids together in order to maximize iron absorption by an acidic gastric pH. Sodium bicarbonate should not be taken due to the risk of metabolic alkalosis and fluid overload in the mother and fetus [25].
Other first-line agents include the nonabsorbable alginates and sucralfate. Alginic acid (a combination of this with antacids is marketed in the US as Gaviscon®) is considered effective and fast-acting in most pregnant patients [32]. The mucosal protectant sucralfate is also safe in pregnancy and is classified as a category B drug by theUS Food and Drug Administration (FDA) Pregnancy Safety Classification (see Chapter 30 for a summary of this classification system). Sucralfate has been shown in a randomized controlled trial in pregnancy to provide greater relief from heartburn and regurgitation than lifestyle and dietary modifications alone [33].
Metoclopramide, FDA pregnancy category class B, may be useful in the treatment of GERD by increasing LES pressure but its main use in pregnancy is in the treatment of nausea and vomiting. Use of metoclopramide is often limited by its poor tolerability and the risk for irreversible extrapyramidal side effects (tardive dyskinesia: irregular involuntary movements of the face), restlessness, dystonias of neck, eyes or tongue and Parkinson-like rigidity, tremor and paucity of spontaneous movement) [5]. Other common side effects include restlessness, drowsiness and fatigue.
H2 receptor antagonists (H2-RA) form the next tier of therapy. The four H2-RA (ranitidine, cimetidine, famotidine and nizatidine) are FDA category B and considered safe in pregnancy. Surveillance studies of infants exposed to ranitidine and cimetidine during early pregnancy have shown an average congenital malformation rate of 4.4%, a rate nearly identical to that among nonexposed infants [34]. In addition, no increased risks for spontaneous abortions, preterm labor or low birthweight have been reported after first-trimester exposure to H2-RA [35].
Despite the longstanding availability of H2-RA, only ranitidine given twice daily has been shown in a randomized, double-blind trial to be efficacious in pregnancy, making it the preferred H2-RA for gestational GERD [36]. Cimetidine is likely equally effective; however, due to the antiandrogenic effects seen in animals and nonpregnant humans, some authors advise against its use in pregnancy [5]. Nizatidine recently changed classification from FDA category C to category B and is considered safe in pregnancy. However, concerns remain over adverse outcomes in pregnant animals exposed to the drug, making it a less preferred option for many experts [25].
Proton pump inhibitors (PPI) are typically reserved for patients with severe symptoms refractory to lifestyle modification and the older generation medications. Concern over the long-term safety of PPI has limited their use. However, there is now a large amount of data supporting their safety in pregnancy. A meta-analysis found no significant increase in risk for major malformations in women taking PPI during the first trimester [37]. Other studies have found no increased risk of low birthweight or number of preterm deliveries [38]. Four of the five PPI (lansoprazole, rabeprazole, pantoprazole and esomprezole) are FDA category B with only omeprazole carrying a class C rating due to fetal toxicity in animal studies.
Anesthesia considerations during labor and delivery
Anesthetics lower LES pressure and delay gastric emptying. The risk of aspiration of gastric contents during regional and general anesthesia may be particularly high in pregnancy. Pulmonary aspiration during labor or Mendelson’s syndrome is a leading cause of obstetric morbidity and mortality due to anesthesia [25]. Due to the serious complications of aspiration, much attention has been given to methods to decrease gastric volume and acidity prior to the administration of anesthesia to prevent lung injury.
Mitigating the effects of aspiration by raising the gastric pH to greater than 2.5 is recommended during labor and delivery [25]. Among the antacids, soluble antacids such as sodium citrate and sodium bicarbonate are preferred over insoluble antacids such as aluminum and magnesium hydroxide, carbonates and trisilicates because they are less likely to cause lung injury if aspirated. Ranitidine and the other H2-RA are also effective in reducing gastric acidity [39]. Prokinetic drugs like metoclopramide may be helpful in decreasing gastric volume by promoting gastric emptying [40]. More recently, omeprazole given orally the evening before surgery has been shown to be safe in obstetric anesthesia and effective in raising gastric pH [41].
Postpartum and lactation considerations
Gastroesophageal reflux disease symptoms typically resolve shortly after delivery but some women may have persistent symptoms. Most medications begun during pregnancy for the treatment of GERD can be continued safely post partum even during lactation. Antacids, sucralfate, and all of the H2-RA except nizatidine are safe for use by breastfeeding women [6]. There are limited safety data concerning PPI use in breastfeeding.
Hyperemesis gravidarum
Hyperemesis gravidarum is discussed in detail in Chapter 48.
Peptic ulcer disease
Background and epidemiology
Estimates from case studies and retrospective series suggest that the incidence of peptic ulcer disease (PUD) is decreased in pregnancy [42]. Older studies from 1939 and 1966 have reported rates of active ulceration as low as 1 in 70,310 hospitalizations of pregnant women and 6 in 23,000 deliveries, respectively [43,44]. However, these studies are limited by their retrospective nature and the fact that they predated flexible endoscopic evaluation. It is likely that PUD is not as uncommon in pregnancy as the literature suggests, but that it is under-recognized. Under-reporting of symptoms by patients and reluctance to perform diagnostic tests by physicians may contribute to this.
Risk factors for PUD in pregnancy are the same as for the general population. They include smoking, nonsteroidal anti-inflammatory drug (NSAID) use, alcoholism, genetic predisposition, gastritis and H. pylori infection [42]. Advanced maternal age is a pregnancy-specific risk factor for PUD.
Pathophysiology
Peptic ulcer disease results from the imbalance of complex interactions between defensive and aggressive factors, the most important of which is likely H. pylori [45]. H. pylori, a motile, urease-producing, gram-negative bacterium, produces inflammation that may lead to ulceration and carcinoma in affected individuals. Host immune factors, genetic predisposition and bacterial virulence likely account for the varied clinical responses to H. pylori infection.
Clinical presentation
Patients with PUD in pregnancy typically present with epigastric pain, anorexia, postprandial nausea and vomiting, abdominal distension and eructation [42]. While these symptoms are similar to those of the general population, PUD symptoms have been reported to be milder in pregnancy [46]. Factors which may account for this include a healthier diet during pregnancy, avoidance of alcohol and cigarette smoking, and increased medical supervision [47].
Patients with PUD and gastrointestinal bleeding present with coffee-grounds emesis, hematemesis or melena. Hematochezia may also be present in the setting of brisk upper gastrointestinal tract bleeding. Patients with perforated PUD may be hypotensive and present with a rigid abdomen from peritonitis.
The differential diagnosis of PUD in pregnancy includes nonulcer dyspepsia, GERD, nausea and vomiting of pregnancy, hyperemesis gravidarum, pancreatitis, acute cholecystitis, viral hepatitis, appendicitis, and acute fatty liver of pregnancy [42].
Diagnosis
Diagnosis of peptic ulcer disease relies on a high index of suspicion. The physical exam may be normal or may reveal only minimal tenderness despite severe ulceration. Fluoroscopic evaluation of the upper gastrointestinal tract with barium is to be avoided if possible during pregnancy. Abdominal radiographs and computed tomography of the abdomen are also generally avoided; however, they should be performed if there is suspicion for perforation.
Esophagogastroduodenoscopy (EGD) can be performed safely in pregnancy when necessary and is the diagnostic modality of choice. Considerations regarding maternal and fetal monitoring, medication used and hemostasis are necessary prior to performing the procedure (see “Endoscopy in pregnancy and lactation” below).
Management
General treatment guidelines are unaffected by pregnancy. Treatment begins with lifestyle and dietary modification. Conservative measures such as abstinence from smoking and alcohol, avoiding trigger foods and diminishing psychologic stress are recommended. Patients not responding to these measures are advised to take antacids and/or sucralfate. In nonpregnant populations, high-dose antacid therapy has been shown to heal 75% of duodenal ulcers [48].
In patients not responding to antacids, empiric therapy with H2-RA may be tried. Patients who remain symptomatic on H2-RA may be switched to PPI therapy. They should also be strongly considered for diagnostic EGD to rule out complications from PUD such as stricture and gastrointestinal bleeding.
Patients who are found to have active H. pylori infection during pregnancy as part of their evaluation for PUD or hyperemesis gravidarum (see section on pathophysiology of hyperemesis gravidarum) can be treated [49]. The most common treatment regimen for nonpregnant patients is a 10–14-day course of twice-daily PPI, amoxicillin, and clarithromycin. For nonpregnant patients who are allergic to penicillin or have resistant infection, “quadruple” therapy with metronidazole, bismuth, tetracycline and high-dose acid suppression by PPI or H2-RA therapy can be used. For pregnant patients who are penicillin tolerant, the usual regimen may be given in pregnancy (amoxicillin is category B and clarithromycin is category C) although concern about the safety of clarithromycin in pregnant animals has led some experts to delay its use until after the first trimester or even until after the pregnancy [50]. However, treatment of penicillin-allergic patients during pregnancy is limted by the teratogenicity of tetracycline (category D) and the possible association of bismuth (category C) with fetal ductus arteriosus closure. Metronidazole (category B) is generally considered safe for use in the second and third trimesters of pregnancy but is less effective in the eradication of H. pylori when not used as part of quadruple therapy. Fortunately, most peptic ulcers will readily heal with PPI or H2-RA therapy and H. pylori treatment, which will help prevent recurrence, can usually be safely delayed until after delivery.
Disorders of the intestinal tract
Diarrheal illness
Acute diarrhea
Most cases of acute diarrhea are due to infections with viruses and bacteria and are self-limited. Noninfectious etiologies are more likely in patients with chronic symptoms. Table 10.1 represents some common etiologies of acute diarrhea, with a brief outline of clinical features and management of each.
Table 10.1 Common diarrheal agents
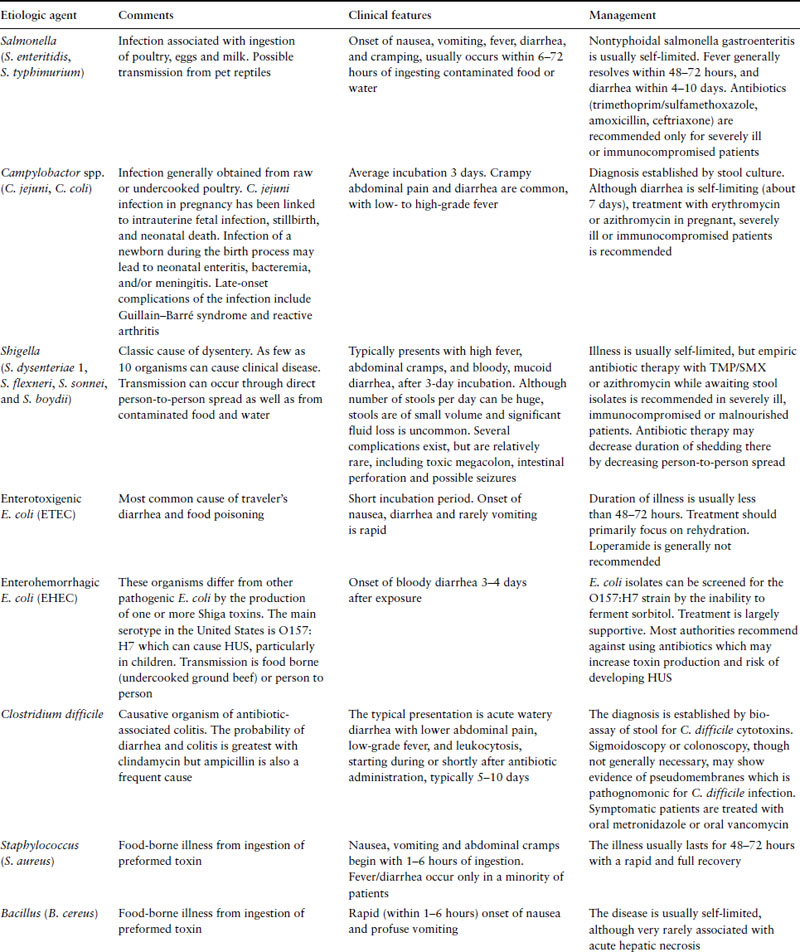
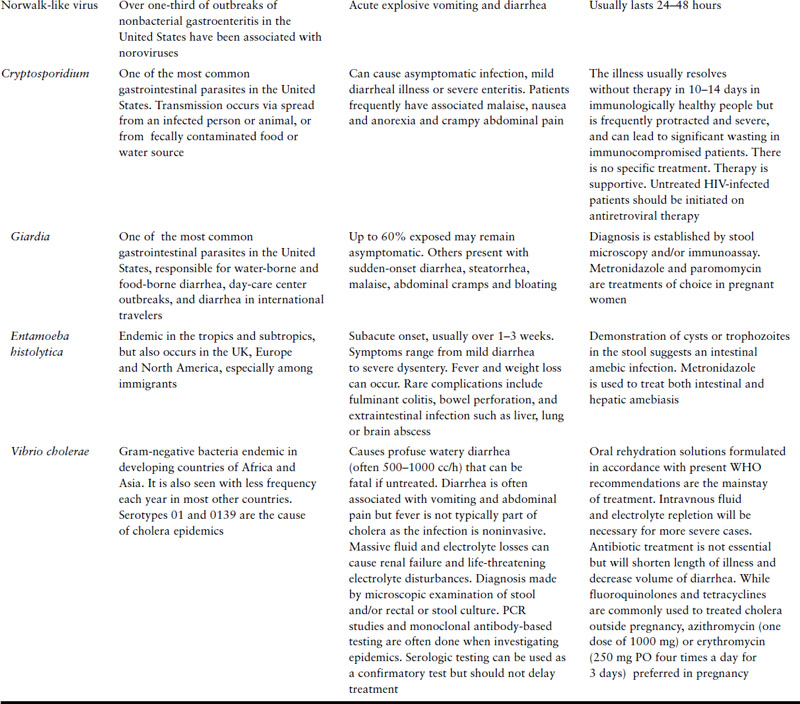
In general, a diagnostic evaluation is only indicated in patients presenting with relatively severe symptoms such as profuse watery diarrhea, hypovolemia, bloody diarrhea, fever, severe abdominal pain, recent use of antibiotics or in an immunocompromised patient. Diagnostic evaluation should likely also occur if diarrhea persists for more than a week and is associated with weight loss. Such an evaluation should include fecal leukocytes and a stool culture and testing for stool ova and parasites, especially in patients with history of exposure to infants, in international travelers or in patients with AIDS. Endoscopy is not indicated very often in acute diarrhea, but should be considered in patients with bloody diarrhea to differentiate infectious causes from inflammatory bowel disease or colonic ischemia.
Recommendations from the Infectious Diseases Society of America with regard to investigation of acute diarrheal illnesses are presented in Table 10.2.
Table 10.2 Investigations for acute infectious diarrhea in pregnancy
| Syndrome | Comments | Testing |
| Community-acquired or traveler’s diarrhea | Often self-resolving without treatment in a few days Investigate especially if fever or blood in stool Fecal leukocytes or lactoferrin may help suggest inflammatory/invasive cause Traveller’s diarrhea may be treated empirically with azithromycin 1 g PO×1 dose and investigate only if symptoms persist. Alternative treatment with fluoroquinolones not recommended in pregnancy | Culture or test for:
|
| Nosocomial diarrhea (onset after 3 days in hospital) | Only consider nosocomial rather than community acquired if onset after 3 days in hospital | Test for:
|
| Persisitent diarrhea >7 days | Investigate especially if immunocompromised host Fecal leukocytes or lactoferrin may help suggest inflammatory/invasive cause | Test for parasites:
|
If HIV positive, test for:
|
The initial management of patients with acute diarrhea must include hydration and alteration of diet. Antibiotic therapy is not required in most cases since the illness is usually self-limited. Loperamide may be used for the symptomatic treatment of acute diarrhea, but only when fever is absent or low grade and the stools are not bloody.
Chronic diarrhea
Diarrhea lasting for 3 weeks or more can result from several etiologies including irritable bowel syndrome, lactose intolerance, inflammatory bowel disease, microscopic colitis, malabsorption and chronic infections. Box 10.1 lists some differential diagnoses for chronic diarrhea. Box 10.2 summarizes the investigation of chronic diarrhea. A thorough medical history and physical exam are important and may help to narrow the differential. The minimum laboratory tests indicated in most patients include a complete blood count, electrolytes, thyroid function tests, total protein and albumin and stool occult blood. Most patients will need referral to gastroenterology for endoscopic evaluation, especially if blood is found in the stool.
Celiac disease
Background and epidemiology
Celiac disease is an immune-mediated disease of the small intestine with associated systemic manifestations and complications. It is characterized by intestinal inflammation and loss of absorptive capacities with resulting nutrient, mineral and vitamin deficiency. It is not known whether pregnancy exacerbates the disease; however, due to increased nutritional demands in pregnancy, compromised absorption of nutrients may become evident and unmask underlying disease.
Celiac disease had been considered to be a rare disease, especially in the United States. Due to the recognition of silent and atypical disease, it is now estimated that as many as 1– 3% of the general population in Europe and the United States are affected with celiac disease [51]. Celiac disease has been diagnosed in individuals of all ages and ancestry. Women are more likely to be affected than men with a female-to-male ratio of 2.9:1 [52].
Family history of celiac disease is a strong risk factor for the development of the disease as 10–20% of first-degree relatives of individuals with celiac disease are also affected [53]. Other risk factors include human leukocyte antigen (HLA) class II antigens DQ2 and DQ8 which are expressed in over 95% of patients, early and massive gluten exposure and other autoimmune diseases [54]. Type I diabetes mellitus (DM), in particular, is strongly associated with celiac disease with an estimated 1–10% of type I diabetic patients having celiac disease [55]. Individuals with Down’s syndrome, Turner’s syndrome, Williams’ syndrome, and selective IgA deficiency are also at increased risk [56].
Pathogenesis
Celiac disease is the result of an inflammatory cascade incited by dietary gluten proteins in genetically susceptible individuals [57]. Gluten proteins found in wheat, barley and rye cross intercellular tight junctions of enterocytes to reach the lamina propria. There they are degraded into peptides and modified by the enzymatic activity of tissue transglutaminase (tTG). Postmodification of the peptides allows binding to HLA-DQ molecules on antigen-presenting cells. The gluten–HLA-DQ complexes are presented to gluten-reactive CD4 + T-cells and recognized as foreign. This triggers an abnormal mucosal response and induces tissue damage.
Clinical presentation
The clinical presentation of celiac disease varies greatly. In the classic presentation patients are severely symptomatic. Gastrointestinal symptoms such as dyspepsia, upper abdominal pain, chronic diarrhea, steatorrhea and failure to thrive predominate. However, diarrhea and weight loss are uncommon with less than 50% of patients reporting diarrhea at the time of diagnosis and approximately 30% of patients presenting overweight [58]. Atypical presentations in which extraintestinal symptoms predominate are becoming increasingly recognized [59]. These include osteoporosis, iron deficiency anemia, and neurologic disorders [57]. Obstetric and gynecologic presentations may also occur in the form of delayed menarche, early menopause, secondary amenorrhea, infertility, recurrent miscarriage, and low birthweight infants [60]. Box 10.3 provides a list of clinical presentations that should be investigated for celiac disease. Currently, iron deficiency anemia due to both iron malabsorption and occult gastrointestinal bleeding is the most common clinical presentation of celiac disease [61].
Box 10.1 Differential diagnosis of chronic diarrhea
- Osmotic diarrhea (ssmotic laxatives, lactose intolerance, sorbitol or mannitol (in many “sugar-free” foods) ingestion)
- Secretory diarrhea (bacterial toxins, inflammatory bowel disease)
- Disordered motility (irritable bowel syndrome, diabetic neuropathy)
- Endocrine causes (hyperthyroidism, gastrinoma, Addison’s disease)
- Fatty diarrhea (Whipple’s disease, celiac disease, pancreatic exocrine insufficiency)
- Inflammatory diarrhea (inflammatory bowel disease (IBD), ischemic or radiation colitis, colon cancer, vasculitis)
- Infections (pseudomembranous colitis, tuberculosis, yersiniosis, HSV, CMV, amebiasis and strongyloidosis)
- Laxative abuse
Box 10.2 Suggested evaluation for chronic diarrhea
- History (to differentiate functional from organic cause): duration greater than 1 year, lack of significant weight loss, absence of nocturnal diarrhea and straining with defecation suggest functional cause
- CBC with differential, thyroid function tests, electrolytes, LFT
- Stool analysis: for occult blood, WBC, presence of fat, ova and parasites
- Temporary cessation of lactose-containing foods (when lactose intolerance is suspected). Pregnant women frequently increase their milk product intake and may be unable to accommodate the increased lactose load
- Quantitative stool measurement and analysis for pH, electrolytes and laxatives
- Colonoscopy/sigmoidoscopy and mucosal biopsy
- Radiologic studies: barium follow-through (to evaluate small intestine; this study is rarely warranted in pregnancy), mesenteric angiography (when mesenteric ischemia is suspected), CT scan of the abdomen
Box 10.3 Persons who should be tested for celiac disease
- Individuals with gastrointestinal symptoms, including chronic diarrhea, malabsorption, weight loss, and abdominal distension
- Individuals without other explanations for persistent elevations of transaminases, short stature, delayed puberty, iron deficiency anemia, recurrent fetal loss and infertility
- Discuss benefits and risks of screening in asymptomatic, high-risk populations
- Persons with type 1 diabetes mellitus
- Persons with other autoimmune endo-crinopathies
- First- and second-degree relatives of persons with celiac disease
- Persons with Turner’s syndrome
- Persons with Williams’ syndrome (a rare congenital condition due to deletions in the genes for elastin and LIM kinase)
- Persons with type 1 diabetes mellitus
Adapted from James SP. National Institutes of Health Consensus Development Statement on Celiac Disease, June 28–30, 2004. Gastroenterology 2005;128(4)(suppl 1): S1–S9.
Nearly every organ system can be affected by celiac disease as a result of malabsorption of nutrients and both fat-soluble and water-soluble vitamins. Severe extraintestinal manifestations may be seen after years of poorly controlled disease. Some of these findings are short stature, failure to thrive, aphthous stomatitis, hair loss, fractures, dental enamel hypoplasia, chronic fatigue, muscular atrophy, peripheral neuropathy, ataxia and depression [62]. Dermatitis herpetiformis, a pruritic, symmetric papulovesicular rash that is seen on the elbows, knees, buttocks and scalps in patients with celiac disease, is seen exclusively in patients with celiac disease and is nearly pathognomonic for the disease [59].
Diagnosis
Accurate serologic tests are now available for screening for celiac disease. These include antigliadin (AGA) IgA and IgG, antiendomysial (EMA) IgA and antitissue transglutaminase (tTG) IgA. A recent review of the diagnostic accuracy of the serologic tests for celiac disease found EMA and tTG to be superior to AGA [63]. False-negative results of the serologic tests may be seen in patients with selective IgA deficiency and patients on a gluten-free diet at the time of testing. Anti-tTG IgA with total serum IgA are currently recommended in the US as screening tools for celiac disease.
Endoscopy with small bowel biopsy remains the gold standard for the diagnosis of celiac disease [56] and should be performed on all patients having positive serologic tests. It is recommended that multiple biopsies be obtained from the second portion of the duodenum or beyond, due to the histologic patchiness of the disease, in order to increase the diagnostic yield. Histologic confirmation by the presence of intraepithelial lymphocytosis and villous atrophy with associated crypt hyperplasia is necessary for the diagnosis [64]. As with the serologic testing, patients must be on a gluten-containing diet to prevent false-negative biopsies.
The differential diagnosis includes infectious gastroenteritis, bacterial overgrowth, lactose intolerance, anorexia nervosa, ischemic enteritis, tropical sprue, hypogammaglobulinemia, Whipple’s disease, intestinal lymphoma and Zollinger–Ellison syndrome [65].
Management
Treatment of celiac disease is a strict gluten-free diet for life. Wheat, rye and barley, all of which contain gluten, must be excluded from the diet. Oats have been shown to be safe but their inclusion in the gluten-free diet remains controversial due to cross-contamination with gluten-containing starches during processing [66]. Due to the complexity of the gluten-free diet and the need for strict adherence, referral to an expert dietitian and to celiac disease advocacy groups is recommended.
Nutrient deficiencies are common in celiac disease. Newly diagnosed patients should be screened for deficiencies of the fat-soluble vitamins (A, D, E and K) and water-soluble vitamins (folate, B6, and B12). Patients should also be screened for iron, calcium, phosphorus and zinc deficiency. Vitamin and mineral supplementation is required in most patients. This includes a multivitamin, calcium (1000–1500 mg/day), vitamin D (400–800 IU/day) and iron, if needed. Caution must be taken not to consume vitamin or mineral preparations that contain gluten.
Bone density scanning to screen for osteoporosis is recommended in all newly diagnosed patients [67]. Screening for other autoimmune disorders such as diabetes mellitus, autoimmune thyroid disorder, and rheumatoid arthritis is not routinely recommended, but may be applied on a case-by-case basis.
Lifelong follow-up of affected individuals is necessary to monitor for dietary compliance and response as patients who are noncompliant or become refractory are at increased risk for malignancy [68]. These malignancies include lymphomas, mostly T-cell type, as well as adenocarcinoma of the small bowel, stomach and esophagus, primary hepatocellular carcinoma and melanoma [69].
Dietary avoidance of gluten leads to symptom improvement in 70% of patients with celiac disease within 2 weeks [70], although based on clinical experience, complete resolution of symptoms can take as long as 6–9 months after initiation of a strict gluten-free diet. Serologic testing can be used to approximate dietary compliance as antibody titers decrease with initiation of the gluten free-diet within 4–6 weeks [71]. In contrast, mucosal recovery with histologic resolution of inflammation may take up to 2 years [72].
Rarely, patients do not respond to nutritional therapy. Abdominal bloating and pain may indicate refractory sprue, concomitant irritable bowel syndrome or bacterial overgrowth. In these refractory cases repeat endoscopy is indicated. Persistent histologic damage, despite dietary compliance, indicates refractory sprue. Intravenous steroids [73], azathioprine [74], cyclosporine [75] and infliximab [76] have been used in these cases in nonpregnant patients with success. Patients with histologic improvement require investigation for other causes of their symptoms.
Prenatal considerations
Infertility is a reported complication of celiac disease [77]. Several European studies have suggested a higher prevalence of celiac disease among infertile women (4–8%) who are otherwise asymptomatic compared with the general population (less than 1%) [78,79]; however, these findings have not been consistent. A recent study from northern California found that only one of 121 (0.8%) women attending an infertility clinic screened positive for celiac disease using tissue transglutaminase and antiendomysial antibody [80], suggesting no difference in the prevalence of celiac disease among women with infertility compared with the general population. Male celiac patients have also been reported to experience infertility secondary to hypogonadism, sexual dysfunction and poor semen quality [81]. Consideration of the diagnosis of celiac disease and testing in those with any possibly associated symptoms is likely warranted in any patient with infertility of recurrent pregnancy losses.
For individuals with known celiac disease, compliance with the gluten-free diet and correction of all vitamin and mineral deficiencies should be achieved before attempting pregnancy.
Pregnancy considerations
Celiac disease should be considered in any pregnant patients presenting with chronic diarrhea, severe iron deficiency, failure to thrive or unexplained abdominal pain. Serologic tests are accurate in pregnancy and confirmatory endoscopy can be performed safely when indicated (see “Endoscopy in pregnancy and lactation” below).
Prompt recognition of de novo celiac disease in pregnancy is important because uncontrolled disease carries an increased risk of adverse pregnancy-related events. These include an increased risk for spontaneous abortion, intrauterine growth retardation and low birthweight [82]. These complications are most likely secondary to nutritional deficiency and are preventable with dietary changes and vitamin and mineral replacement. The risk of cesarean section was also found to be moderately higher for women with celiac disease (odds ratio (OR) 1.33, 95% confidence interval (CI) 1.03–1.70) in a British study, possibly due to socio-economic or educational advantages of women with celiac disease [83].
It is recommended that pregnant women with celiac disease be followed by high-risk obstetrics as well as a dietitian with expertise in celiac disease to ensure total adherence to the gluten-free diet. Malabsorption and steatorrhea, the hallmark characteristics of uncontrolled disease, are associated with oxalate kidney stone formation, fat-soluble vitamins deficiency and calcium malabsorption. Thus, pregnant women with celiac disease should be monitored for dietary compliance with serial tTG. Levels of the fat-soluble vitamins (A, D, E, and K) should also be monitored and replacement should be initiated if levels are low.
Inflammatory bowel disease
Background and epidemiology
The inflammatory bowel diseases (IBD) are a group of chronic, idiopathic, inflammatory conditions of the gastrointestinal tract. The term is used most commonly to refer to Crohn’s disease (CD) and ulcerative colitis (UC). Other inflammatory bowel diseases are indeterminate colitis and the microscopic colitides: lymphocytic and collagenous colitis. Inflammation leads to ulcerations, loss of absorptive capacity and fibrostenotic areas with intestinal obstruction.
The incidence and prevalence of CD and UC vary greatly with population. Incidence rates are considerably higher in northern and western Europe and in North America compared with Africa, South America and Asia. The incidence is also greater (3–6 times greater) in Jewish populations [84].
It is estimated that the worldwide incidence of UC is 0.5–24.5 per 100,000 persons and the worldwide incidence of CD is 0.1–16 per 100,000 persons [85]. Interestingly, in western, industrialized nations the incidence of UC has remained relatively constant, whereas the incidence of CD rose substantially between the 1960s and 1980s [85,86]. Whether this increase is due to better detection or to environmental and dietary changes leading to a true rise in new cases has yet to be determined. The overall prevalence of UC and CD in western countries is 150–250 per 100,000 persons and 100–200 per 100,000 persons, respectively [87].
The incidence and prevalence of IBD also vary with age. CD and UC are both diseases of young people, with the highest incidence seen between 20 and 40 years of age. A second, smaller peak is seen in men and women between the sixth and ninth decades of life [88]. Whether this second peak represents a delayed diagnosis, misdiagnosed ischemic colitis or true de novo disease has been controversial.
Risk factors for IBD include high socio-economic status and urban environment. Male gender confers a slightly higher risk of UC with a male-to-female ratio of 1.7:1, whereas female gender may confer a small excess risk of CD with a male-to-female ratio between unity and 1:1.2. Smoking is a definite risk factor for CD. It doubles the risk for developing CD and increases the risk of having an aggressive disease course [89]. In contrast, smoking is protective for UC. A meta-analysis found that the risk of developing UC was 60% lower in smokers compared with nonsmokers [90].
The relationship between oral contraceptives and IBD is unclear. Early studies which did not account for tobacco use suggested an increased risk for the development of CD and UC in women using oral contraceptives. A more recent large Italian population-based study also found that women who used oral contraceptives for at least 1 month before the onset of symptoms had a threefold higher risk of CD [91]. However, no significant risk was found for UC and no dose–response relationship was seen. Other case–control studies have found a lack of association between oral contraceptives and both UC and CD [92,93].
The role of diet has been investigated in multiple studies. Associations have been reported for dairy products, fast food, cold drinks and refined sugars and both UC and CD; however, no causal explanation has been found between any specific dietary factor and IBD [94]. NSAID and cyclo-oxygenase-2 inhibitors may precipitate colitis and worsen the disease course of IBD [95]. However, as in the case of diet, no definite risk between NSAID use and the development of IBD has been found.
Appendectomy has been shown to be protective for UC in several studies, especially if appendicitis occurred before age 20 [96]. This protective effect may be due to postoperative changes to the immune system. Several infectious agents, most notably Mycobacterium paratuberculosis and measles virus, have been proposed to cause CD [97].
Pathophysiology
The exact pathogenesis of IBD is unknown. It is likely that IBD is due to complex interactions between genetic, immunologic and environmental factors. One theory that has been proposed is based on the inverse relationship between the incidence of IBD and the rates of enteric infection at the population level [98]. Dubbed the “hygiene hypothesis,” this theory suggests that early exposure to enteric infections protects against the future development of IBD.
It has long been theorized that genetic factors predispose to the development of IBD. Familial aggregation, ethnic variations in disease frequency and twin studies showing higher disease rates between monozygotic twins than dizygotic twins all suggest a role for genetic factors. Recently, specific susceptibility genes for CD and UC have been identified. The NOD2 gene located on chromosome 16 was the first of such genes [99,100]. Loss-of-function mutations in the NOD2 gene, as detected in some patients with CD, may impair intracellular bacterial sensing and immune response. CD patients who are carriers of NOD2 mutations display a specific phenotype. They are more likely to have small bowel disease as well to have early onset of disease, strictures and fistulas [101]. Other genetic loci that have been linked to IBD include the multidrug resistance 1 (MDR-1) gene on chromosome 7, SLC22A4/5 genes on chromosome 5 and DLG5 gene on chromosome 10.
In addition to genetic and environmental factors, immune dysregulation is also likely to be necessary for the development of IBD. Studies in CD have demonstrated disruptions in mucosal homeostasis with an excessive and persistent T- helper cell type 1 (Th1) immune response in the gut mucosa [102]. This creates a deficiency in regulatory T-cells, allowing patients with CD to react to their own microflora. In contrast, an atypical Th2 response has been described in UC [103].
Further evidence of the important role of the immune system in the pathogenesis of IBD is provided by the marked elevation of tumor necrosis factor (TNF)-alpha, an important mediator of intestinal inflammation, in patients with CD [104]. The central role of TNF in the pathogenesis of IBD is substantiated by the recent development of effective targeted pharmacotherapy using anti-TNF antibodies.
Clinical presentation
Patients with IBD present with a range of symptoms, depending on the extent and severity of the disease. While CD and UC have many overlapping signs and symptoms, substantial differences exist (Table 10.3). In UC, patients generally present with frequent, small-volume diarrhea [105]. Nocturnal stools may also be present. Stools are generally bloody although the amount of blood passed per rectum may vary from scant to copious. Patients may also report tenesmus and fecal urgency due to rectal involvement. Abdominal pain and cramping are also often present. Systemic symptoms such as fever, anorexia, weight loss, fatigue and malaise may be present in individuals with diffuse involvement of the colon (i.e. pancolitis). Patients with severe, diffuse colonic involvement, termed fulminant colitis, may have severe abdominal distension due to toxic megacolon.
Table 10.3 Distinguishing features of ulcerative colitis and Crohn’s disease
Reproduced with permission from Baumgert, DC, Sandborn, WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007;369:1641.
| Ulcerative colitis | Crohn’s disease | |
| Clinical features | ||
| Hematochezia | Common | Rare |
| Passage of mucus or pus | Common | Rare |
| Small bowel disease | No (except in backwash ileitis) | Yes |
| Can affect upper gastrointestinal tract | No | Yes |
| Abdominal mass | Rare | Sometimes |
| Extraintestinal manifestations | Common | Common |
| Small bowel obstruction | Rare | Common |
| Colonic obstruction | Rare | Common |
| Fistulas and perianal disease | No | Common |
| Biochemical features | ||
| Antineutrophil cytoplasmic antibodies | Common | Rare |
| Anti-Sacchromyces cervisiae antibodies | Rare | Common |
| Pathologic features | ||
| Transmural mucosal inflammation | No | Yes |
| Distorted crypt architecture | Yes | Uncommon |
| Cryptitis and crypt abscesses | Yes | Yes |
| Granulomas | No | Yes (but rarely in mucosal biopsies) |
| Fistulas and skip lesions | No | Yes |
Patients with small bowel and colonic CD may also present with diarrhea and abdominal pain. Perianal disease in the form of fissures, abscesses, and fistulas is common in CD and may be present in the absence of other gastrointestinal symptoms [106]. Intestinal obstruction from inflammation and fibrostenotic disease is also common in CD. Patients with esophageal and gastric involvement may report odynophagia, gastroesophageal reflux, dyspepsia, epigastric pain, and chest pain and upper gastrointestinal tract bleeding [107]. They also commonly present with anorexia, weight loss and cachexia.
Extraintestinal manifestations are seen in 20–40% of patients with IBD and may affect nearly every organ system [108]. They may precede the development of gastrointestinal disease. Some manifestations respond to treatment of the underlying bowel disease whereas others do not. Organ systems commonly involved include the skin, joints, and eyes [109]. Extraluminal gastrointestinal organs may also be involved. Dermatologic manifestations include erythema nodosum (reddish painful bumps, generally on the front of the leg below the knees) and pyoderma gangrenosum (deep ulcers with a violaceous border, usually on the legs). Sacroiliitis, ankylosing spondylitis, and peripheral arthritis are frequently encountered rheumatologic manifestations. Patients with ocular involvement may have iritis, uveitis and/or episcleritis. Primary sclerosing cholangitis and bile duct carcinoma are extraluminal gastrointestinal conditions associated with UC.
Diagnosis
The diagnosis of IBD relies on laboratory, radiographic, endoscopic and histologic data. Hematologic abnormalities that may be present include low hemoglobin, high white blood cell count with left shift and high platelet count [110]. C-reactive protein (CRP), a nonspecific marker for inflammation, has been shown to correlate well with disease activity in IBD, especially in CD [111]. CRP has been shown to be a more sensitive marker for detecting IBD compared to the erythrocyte sedimentation rate (ESR), albumin, and complete blood count abnormalities.
Stool examination may be positive for fecal leukocytes in IBD. This is a nonspecific finding and not helpful in the diagnosis of IBD [112]. However, newer fecal markers have emerged that have a higher sensitivity and specificity for IBD. These include fecal lactoferrin, elastase, myeloperoxidase and calprotectin [113]. Their utility as noninvasive markers of intestinal inflammation is the subject of active research. In the future, they may be used to avoid invasive examinations such as colonoscopy which may be particularly helpful during pregnancy. Radiographic imaging plays an important role in the diagnosis of IBD. Plain films are often obtained to rule out toxic megacolon (acute severe colitis with potentially life-threatening dilation of the colon), perforation and obstruction. Air-contrast barium studies are frequently obtained to evaluate for bowel wall thickening, loss of smooth mucosa, ulcers, dilated bowel, strictures, fistulas and perirectal disease [114]. However, their use is limited in pregnancy by high radiation exposure and variations in examiner experience [115]. Computed tomography (CT) is used widely to identify bowel wall thickening, intra-abdominal abscesses and other extraluminal complications of IBD. Over the last decade, bowel ultrasound has become an increasingly accepted mode of evaluating IBD, particularly in Europe. Ultrasound can detect transmural inflammation and determine the anatomic location of CD and its extension within the bowel [116]. Newer cross-sectional imaging techniques, including CT enterography, MRI and MRI enterography are potential future strategies to evaluate intra- and extraluminal changes in IBD.
Despite the development of noninvasive markers of inflammation and novel imaging techniques, endoscopy with biopsy remains the gold standard for the diagnosis of suspected IBD. No endoscopic finding is specific for IBD but the pattern of inflammation can help distinguish UC from CD [117]. UC is characterized by inflammation in the rectum spreading continuously and uniformly to the proximal colon. The extent of colonic involvement is variable, with 50% of patients having disease confined to the rectosigmoid. Disease is generally limited to the colon in UC, although distal small bowel involvement may occur in up to 17% of patients [118]. Termed “backwash” ileitis, this inflammation in the distal terminal ileum is likely due to reflux of colonic contents into the ileum from an incompetent ileocecal valve.
In contrast, CD is characterized by focal intestinal inflammation with segments of complete or relative sparing (i.e. “skip lesions”). One-third of cases of CD involve the small bowel only, while 20% of patients have disease limited to the colon [119]. The majority of remaining cases have ileocolitis. Endoscopic evaluation of the small bowel for CD has been facilitated by the introduction of wireless capsule endoscopy which has been reported to have up to a 70% yield in diagnosing small bowel CD [120].
Pathologic features in IBD include architectural distortion, cryptitis and crypt abscesses. These are nonspecific findings and may be seen in other causes of colitis. Granulomas are a hallmark feature of CD but may be seen in UC as well.
A definitive diagnosis can be made in the majority of cases of chronic IBD but up to 10–15% of adult cases have overlapping features of UC and CD and are deemed indeterminate [121]. In some of these cases, checking the recently characterized IBD-specific antibodies (e.g. ASCA, p-ANCA, omp C, c-Bir 1) may prove helpful but their routine use is still controversial.
The differential diagnosis includes infectious colitis, ischemic colitis, diverticular disease-associated colitis, medication-induced colitis (e.g. NSAID, antineoplastic drugs), radiation colitis/proctitis, and Behçet’s disease [119]. Colonic and small bowel neoplasms may also present with inflammatory changes that resemble IBD. Noninflammatory causes of hematochezia which should be ruled out include hemorrhoids and angiodysplasias of the colon and small bowel. Irritable bowel syndrome may share some clinical features of IBD but is easily distinguished from IBD in most cases due to the absence of laboratory, radiographic, endoscopic and histologic findings.
Fertility
As IBD is a disease of young people, often presenting during the childbearing years, fertility is a major concern. In general, infertility rates for women with IBD, which range from 5% to 14%, are no higher than in the normal population [122–124]. Initial epidemiologic data suggested higher infertility rates in women with CD but these data did not account for higher voluntary childlessness rates in IBD patients presumed secondary to self-image and sexual dysfunction common in affected individuals [125].
Fertility may, however, be compromised in women who have undergone surgery for IBD refractory to medical therapy. While resectional surgery does not decrease and may in fact improve fertility in women with Crohn’s disease [126, 127], ileal pouch–anal anastomosis (IPAA) after total colectomy is associated with a dramatic decrease in female fertility. IPAA has become an option as treatment for pancolitis poorly controlled by medical therapy or for longstanding UC with dysplastic premalignant features. A Scandinavian study of 237 women who had undergone IPAA found that postoperative births were 49% of expected and only 35% of expected when in vitro fertilization was excluded [128]. A subsequent meta-analysis which included eight studies confirmed that the relative risk of infertility was 48% after IPAA [129]. While some of this effect may be due to the fact that women with severe IBD (and therefore more likely to have tubal disease) are more likely to require an IPAA, this decrease in fertility has also been attributed to tubal dysfunction caused by abnormal adhesion formation after deep pelvic dissection and appears to be permanent. No procedural factors have been identified (e.g. one-step versus two-step procedure) that consistently affect the risk of infertility. Women considering having children and requiring proctocolectomy should be informed of this risk for infertility as deferring reconstruction after completing childbearing may be a preferable approach.
Fertility in women with CD may also be impaired due to active inflammation causing direct or indirect complications [130]. Transmural inflammation from CD can create fistulas from the rectum, proximal colon and ileum to the uterus and ovaries. Disease may also directly extend to the fallopian tubes and ovaries, causing granulomatous salpingitis and oophoritis. Indirectly, CD may decrease fertility by causing anovulatory cycles or amenorrhea or by causing dyspareunia and impairing sexual function.
Regarding fertility in men with IBD, long-term treatment with sulfasalazine has been shown to decrease sperm counts and semen quality. This infertility is temporary and reverses with discontinuation of the drug. Male fertility otherwise does not appear to be affected by IBD [131].
Preconception considerations
Preconception counseling and management are of fundamental importance to insure a successful pregnancy in patients with IBD. Indeed, discussion about reproductive issues should be a routine part of the care of women with IBD of childbearing age. In addition to fertility issues, preconception counseling should include educating the patient and her partner that the optimal time to conceive is during a period of clinical remission as this maximizes the chance of maintaining remission during pregnancy without escalating therapy.
During the preconception period, potentially teratogenic drugs should be discontinued. Sulfasalazine interferes with folic acid metabolism and patients wishing to get pregnant should be switched to one of the newer 5-aminosalicylates or increase their dose of folic acid supplementation to 2 mg/day to counter the effects of sulfasalazine on folic acid metabolism, which could theoretically increase the risk of neural tube defects in the fetus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


